Abstract
Purpose of Review
This article will discuss high-resolution CT (HRCT) and MRI of the pediatric temporal bone with a focus on variant anatomy that can mimic pathology or affect surgical planning, as well as some potential pitfalls in image interpretation.
Recent Findings
The latest research shows that with improving imaging technology, there is better visualization of temporal bone structure, both normal and abnormal, on HRCT and MRI. Examples include earlier detection of cochlear obstruction in labyrinthitis ossificans with MRI, the ability to better define ossicular chain abnormalities, and the identification of pericochlear lucency in children without hearing loss.
Summary
Advances in temporal bone imaging have contributed to a greater understanding of normal anatomy as well as temporal bone pathology and its implications for treatment and surgical planning. It is clear that correlation of imaging findings with clinical and surgical findings will be an essential part of future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
HRCT has been the mainstay for detailed imaging evaluation of the diminutive and complex anatomy of the temporal bone for roughly the past 40 years. Over that time, significant advances in CT imaging capabilities and the advent of higher resolution MRI sequences have led to a greater ability to visualize both normal and variant anatomy and pathology. We will discuss vascular variants, both venous and arterial, as well as several common variations of normal bony anatomy that may mimic pathology or have important surgical implications. We will include a brief description of “pseudofractures,” normal canals and sutures that may be mistaken for fractures. Finally, we will discuss potential pitfalls in temporal bone imaging, subtle clues to pathology which may not be readily apparent to the inexperienced observer.
Variant Anatomy
Vascular Variants
Variations of arterial anatomy within the temporal bone are relatively less common than variations of venous anatomy, but are of great importance because of the potential for significant intraoperative bleeding if not recognized prior to surgery. These include an aberrant or dehiscent internal carotid artery (ICA) and the persistent stapedial artery. These variations are well depicted on CT with MR arteriography and venography complimentary in evaluation.
Dehiscent or Aberrant Internal Carotid Artery (ICA)
The normal ICA courses through the petrous temporal bone within the carotid canal, first as a vertical segment located anterior to the cochlea and medial to the tympanic cavity, then continuing anteromedially as the horizontal segment before entering the cavernous sinus [1]. The vertical segment is normally separated from the tympanic cavity by a very thin plate of bone often referred to as the carotid plate, which in one study of 83 adult and pediatric temporal bone specimens by Hasebe et al. [2] was found to measure 0.24 mm on average, and showed a positive correlation of thickness with age in the pediatric population. Dehiscence of this bony plate can result in herniation of the ICA into the tympanic cavity, appearing on HRCT as bony uncovering of a laterally displaced vertical segment with otherwise normal course of the ICA through the temporal bone (Fig. 1). There may also be deficiency of the intervening bone between the petrous carotid canal and the basal turn of the cochlea, resulting in greater risk for vascular injury during cochlear implantation [3, 4].
An aberrant carotid artery is thought to result from agenesis or involution of the upper cervical and vertical ICA segments during embryologic development with persistence of alternate blood flow pathways to maintain carotid circulation. The aberrant vessel is actually an enlarged inferior tympanic artery, a branch of the ascending pharyngeal artery, that courses through the inferior tympanic canaliculus into the tympanic cavity where it anastomoses with the caroticotympanic vessels and feeds into the horizontal ICA via a defect in the carotid plate [1, 5, 6]. An aberrant ICA is identified on HRCT, and differentiated from other retrotympanic masses, by four classic findings: absence of the vertical ICA canal, enlarged inferior tympanic canaliculus, aberrant vessel coursing through the hypotympanum, and dehiscence of the carotid plate where the aberrant vessel joins the horizontal ICA [1, 5].
Persistent Stapedial Artery (PSA)
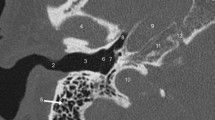
Like the other arterial anomalies of the temporal bone, the PSA is a rare but important variant that can be a source of significant complications if not known prior to surgery. The stapedial artery is a transient embryologic structure that gives rise to several branches that eventually become part of the external carotid system supplying the orbit, meninges and face. The middle meningeal artery (MMA) is one of these branches, eventually supplied by the internal maxillary artery following the normal regression of the stapedial artery. In the setting of a PSA, the MMA remains irrigated by the PSA either via the caroticotympanic artery or inferior tympanic artery. In either case, HRCT shows absence of the foramen spinosum due to the anomalous origin of the MMA. The inferior tympanic canaliculus may be enlarged if the inferior tympanic artery gives rise to the PSA. The PSA can be seen on HRCT arising from the inferior petrous carotid canal, ascending within the tympanic cavity over the cochlear promontory, through the obturator foramen of the stapes and then coursing anteriorly, parallel to or within the facial canal (Fig. 2) [7,8,9].
Persistent stapedial artery. Axial HRCT of the left temporal bone inferior to superior (a–c) and coronal (d). a Inferiorly, there is an aberrant vessel (white arrow) arising from the distal vertical segment of the ICA. b The vessel can be seen ascending within the tympanic cavity coursing over the cochlear promontory (white arrow), then c, coursing anteriorly within the enlarged tympanic facial nerve canal (white arrow) toward the middle cranial fossa. d Coronal HRCT of the left temporal bone shows the aberrant vessel coursing over the cochlear promontory (white arrow)
A dehiscent or aberrant ICA and PSA can present with pulsatile tinnitus and may be visualized at otoscopy as a red pulsatile retrotympanic mass, although, if covered by bone, may look similar to a cholesteatoma. An aberrant ICA may also be a cause of conductive hearing loss. Frequently, however, these arterial anomalies are asymptomatic or nonspecific in their presentation, and may not be suspected clinically, highlighting the importance of identifying the abnormality prior to surgery.
