Abstract
Purpose of Review
To summarize current knowledge with regards to age-related changes in the bone marrow from childhood to senility and discuss how these changes are affected in patients with osteoporosis.
Recent Findings
There is a dramatic increase in marrow fat content in females around the time of the menopause in line with a reduction in bone mass. Marrow fat content is very responsive to physiological changes in estrogen and increase in marrow fat can be reversed by estrogen replacement. Marrow fat composition, rather than content, seems to be related to insufficiency fracture and diabetes.
Summary
MR imaging is a very good way of non-invasively studying marrow fat changes throughout life—both content and composition. Marrow fat content is an inverse marker of red marrow content. Red marrow content drives bone perfusion. Bone perfusion affects marrow nutrition and bone healing. Research into the fascinating bone-fat-perfusion relationship has only just began.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of the relationship between bone, marrow fat, and bone perfusion is a fascinating one which has only just began. Imaging-based studies have shown how changes in bone, marrow fat/red marrow, and bone perfusion parallel each other throughout life, while laboratory-based studies have identified pluripotent marrow stem cells with the ability to differentiate along adipocytic, osteoblastic, and hematopoietic cell lines. One needs to first appreciate the physiological age-related changes that occur in the bone marrow to better understand this bone marrow perfusion connect as a prelude to recognizing changes that occur in this relationship with diseases such as osteoporosis and insufficiency fracture.
The Bone Marrow
The bone marrow is supported by bony trabeculae and a fibrous retinaculum and contained within a bone cortex of variable thickness. The bone marrow accounts for 4% body weight or 2.8 kg in a 70 kg man, such that it only behind the bony skeleton, the skin, and subcutaneous fat in terms of weight [1, 2]. A ‘normal’ bone marrow cavity is made up of trabecular bone (20%), marrow fat (50%), non-fatty cellular elements (25%), and vascular channels (5%). The actual composition varies greatly with age, anatomical location, and physiological state [2]. The non-fatty cellular elements comprise stem cells, hemopoietic cells (erythrocytes, granulocytes, lymphocytes, monocytes, and platelets), and their precursors. Erythropoiesis takes place in distinct anatomical units (‘erythroblastic islands’); granulopoiesis takes place in less distinct foci, and platelet production (megakaryocytosis) occurs adjacent to the sinus epithelium [1]. Upon maturation, hemopoietic cells transverse the wall of the venous sinuses to enter the bloodstream, while platelets enter the bloodstream by cytoplastic processes from megakaryocytes penetrating the sinus wall into the sinus lumen [1]. Hematopoietic stem cells, which have the capacity to build the entire adult hematopoietic system, reside particularly in the metaphyseal regions of tubular bone, less so in the diaphyseal and hardly in the epiphyseal regions [3]. They also preferentially located in the endosteal areas of the medullary canal rather than the central medullary area [4••]. Young hematopoietic stem cells localize closer to the vasculature than more mature stem cells suggesting that their location is affected by aging [5]. With aging, although hematopoietic stem cells are increased in number, their self-renewal capacity and potential for marrow reconstitution is reduced [6••]. Also they tend to become more myeloid in their differentiation potential [6••]. This changing behavior of these aging hematopoietic stem cells may reflect changes in the bone marrow microenvironment [6••].
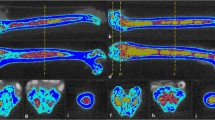
In utero, the bone marrow is almost entirely hematopoietic expect for the epiphyses and apophyses which are nearly all fat. About two-thirds of full term infants show fatty marrow change in the toes at the time of birth [7]. Thereafter with maturation, the appendicular marrow converts to a mainly fatty marrow in a symmetrical centripetal fashion from the periphery to the central skeleton [2]. Simultaneously, red marrow converts to fatty marrow in the tubular bones proceeding from the diaphysis to the metaphysis [2, 8] (Fig. 1). This physiological maturation process is known as ‘conversion’ of the bone marrow. By 10 years of age, marrow conversion of red to fatty marrow has begun in the diaphyses [2] (Fig. 1). By 20 years, nearly all of the long bone marrow is fatty except for the proximal metaphyses [2] (Fig. 1). By thirty years of age, some red marrow remain only in the proximal metaphyses, and the axial skeletal (pelvis, spine, scapulae, clavicles, sternum, and skull). This distribution then persists throughout life though with a gradual lessening of the amount of red marrow and an increase in the amount of fatty marrow in each of these areas. In the event of an increased functional demand for hematopoiesis, this sequence of events can reconvert with fatty marrow reconverting to red marrow in a reverse, symmetrical centrifugal manner [9]. When the marrow conversion is complete, the distribution of red:fatty marrow areas is quite consistent For example in the hip region, red marrow is consistently most abundant in the acetabulum > intertrochanteric region and proximal diaphysis > proximal femoral neck > femoral head > greater trochanter [10••].