High and Dehiscent Jugular Bulb
Venous variants are more likely to be encountered, most commonly the high jugular bulb (HJB) with or without dehiscence. A high and/or dehiscent jugular bulb may present with tinnitus, vestibular symptoms or conductive hearing loss (CHL), and may be seen as a vascular retrotympanic mass at otoscopy, but is more typically asymptomatic and therefore important to recognize on preoperative imaging [7, 8, 10,11,12,13]. The jugular bulb represents the junction of the sigmoid sinus and internal jugular vein. Its normal position is variable, generally described as lying below the hypotympanum [14, 15]; however, multiple definitions exist in the literature to describe a HJB, anywhere from above the lower level of the IAC [10] to above the inferior bony tympanic annulus to above the round window and basal turn of the cochlea, resulting in an incidence of 3.5–34% depending on which definition is used [8, 13, 16, 17]. A HJB can protrude into the tympanic cavity or external auditory canal (EAC) [11] and, when associated with dehiscence of the overlying sigmoid plate, becomes even more vulnerable to injury at the time of surgery (Fig. 3). A jugular bulb diverticulum is differentiated by a more superior medial projection, which is important in the planning of surgery involving the petrous apex.
High/dehiscent jugular bulb. a and b Coronal and axial HRCT of the right temporal bone shows a high jugular bulb at the level of the tympanic cavity overlying the oval window (black arrow in a) with focal dehiscence at the level of the cochlear promontory (white arrow in b). c Coronal HRCT of the right temporal bone in a different patient shows a high right jugular bulb with focal protrusion through a dehiscence (white arrow) into the tympanic cavity
Anteriorly Positioned Sigmoid Sinus
The position of the sigmoid sinus is highly variable and may therefore be at risk during mastoid surgery [8, 17]. There are typically no presenting symptoms of an anteriorly positioned sigmoid sinus unless there is coexistent sigmoid plate dehiscence, which may cause pulsatile tinnitus [18] (Fig. 4). Using the definition of Tomura et al. [13], an anteriorly positioned sigmoid sinus is present on HRCT when no AP distance can be seen between the posterior wall of the EAC and the sigmoid sinus on axial images, which resulted in an incidence of 1.6% in their study. However, sinus-EAC distance of <10 mm on axial images has been considered a significant preoperative finding, present in 12.4% of patients in a study by Atilla et al. [17]. An anteriorly positioned sigmoid sinus may limit the surgical approach and is more vulnerable to injury at mastoid surgery [17, 19].
Dehiscent sigmoid plate. a Axial HRCT of the left temporal bone in a patient with pulsatile tinnitus shows slight bulging of the left sigmoid sinus anteriorly with multifocal dehiscence of the overlying sigmoid plate (white arrow). b Contralateral right temporal bone HRCT for comparison to the normal side
Emissary Veins
There are several transtemporal venous drainage pathways, which serve as additional conduits between the intracranial and extracranial venous circulation. They are the posterior condylar vein, the mastoid emissary veins (MEVs) and the petrosquamosal sinus [20]. Aside from providing collateral venous outflow pathways in some normal individuals, their primary importance lies in their vulnerability during surgery and their occasional function as the only venous outflow in some individuals. Iatrogenic injury to these valveless veins is known to be a source of significant intraoperative bleeding, and potentially air embolism or dural sinus thrombosis at the time of middle ear or skull base surgery. Individuals with markedly hypoplastic or absent jugular veins, as is often the case in congenital skull base malformations such as CHARGE syndrome or achondroplasia, may be completely reliant on these emissary veins for venous drainage [21], and there are reports of venous infarct, hemorrhage and even death related to ligation or coagulation of an emissary vein at surgery [22,23,24].
MEVs act as a conduit between the sigmoid sinus and suboccipital venous plexus. They are commonly seen on HRCT, with a reported incidence of 63–92% [8, 22, 25, 26], appearing as a single or multiple bony canals traversing the retromastoid temporal bone (Fig. 5). The size of the MEV is variable, reported as measuring 2.15–3.5 mm average diameter [22, 27] with enlargement possible in the setting of high flow vascular lesions, severely hypoplastic or absent jugular veins and/or certain syndromic patients.
Persistent Petrosquamosal Sinus (PPS)
The petrosquamosal sinus is an emissary vein that normally regresses during fetal development. A PPS becomes a conduit between the intracranial and extracranial venous drainage, linking the transverse sinus to the retromandibular vein and pterygoid venous plexus [20]. The bony canal or groove containing the sinus can be visualized on HRCT coursing anteriorly from the far lateral transverse sinus, along the superolateral margin of the petrous bone at the superior margin of the petrosquamosal suture. It then exits the skull base through a post-glenoid foramen [28]. It is typically quite small but has been reported as large as 2–4 mm diameter [20]. There is a reported association with semicircular canal aplasia and congenital skull base malformations [28]. Importantly, both SSC aplasia and PPS have been reported in association with CHARGE syndrome, patients in whom middle ear surgery is often considered [20, 28,29,30].
Nonvascular Variants
Deep Sinus Tympani
The sinus tympani is the posterior tympanic cavity bony recess located medial to the pyramidal eminence, stapedial muscle and descending facial canal. It may be involved in the setting of chronic middle ear infection and acquired cholesteatoma [31]. A deep sinus tympani has been defined as a depth >6 mm in the axial plane on HRCT [31]. Using this definition, a deep sinus tympani was present on HRCT in 5.9% of cases in a study by Tomura et al. [13]. A deep sinus tympani may come in close proximity to the posterior semicircular canal or facial nerve, resulting in greater vulnerability of these structures during middle ear surgery, when the full extent of the sinus tympani may be very difficult for the surgeon to directly visualize (Fig. 6). Knowledge of the sinus tympani morphology and relation to surrounding structures can aid in determining surgical approach [13, 31].
Enlarged Internal Auditory Canal (IAC)
An enlarged IAC has been described as a sign of pathology on imaging, as in the case of bony expansion from a vestibular schwannoma. However, there is great variability in the size of the IAC reported in studies of both temporal bone specimens and HRCT, ranging from 4 to 8 mm at the porus acousticus, to 2–5.8 mm at its midportion, to 2–8 mm at its lateral aspect [32,33,34]. Greater diameters have been reported in normal patients [13, 19]. If an intact crista falciformis can be visualized on coronal images and the cortical outline of the IAC is normal, the likelihood of underlying pathology in a patient with isolated IAC enlargement is very low [13, 19]. It is important to note, however, that an enlarged funnel-shaped IAC may be seen in syndromic conditions such as Pendred syndrome or branchio-oto-renal syndrome, and should always prompt a search for associated abnormalities (Fig. 7).
Funnel-shaped IACs. a Axial HRCT of the right temporal bone shows unusual morphology of the IAC, a bilateral finding, without cortical erosions. No underlying etiology was found. b Axial HR 3D T2-weighted MRI of the IACs in a patient with Pendred syndrome shows bilateral funnel-shaped IACs. Labyrinthine dysplasia and enlarged endolymphatic duct and sac are present (not shown)
Pericochlear Lucency
Regions of lucency within the otic capsule on CT are classically thought to be evidence of a demineralizing process such as otosclerosis or osteogenesis imperfecta (OI) (Fig. 8a, b). These foci typically occur in the region of the fissula ante fenestrum, a small region of fibrocartilaginous tissue between the middle and inner ear just anterior to the oval window, and patients typically present in late adolescence with a conductive hearing loss (CHL), sensorineural hearing loss (SNHL) or a mixed hearing loss [35]. However, the significance of this finding in pediatric patients has been called into question, based on reports of pericochlear lucencies in asymptomatic infants and children. Several studies of pediatric temporal bones have shown a greater prevalence of this finding in children, no significant correlation with SNHL, and a positive correlation of bone density of the otic capsule with age [36∙, 37, 38]. Sanverdi et al. [36]∙ found pericochlear lucencies in children up to 15 years of age with much greater degree of lucency seen in those <6 months of age. Moser et al. [39] studied the histologic and CT appearance of temporal bones from 66 cadavers and found foci of hypoattenuation of both the fissula ante finestrum and the anterior otic capsule in all fetal specimens and pediatric specimens up to 4 months of age that corresponded to regions of normal cancellous bone. In children, foci of pericochlear lucency in the absence of clinical signs of otosclerosis or OI may simply relate to incomplete endochondral ossification of the otic capsule (Fig. 8c) [36∙, 39].
Pericochlear lucencies. a Axial HRCT of the right temporal bone in a patient with osteogenesis imperfect shows pericochlear lucency (white arrow) in the region of the fissula ante finestrum. b Axial HRCT of the right temporal bone in a patient without hearing loss shows similar pericochlear lucency (white arrow)
Pseudofractures
Within the temporal bone there are a number of normal structures that are not consistently visualized on imaging and may be mistaken for fracture when visible on HRCT. These “pseudofractures” include several small canals and sutures whose visibility is dependent on their size and imaging parameters, such as slice thickness. They are the singular canal, arcuate artery canal, mastoid canaliculus, inferior tympanic canaliculus, the vestibular aqueduct (VA), and the multiple intrinsic and extrinsic sutures of the temporal bone. The VA deserves special attention, not only as a potential pseudofracture but also for its association with certain pathology when abnormally enlarged.
The VA is normally seen as a J-shaped bony canal running from the vestibule to the posterior margin of the petrous bone. It contains the endolymphatic duct and has a slight prominence at its distal end where it contains the endolymphatic sac. Abnormal enlargement of the VA is seen in association with SNHL in the appropriately named large vestibular aqueduct syndrome (LVAS) [40]. Patients with LVAS typically present with progressive SNHL at birth or in early childhood. Sudden onset of rapidly progressive hearing loss can occur with minor head trauma. Due to the resultant third window phenomenon from an abnormally large communication between the vestibule and VA, patients may also present with a CHL [41]. Enlarged VA is seen in association with other inner ear malformations of the cochlea and vestibule [42] and several syndromes, including Pendred syndrome, BOR syndrome, and CHARGE syndrome (Fig. 9) [41, 43, 44]. Several criteria have been proposed for defining an enlarged VA; however, the authors prefer the Boston–Cincinnati Criteria which define a large VA as measuring >0.9 mm mid-aperture and >1.9 mm at the operculum in children [40, 43, 45,46,47].
Pitfalls
Ossicular Chain Anomalies
There are myriad congenital abnormalities of the ossicular chain that can result in congenital hearing loss. Ossicular chain anomalies (OCA) may be seen in isolation or in conjunction with other malformations of the middle ear, external canal, and auricle. Any child with unexplained CHL may benefit from a HRCT to determine the etiology and potentially direct surgical reconstruction of the ossicular chain.
In the setting of isolated OCA (without EAC stenosis), hearing loss is related to abnormal fixations or discontinuities of the ossicular chain (Figs. 10, 11). The stapes is most frequently involved, with fixation of the stapes footplate being the most common isolated congenital middle ear malformation [48,49,50]. In the preoperative evaluation of stapes fixation, it is important to note the patency of the round window, as round window atresia has been reported in association with stapes fixation and stapedectomy alone may not improve hearing in this setting [51]. Evidence of oval window atresia should also be sought on preoperative HRCT in the setting of congenital hearing loss as oval window development is induced by contact with the stapes footplate [52]. Absence, malposition, or severe dysplasia of the stapes has been described in association with oval window atresia [52, 53]. Because the stapes superstructure, the lenticular process of the incus and the facial nerve are all derived from the second branchial arch, anomalies of these structures often coexist with oval window atresia (Fig. 12) [50, 52,53,54].
Multiple ossicular fixation in an asymmetrically small tympanic a cavity. a Coronal HRCT of the right temporal bone shows fixation of the head of the malleus to the tegmen tympani (white arrow). b Axial HRCT of the right temporal bone shows fixation of the body of the incus to the bony covering of the tympanic facial nerve (white arrow). c Axial HRCT of the right temporal bone shows discontinuity of the incudostapedial joint (white arrow)
Absent lenticular process of the incus. a Axial HRCT of the right temporal bone shows demineralization distal incus (white arrow) extending toward a dysplastic stapes which is abnormally inferiorly displaced with respect to the oval window. b Axial HRCT of the left temporal bone for comparison shows normal orientation of the distal incus (white arrow) in relation to the normally positioned stapes
Facial nerve malposition in the setting of multiple ossicular chain anomalies. a Axial HRCT of the right temporal bone shows abnormal lateral position of the distal tympanic segment of the facial nerve (white arrow). b Coronal HRCT in the same patient shows the proximal descending facial nerve (black arrow) anteriorly positioned at the level of the oval window. The stapes superstructure is not visualized in either plane
Several imaging clues have been described that may help in identification of subtle ossicular chain abnormalities on HRCT [55]. These include increased distance of the malleus handle from the cochlear promontory, indicating lateral displacement or abnormal rotation (Fig. 13); increased incudostapedial angle, indicating abnormal ossicular position or morphology; and narrowing or widening of Prussak space, suggesting abnormal ossicular position.
Malleus fixation in a patient with mild EAC stenosis. a Axial HRCT of the left temporal bone shows increased distance of the malleus handle (white arrow) from the cochlear promontory due to abnormal vertical orientation in the setting of lateral fixation at the level of the anterior tympanic annulus. b Axial HRCT of the normal right temporal bone for comparison shows the normal position of the malleus handle (white arrow)
Semicircular Canal Dehiscence (SCD)
The etiology of SCD may be idiopathic, although several risk factors have been proposed, including chronic otitis media with cholesteatoma, pressure erosions from vascular pulsations, developmentally thin bone covering the semicircular canal (SCC) and trauma [41, 56]. SCD leads to the so-called third window phenomenon, via abnormal communication between the bony labyrinth and either the cranial cavity or middle ear or vascular structures such as the superior petrosal sinus, depending on which SCC is dehiscent. The abnormality is suggested clinically by specific audiometric findings, CHL characterized by an air-bone gap particularly at lower frequencies, and clinical symptoms including vertigo and nystagmus induced by noise or pressure (Tullio phenomenon and Hennebert sign, respectively) [57]. It is recognized on HRCT by absence of the bony covering of the semicircular canal (SCC), best seen on reformatted images parallel and perpendicular to the involved semicircular canal (Figs. 14, 15) [41, 58, 59]. The superior SCC is most commonly involved.
However, several studies have shown HRCT to be sensitive but not specific for SCD, particularly prone to false positives in children even with appropriate slice thickness and reformatted images [56, 58, 60, 61]. Hagiwara et al. [56] found a higher incidence of radiographic SCD in children without clinical symptoms consistent with SCD syndrome. This difference was most pronounced in the <2 years age group. A study of temporal bone specimens by Carey et al. [62] showed that the bone overlying the SCC in children is extremely thin, such that it may be below the resolution of a HRCT slice thickness of 0.5 mm, and that it continues to increase in thickness over the first 3 years of life. These findings have led some to hypothesize that volume averaging and immature bone in the pediatric population may lead to an overdiagnosis of SCD [56, 62]. Furthermore, it has been suggested that development of SCD syndrome, which typically presents in older patients, is the result of a two-hit process: first, an arrest of bone maturation early in life followed by a second hit such as trauma or pressure erosion later in life [56, 62, 63]. The significance of radiographic SCD in the pediatric population is not fully known; however, it is clear from the literature that HRCT may result in false positives, especially in very young children, and care should be taken when interpreting these studies.
Perilymphatic Fistula/Gusher
A perilymphatic fistula (PLF) is an abnormal communication between the perilymph and middle ear through a defect in the otic capsule, the round window or oval window. The fistula may be congenital or acquired, a potential consequence of surgery, trauma (fracture involving the otic capsule), or infectious/inflammatory processes (cholesteatoma eroding into the lateral SCC or cochlea) [41, 64]. A CSF leak may develop through the oval window as a result of chronically increased pressure within the perilymphatic space as may occur in the setting of an abnormal communication between the perilymphatic space and CSF [65]. Patients may present with vertigo, hearing loss, otorrhea, and even recurrent meningitis due to abnormal communication between the subarachnoid space, perilymph, and middle ear. Some cases may result in third window phenomenon, depending on the site of fistula. HRCT findings suggestive of a PLF include a defect in the bone overlying the lateral SCC or cochlear promontory, bulging of the oval window into the tympanic cavity (if there is perilymphatic hydrops) and deficient lamina cribrosa (Fig. 16) [65]. HRCT may show pneumolabyrinth in the setting of traumatic perilymphatic fistula, with pneumocochlea portending a worse outcome [64].
Common cavity deformity with gusher. a Axial HRCT of the right temporal bone shows dysplasia of the otic capsule with a single large common cavity (black arrow) and deficiency of the lamina cribrosa between the IAC (white arrow) and cochlea. b Axial HRCT more inferiorly shows bulging of the oval window into the tympanic cavity (white arrow), a sign of perilymphatic hydrops and risk for gusher
Patients with abnormal communication between the CSF space and perilymph are at risk for a perilymphatic gusher, also referred to as a CSF gusher, a sudden rapid flow of fluid from the cochlea that may occur during stapedectomy or cochlear implantation. The abnormal communication may be a result of congenital deficiency of bone (lamina cribrosa) between the cochlea and IAC, modiolar deficiency associated with cochlear malformations, or an enlarged endolymphatic duct/sac (Fig. 17) [66,67,68,69,70]. Erosion of the lateral SCC or cochlea by a cholesteatoma may also result in a gusher at surgery (Fig. 18) [41]. The margins of the bony labyrinth and middle ear cavity should always be inspected closely, especially at the level of the cochlear promontory and lateral SCC.
Lateral SCC erosion in the setting of a middle ear mass. a Coronal HRCT of the left temporal bone shows focal erosion of the bony covering of the lateral SCC (white arrow) with abnormal soft tissue filling the adjacent tympanic cavity. b Coronal HRCT of the contralateral side for comparison shows the normal lateral SCC (white arrow)
A deficient lamina cribrosa between the basal turn of the cochlea and the lateral aspect of the IAC is thought to be the etiology of a gusher at the time of cochleostomy during cochlear implantation in patients with certain malformations of the bony labyrinth, including common cavity deformity, incomplete partition type I, incomplete partition type II (Mondini malformation), or cochlear hypoplasia [68, 71]. It is also thought to be the etiology of a stapes gusher during stapedectomy in patients with X-linked congenital mixed hearing loss with stapes gusher, a syndrome first described by Nance et al. [70] in 1971, now classified as incomplete partition type III (IP III). The most consistently described HRCT finding in IP III is an enlarged IAC and incomplete separation of the IAC from the basal turn of the cochlea (Fig. 19) [72,73,74,75]; other inconsistently described features include a deficient modiolus, enlarged labyrinthine facial nerve canal, and abnormal vestibular aqueduct [67, 72, 74,75,76,77]. Preoperatively, it is important to recognize findings suggestive of this association in order to avoid a stapes gusher which can lead to worsening of SNHL [69]. These findings may also direct the clinician to an etiology of meningitis and the ability to eliminate the morbidity related to recurrent meningitis [78].
A normal modiolus, aside from providing a pathway for the cochlear nerve, acts as a barrier to CSF flowing from the IAC into the cochlea [79]. In the absence of a normal modiolus, a more direct communication exists between the IAC and cochlea, resulting in a source of possible gusher at cochlear implantation surgery. Although bony structures such as the modiolus are typically better evaluated by CT, recent studies have shown a greater sensitivity to detecting modiolar deficiency with MRI [79].
Patients with LVAS are at risk for gusher at cochlear implant not only because of the abnormally patent vestibular aqueduct but also because of the frequent association of LVAS with modiolar deficiency [79,80,81].
Labyrinthitis Ossificans
Patients with labyrinthitis ossificans typically present with SNHL and a history of meningitis, although trauma, sickle cell disease, autoimmune diseases, and adjacent chronic infection/inflammation (otitis media and cholesteatoma) have also been reported [82∙, 83]. The CT findings of labyrinthitis ossificans (LO) were first described in a report by Swartz et al. [84] in 1985, which demonstrated regions of labyrinthine ossification, representing the late ossification stage of the disease. However, it is not only the late stage but also the early fibrous stage of LO that can make electrode insertion at cochlear implantation difficult or even impossible. LO can occur as early as 2 weeks following meningitis [85]. Several studies have shown that HRCT is insensitive to the fibrous stage, while it may be detected on MRI by a loss of normal fluid signal within the membranous labyrinth (Fig. 20) [79, 83, 86, 87]. The proximal scala tympani within the inferior basal turn is commonly involved in isolation. High-resolution MRI is imperative to delineate the two main scalar chambers and degree of involvement, which is helpful in determining surgical approach [82∙, 83]. At the time of MR imaging in children for meningitis, axial 2–3 mm post-contrast images through the temporal bones may be helpful to detect the acute inflammatory phase and predict the development of SNHL, which can be suggested by presence of labyrinthine enhancement [88]. This may help to rapidly direct these children to the otolaryngologist for implant evaluation. Once osseous obstruction is present, the success of cochlear implantation decreases.
Labyrinthitis ossificans. a Axial HR 3D T2-weighted image of the temporal bones shows loss of normal fluid signal in the inferior basal turn of the right cochlea (white arrow). b Axial HR 3D T2-weighted image of the temporal bones in a different patient shows loss of normal fluid signal within the inferior basal turn of the cochlea bilaterally (white arrows)
Facial Nerve
It is imperative to evaluate the position of the facial nerve on temporal bone CT. Abnormalities of the facial nerve as it courses through the temporal bone are best evaluated with HRCT which allows assessment of the size and morphology of the facial canal. The facial nerve canal may be dehiscent, most commonly along the inferolateral or medial wall of the tympanic segment, although dehiscence at the level of the anterior epitympanic air cell or jugular fossa has been described [89]. Dehiscence is relatively frequent, with a prevalence ranging from 55 to 74% in histologic studies of temporal bone specimens [90,91,92]. Not only does this place the facial nerve at greater risk during surgery but the nerve may protrude through the defect and may be mistaken for a soft tissue mass [89, 93,94,95]. Identification of focal dehiscence by HRCT becomes somewhat more difficult when there is abnormal soft tissue around the facial canal [96] and although HRCT is the best modality for evaluating for dehiscence it is not 100% sensitive either with or without soft tissue in the middle ear [96].
The facial nerve may have an abnormal course through the temporal bone. Because the second branchial arch, which gives rise to the facial nerve, and the first pharyngeal pouch, which gives rise to the EAC, are developing simultaneously in utero, an abnormal course of the facial nerve is commonly seen in patients with congenital aural atresia and microtia (Fig. 21) [93, 97]. The tympanic segment of the facial nerve may be anteriorly and medially displaced such that it overlies the oval window. It is thought that the facial nerve may even contribute to oval window atresia in this setting by interfering with its normal induction by the stapes footplate (Fig. 22) [93]. The mastoid segment of the facial nerve may be anterolaterally displaced, with a slightly more horizontal course as it descends [97]. Any aberrant course of the facial nerve results in greater vulnerability and may obstruct access to the middle ear and/or oval window during atresia repair surgery. As in the case of dehiscence, HRCT is the most appropriate preoperative imaging modality for determining facial nerve position; however, it is not 100% sensitive, and abnormalities may be seen intraoperatively that are not apparent on CT [97,98,99,100]. In the setting of EAC atresia or stenosis, it is important to look for coexistent abnormalities of the ossicular chain, most commonly incudomalleal fusion, bony fusion of the malleus neck to the atretic plate, laterally displaced malleus handle with fixation to the anterior tympanic annulus, and a hypoplastic handle of the malleus [55, 89].
Malpositioned facial nerve in the setting of EAC atresia. Coronal HRCT of the right temporal bone shows the descending segment of the facial nerve abnormally anteriorly positioned with an abnormal anterolateral course through the temporal bone, exiting the bony atretic plate (white arrow) at the level of the cochlea
Conclusion
Though only a small part of the human body, the temporal bone represents a significant source of pathology. For the radiologist, it can present substantial diagnostic difficulty with its complex and variable anatomy and pathology. As advances in imaging technology allow better visualization of the temporal bone anatomy, it is imperative to not only have an in-depth understanding of normal imaging appearance to avoid over diagnosis, but also to be able to recognize temporal bone pathology and its potential implications.
References
Papers of particular interest, published recently, have been highlighted as: ∙ Of importance
Sauvaget E, Paris J, Kici S, Kania R, Guichard J, Chapot R, et al. Aberrant internal carotid artery in the temporal bone. Arch Otolaryngol-Head Neck Surg. 2006;132:86.
Hasebe S, Sando I, Orita Y. Proximity of carotid canal wall to tympanic membrane: a human temporal bone study. Laryngoscope. 2003;113:802–7.
Young R, Shatzkes D, Babb J, Lalwani A. The carotid-cochlear interval: anatomic variation and potential clinical implications. Am J Neuroradiol. 2006;27:1486–90.
Wysocki J, Skarzyñski H. Distances between the cochlea and adjacent structures related to cochlear implant surgery. Surg Radiol Anat. 1998;20:267–71.
Lo W, Solti-Bohman L, McElveen J. Aberrant carotid artery: radiologic diagnosis with emphasis on high-resolution computed tomography. RadioGraphics. 1985;5:985–93.
Lasjaunias P, Moret J, Manelfe C, Theron J, Hasso T, Seeger J. Arterial anomalies at the base of the skull. Neuroradiology. 1977;13:267–72.
Glasscock M, Dickins J, Jackson C, Wiet R. Vascular anomalies of the middle ear. Laryngoscope. 1980;90:77–88.
Koesling S, Kunkel P, Schul T. Vascular anomalies, sutures, and small canals of the temporal bone on axial CT. Clin Imaging. 2005;29:444–5.
Thiers F, Sakai O, Poe D, Curtin H. Persistent stapedial artery: CT findings. Am J Neuroradiol. 2001;21:1551–4.
Atmaca S, Elmali M, Kucuk H. High and dehiscent jugular bulb: clear and present danger during middle ear surgery. Surg Radiol Anat. 2013;36:369–74.
Ball M, Elloy M, Vaidhyanath R, Pau H. Beware the silent presentation of a high and dehiscent jugular bulb in the external ear canal. J Laryngol Otol. 2009;124:790–2.
Moore P. The high jugular bulb in ear surgery: three case reports and a review of the literature. J Laryngol Otol. 1994;108.
Tomura N, Sashi R, Kobayashi M, Hirano H, Hashimoto M, Watarai J. Normal variations of the temporal bone on high-resolution CT: Their incidence and clinical significance. Clin Radiol. 1995;50:144–8.
Haginomori S, Sando I, Miura M, Orita Y, Hirsch B. Medial high jugular bulb. Otol Neurotol. 2001;22:423–5.
Overton S, Ritter F. A high placed jugular bulb in the middle ear: a clinical and temporal bone study. Laryngoscope. 1973;83:1986–91.
Towbin R, Ball W, Benton C, Han B. Pediatric case of the day. I. Dehiscent jugular bulb. II. Mondini malformation.III. Aural atresia. RadioGraphics. 1988;8:1221–6.
Atilla S, Akpek S, Uslu S, Ilgit E, Işik S. Computed tomographic evaluation of surgically significant vascular variations related with the temporal bone. Eur J Radiol. 1995;20:52–6.
Zhao P, Lv H, Dong C, Niu Y, Xian J, Wang Z. CT evaluation of sigmoid plate dehiscence causing pulsatile tinnitus. Eur Radiol. 2016;26:9–14.
Visvanathan V, Morrissey M. Anatomical variations of the temporal bone on high-resolution computed tomography imaging: how common are they? J Laryngol Otol. 2015;129:634–7.
Marsot-Dupuch K, Gayet-Delacroix M, Elmaleh-Berges M, Bonneville F, Lasjaunias P. The petrosquamosal sinus: CT and MR findings of a rare emissary vein. Am J Neuroradiol. 2001;22:1186–93.
Hoffmann O, Klingebiel R, Braun J, Katchanov J, Valdueza J. Diagnostic pitfall: atypical cerebral venous drainage via the vertebral venous system. Am J Neuroradiol. 2002;23:408–11.
Reis C, Deshmukh V, Zabramski J, Crusius M, Desmukh P, Spetzler R, et al. Anatomy of the mastoid emissary vein and venous system of the posterior neck region: neurosurgical implications. Oper Neurosurg. 2007;61:193–201.
Rivet D, Goddard J, Rich K, Derdeyn C. Percutaneous transvenous embolization of a dural arteriovenous fistula through a mastoid emissary vein. J Neurosurg. 2006;105:636–9.
Hoshi M, Yoshida K, Ogawa K, Kawase T. Hypoglossal neurinoma. Two case reports. Neurol Med Chir. 2000;40:489–93.
San Millan Ruiz D, Gailloud P, Rufenacht D, Delavelle J, Henry F, Fasel J. The craniocervical venous system in relation to cerebral venous drainage. Am J Neuroradiol. 2002;23:1500–8.
Shelling F. The emissaries of the human skull. Anat Anz. 1978;143:340–82.
Louis R, Loukas M, Wartmann C, Tubbs R, Apaydin N, Gupta A, et al. Clinical anatomy of the mastoid and occipital emissary veins in a large series. Surg Radiol Anat. 2008;31:139–44.
Giesemann A, Goetz G, Neuburger J, Lenarz T, Lanfermann H. Persistent petrosquamosal sinus: high incidence in cases of complete aplasia of the semicircular canals. Radiology. 2011;259:825–33.
Lemmerling M, Dhooge I, Mollet P, Mortier G, Van Cauwenberge P, Kunnen M. CT of the temporal bone in the CHARGE association. Neuroradiology. 1998;40:462–5.
Morimoto A, Wiggins R III, Hudgins P, Hedlund G, Hamilton B, Mukherji H, et al. Absent semicircular canals in CHARGE syndrome: radiologic spectrum of findings. Am J Neuroradiol. 2006;27:1663–71.
Saito R, Igarashi M, Alford B, Guilford F. Anatomical measurement of the sinus tympani. Arch Otolaryngol Head Neck Surg. 1971;94:418–25.
Ebenius B. The results of examination of the petrous bone in auditory nerve tumors. Acta Radiol. 1934;15:284–90.
Olivares F, Schuknecht H. Width of the internal auditory canal. Ann Otol Rhinol Laryngol. 1979;88:316–23.
Valvassori C. The radiological diagnosis of acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1966;83:582–7.
Purohit B, Hermans R, Op De Beeck K. Imaging in otosclerosis: a pictorial review. Insights Imaging. 2014;5:245–52.
∙ Sanverdi S, Ozgen B, Dolgun A, Sarac S. Incomplete endochondral ossification of the otic capsule, a variation in children: evaluation of its prevalence and extent in children with and without sensorineural hearing loss. Am J Neuroradiol. 2014;36:171–175. This study showed no correlation between degree of endochondral ossification of the otic capsule and sensorineural hearing loss in children. Pericochlear lucency may be seen as a normal finding in children, especially in the very young.
Pekkola J, Pitkäranta A, Jappel A, Czerny C, Baumgartner W, Heliövaara M, et al. Localized pericochlear hypoattenuating foci at temporal-bone thin-section ct in pediatric patients: nonpathologic differential diagnostic entity? Radiology. 2004;230:88–92.
Kroeker A, Nelson M, Thorne M. Pediatric hearing loss and radiographic pericochlear hypoattenuation. Otol Neurotol. 2013;34:726–8.
Moser T, Veillon F, Sick H, Riehm S. The hypodense focus in the petrous apex: a potential pitfall on multidetector ct imaging of the temporal bone. Am J Neuroradiol. 2008;29:35–9.
Valvassori G, Clemis J. The large vestibular aqueduct syndrome. Laryngoscope. 1978;88:723–8.
Ho M, Moonis G, Halpin C, Curtin H. Spectrum of third window abnormalities: semicircular canal dehiscence and beyond. Am J Neuroradiol. 2016;38:2–9.
Tong K, Harnsberger H, Dahlen R, Carey J, Ward K. Large vestibular aqueduct syndrome: a genetic disease? Am J Roentgenol. 1997;168:1097–101.
Nordström C, Laurell G, Rask-Andersen H. The human vestibular aqueduct: anatomical characteristics and enlargement criteria. Otol Neurotol. 2016;37:1637–45.
Reardon W. Enlarged vestibular aqueduct: a radiological marker of pendred syndrome, and mutation of the PDS gene. QJM. 2000;93:99–104.
Boston M, Halsted M, Meinzen-Derr J, Bean J, Vijayasekaran S, Arjmand E, et al. The large vestibular aqueduct: a new definition based on audiologic and computed tomography correlation. Otolaryngol-Head Neck Surg. 2007;136:972–7.
Vijayasekaran S, Halsted M, Boston M, Meinzen-Derr J, Bardo D, Greinwald J, et al. When is the vestibular aqueduct enlarged? A statistical analysis of the normative distribution of vestibular aqueduct size. Am J Neuroradiol. 2007;28:1133–8.
Juliano A, Ting E, Mingkwansook V, Hamberg L, Curtin H. Vestibular aqueduct measurements in the 45 oblique (Pöschl) plane. Am J Neuroradiol. 2016;37:1331–7.
Esteves S, Silva A, Coutinho M, Abrunhosa J, Sousa C. Congenital defects of the middle ear—uncommon cause of pediatric hearing loss. Braz J Otorhinolaryngol. 2014;80:251–6.
Park K, Choung Y. Isolated congenital ossicular anomalies. Acta Otolaryngol. 2009;129:419–22.
Swartz J, Faerber E. Congenital malformations of the external and middle ear: high-resolution CT findings of surgical import. Am J Roentgenol. 1985;144:501–6.
Pappas D, Pappas D, Hedlin G. Round window atresia in association with congenital stapes fixation. Laryngoscope. 1998;108:1115–8.
Booth T, Vezina L, Karcher G, Dubovsky E. Imaging and clinical evaluation of isolated atresia of the oval window. Am J Neuroradiol. 2000;21:171–4.
Herman H, Kimmelman C. Congenital anomalies limited to the middle ear. Otolaryngol-Head Neck Surg. 1992;106:285–7.
Huang T. Anomalously coursing facial nerves above and below the oval window: three case reports. Otolaryngol Head Neck Surg. 1997;116:438–41.
Jacob R, Gupta S, Isaacson B, Kutz J, Roland P, Xi Y, et al. High-resolution CT findings in children with a normal pinna or grade I microtia and unilateral mild stenosis of the external auditory canal. Am J Neuroradiol. 2014;36:176–80.
Hagiwara M, Shaikh J, Fang Y, Fatterpekar G, Roehm P. Prevalence of radiographic semicircular canal dehiscence in very young children: an evaluation using high-resolution computed tomography of the temporal bones. Pediatr Radiol. 2012;42:1456–64.
Minor L. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–27.
Belden C, Weg N, Minor L, Zinreich S. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology. 2003;226:337–43.
Chien W, Carey J, Minor L. Canal dehiscence. Curr Opin Neurol. 2011;24:25–31.
Cloutier J, Bélair M, Saliba I. Superior semicircular canal dehiscence: positive predictive value of high-resolution CT scanning. Eur Arch Otorhinolaryngol. 2008;265:1455–60.
Sequeira S, Whiting B, Shimony J, Vo K, Hullar T. Accuracy of computed tomography detection of superior canal dehiscence. Otol Neurotol. 2011;32:1500–5.
Carey J, Minor L, Nager G. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol-Head Neck Surg. 2000;126:137–47.
Zhou G, Ohlms L, Liberman J, Amin M. Superior semicircular canal dehiscence in a young child: Implication of developmental defect. Int J Pediatr Otorhinolaryngol. 2007;71:1925–8.
Prisman E, Ramsden J, Blaser S, Papsin B. Traumatic perilymphatic fistula with pneumolabyrinth: diagnosis and management. Laryngoscope. 2011;121:856–9.
Ehmer D, Booth T, Kutz J, Roland P. Radiographic diagnosis of trans-stapedial cerebrospinal fluid fistula. Otolaryngol-Head Neck Surg. 2010;142:694–8.
Merchant S, Rosowski J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29(3):282–9.
Emmett J. Cerebrospinal fluid gusher during stapedectomy. Oper Tech Otolaryngol-Head Neck Surg. 2003;14(4):300–2.
Micco A. Post-cochlear implant gusher and CSF leak. Oper Tech Otolaryngol-Head Neck Surg. 2003;14:297–9.
Wahba H, Youssef T. Stapedectomy gusher: a clinical experience. Int Adv Otol. 2010;6:149–54.
Nance W, Setleff R, McLead A, Sweeney D, Cooper C, McConnell F. X-linked mixed deafness with congenital fixation of the stapedial footplate and perilymphatic gusher. Birth Defects. 1971;7:64–9.
Gupta S, Maheshwari S, Kirtane M, Shrivastav N. Pictorial review of MRI/CT Scan in congenital temporal bone anomalies, in patients for cochlear implant. Indian J Radiol Imaging. 2009;19:99–106.
Phelps P, Reardon W, Pembrey M, Bellman S, Luxom L. X-linked deafness, stapes gushers and a distinctive defect of the inner ear. Neuroradiology. 1991;33:326–30.
Talbot J, Wilson D. Computed tomographic diagnosis of X-linked congenital mixed deafness, fixation of the stapedial footplate, and perilymphatic gusher. Am J Otol. 1994;15:177–82.
Tang A, Parnes L. X-linked progressive mixed hearing loss: computed tomography findings. Ann Otol Rhinol Laryngol. 1994;103:655–7.
Papadaki E, Prassopoulos P, Bizakis J, Karampekios S, Papadakis H, Gourtsoyiannis N. X-linked deafness with stapes gusher in females. Eur J Radiol. 1998;29:71–5.
Cremers C, Hombergen G, Scaf J, Huygen P, Volkers W, Pinckers A. X-linked progressive mixed deafness with perilymphatic gusher during stapes surgery. Arch Otolaryngol Head Neck Surg. 1985;111:249–54.
Glasscock M. The stapes gusher. Arch Otolaryngol Head Neck Surg. 1973;98:82–91.
Clarós P, Guirado C, Clarós A, Clarós A, Clavería A, Wienberg P. Association of spontaneous anterior fossa CSF rhinorrhea and congenital perilymphatic fistula in a patient with recurrent meningitis. Int J Pediatr Otorhinolaryngol. 1993;27:65–71.
Parry D, Booth T, Roland P. Advantages of magnetic resonance imaging over computed tomography in preoperative evaluation of pediatric cochlear implant candidates. Otol Neurotol. 2005;26:976–82.
Lemmerling M, Mancuso A, Antonelli P, Kubilis P. Normal modiolus: CT appearance in patients with a large vestibular aqueduct. Radiology. 1997;204:213–9.
Aschendorff A, Marangos N, Laszig R. Large vestibular aqueduct syndrome and its implication for cochlear implant surgery. Am J Otol. 1997;18(6 Suppl):S57.
∙ Booth T, Roland P, Kutz J, Lee K, Isaacson B. High-resolution 3-D T2-weighted imaging in the diagnosis of labyrinthitis ossificans: emphasis on subtle cochlear involvement. Pediatr Radiol. 2013;43:1584–1590. High resolution MRI was shown to have greater sensitivity for the early stages of cochlear obstruction and better able to show the full extent of involvement.
Isaacson B, Booth T, Kutz J, Lee K, Roland P. Labyrinthitis ossificans: How accurate is MRI in predicting cochlear obstruction? Otolaryngol-Head Neck Surg. 2009;140:692–6.
Swartz J, Mandell D, Faerber E, Popky G, Ardito J, Steinberg S, et al. Labyrinthine ossification: etiologies and CT findings. Radiology. 1985;157:395–8.
Tinling S, Colton J, Brodie H. Location and timing of initial osteoid deposition in postmeningitic labyrinthitis ossificans determined by multiple fluorescent labels. Laryngoscope. 2004;114:675–80.
Young N, Hughes C, Byrd S, Darling C. Postmeningitic ossification in pediatric cochlear implantation. Otolaryngol Head Neck Surg. 2000;122:183–8.
Phelps P, Proops D. Imaging for cochlear implants. J Laryngol Otol. 1999;113:21–3.
Kopelovich J, Germiller J, Laury A, Shah S, Pollock A. Early prediction of postmeningitic hearing loss in children using magnetic resonance imaging. Arch Otolaryngol-Head Neck Surg. 2011;137:441–7.
Swartz J. The facial nerve canal: CT analysis of the protruding tympanic segment. Radiology. 1984;153:443–7.
Takahashi H, Sando I. Facial canal dehiscence: histologic study and computer reconstruction. Ann Otol Rhinol Laryngol. 1992;101:925–30.
Moreano E, Paparella M, Zelterman D, Goycoolea M. Prevalence of facial canal dehiscence and of persistent stapedial artery in the human middle ear. Laryngoscope. 1994;104:309–20.
Baxter A. Dehiscence of the fallopian canal: an anatomical study. J Laryngol Otol. 1971;85:587–94.
Ho M, Juliano A, Eisenberg R, Moonis G. Anatomy and pathology of the facial nerve. Am J Roentgenol. 2015;204:W612–9.
Johnsson L, Kingsley T. Herniation of the facial nerve in the middle ear. Arch Otolaryngol Head Neck Surg. 1970;91:598–602.
Nager G, Proctor B. II: Anatomical variations and anomalies involving the facial nerve canal. Ann Otorhinolaryngol. 1978;88(Suppl 1):45–61.
Fuse T, Tada Y, Aoyagi M, Sugai Y. CT detection of facial canal dehiscence and semicircular canal fistula: comparison with surgical findings. J Comput Assist Tomogr. 1996;20:221–4.
Goldsztein H, Roberson J. Anatomical facial nerve findings in 209 consecutive atresia cases. Otolaryngol-Head Neck Surg. 2013;148:648–52.
Yu Z, Han D, Gong S, Wang Z, Dai H, Zhao S, et al. Facial nerve course in congenital aural atresia—identified by preoperative CT scanning and surgical findings. Acta Otolaryngol. 2008;128:1375–80.
Dedhia K, Yellon R, Branstetter B, Egloff A. Anatomic variants on computed tomography in congenital aural atresia. Otolaryngol-Head Neck Surg. 2012;147:323–8.
Yellon R, Branstetter B. Prospective blinded study of computed tomography in congenital aural atresia. Int J Pediatr Otorhinolaryngol. 2010;74:1286–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rebekah Clarke and Timothy Booth each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Neuroimaging.
Rights and permissions
About this article
Cite this article
Clarke, R., Booth, T. CT and MR Imaging of the Pediatric Temporal Bone: Normal Variants and Pitfalls. Curr Radiol Rep 5, 34 (2017). https://doi.org/10.1007/s40134-017-0225-9
Published:
DOI: https://doi.org/10.1007/s40134-017-0225-9