From birth, red marrow converts to fatty marrow from the periphery to the central skeleton. Superimposed on this, red marrow converts to fatty marrow in the tubular bones proceeding from diaphysis to metaphysis until by the age of about 30 years only the proximal metaphysis contains appreciable red marrow (Color figure online)
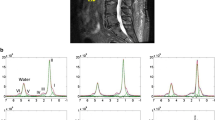
Red and fatty marrow areas are not composed purely of either non-fatty cells or fat cells, respectively. ‘Red marrow’ typically contains about 60% hematopoietic cells and about 40% fat cells (Fig. 2a, b) while ‘fatty marrow’ contains about 5% hematopoietic cells and about 95% adipocytes [2, 11]. In other words, ‘fatty marrow’ tends to be fattier than ‘red marrow’ is red. Fat cells (adipocytes) as expected contain more lipid than hematopoietic cells. Hematopoietic cells contain slightly more water and protein than adipocytes. The approximate chemical composition of fatty marrow is about 80% lipid, 15% water, and 5% protein, while that of red marrow is about 50% lipid, 35% water, and 15% protein [2, 11] (Fig. 3). This is relevant as MR quantification techniques such as MR spectroscopy use the fat:water ratio to determine marrow fat fraction (also referred to as fat content %). Twenty-two different fatty acids have been identified in the bone marrow and subcutaneous fat [12]. Significant differences in fatty acid composition do exist between marrow and subcutaneous fat as well as between marrow fat samples obtained from the proximal femur (predominantly hematopoietic marrow site) and the proximal tibia (predominantly fatty marrow site) [12].
Bone Marrow Fat
MR spectroscopy (MRS) is the most widely used method to measure marrow fat. In the bone marrow, the focus of MRS is the hydrogen nucleus (proton MRS or 1H-MRS), since this is the most abundant nucleus and emits the most intense radiofrequency signal. MRS readout utilizes a “parts per million or “ppm” scale to describe the “position” of the resonance frequencies on the x axis. Because the position on the x axis is influenced by the microenvironment of the hydrogen nucleus, MRS has the capacity to analyze the type of fat as well as the quantity of fat present in the bone marrow. Non-spectroscopic, yet precise, methods of quantifying fat fraction are also available such as the three-point IDEAL (Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation) or the 2-point Dixon method which involves sequential suppression or fat and water. The reproducibility of (1)H-MR spectroscopy and fat/water suppression imaging is high, tending to be best in those areas with the highest inherent fat fraction [13, 14].
Age-Related Changes in Marrow Fat Content and Composition
Lifelong studies addressing physiological changes in marrow fat content show a gradual physiological increase in percentage marrow fat content with advancing years [15, 16]. An easily remembered approximation is that vertebral body marrow fat content increases from 25% at 25 years of age to 65% at 65 years of age [15–18]. The mean volume of a lumbar vertebral body is about 16 ± 1.5 cm3 [17, 18]. The fat content of the vertebral bodies tends to increase from L1 to L4.
Over and above this increase in marrow fat content with age, MR-based studies have shown how a distinct sex difference does exist in marrow fat content [15, 19]. From the time of skeletal maturation to extreme old age, males show a gradual increase in lumbar vertebral marrow fat content of 7% per decade throughout life [15, 19] (Table 1). In contrast, females show a less steep increase in marrow fat of about 5% per decade up to 55 years though a dramatic increase in marrow fat content between the ages of 55 and 65 years [15, 19] (Table 1; Fig. 4). Male lumbar vertebrae possess about 10% more fat than female vertebrae of comparable age from skeletal maturation up to 50 years [15]. Between 50 and 60 years, this sex difference narrows and disappears [15]. By 60 years of age, healthy females tend to possess about 10% more marrow fat in their vertebrae than males [19] (Table 1; Fig. 4).
Marrow fat content (%) ±SD of L3 lumbar vertebral body stratified for age. There is a sharp increase in female marrow fat content between 55 and 65 years of age [20]
Marrow fat increases as a result of the increase in fat cell number rather than fat cell size. In the bone marrow, there are both mesenchymal (i.e., stromal) stem cells and hematopoietic stem cells. Mesenchymal stem cells have the potential to differentiate along adipocytic or osteoblastic cell lines, while hematopoietic stem cells differentiate along various hematological cell lines. A highly regulated balance of stem cell proliferation and differentiation maintains a steady-state functioning marrow. Disease or aging do affect this homeostatic balance. It is clear that a shift in this stem cell differentiation process towards the adipocytic cell line and away from the osteoblastic and hematopoietic cell lines can result in the change in marrow constituents seen with increasing age. Over and above this change in marrow composition, the general ability of the bone marrow to replenish itself from its own stem cell populations to seems to diminish with aging [6].
Increased age-related oxidative stress, resulting from an imbalance between antioxidant function and free radical production, is a potential influencer of stem cell function as well as a reduction in circulating estrogen. The bone marrow is very responsive to changing estrogen levels. Relatively young women who undergo surgical oophorectomy, will experience a sharp rise (92%) in marrow fat that occurs along with a sharp decline (12.5%) in vertebral bone mass [21••]. This deterioration will continue at a slower rate during over the next two years post-surgery [21••]. Marrow fat content even changes during the menstrual cycle [22••]. Vertebral bone marrow fat fraction increased by 2% during the follicular phase and decreased by a comparable degree during the luteal phase [22••]. Similarly, bone marrow fat fraction decreased by 5% during hormone replacement therapy (HRT) in post-menopausal women and increased after cessation [22••]. Estrogen seems to regulate bone marrow fat independent of bone mass and can rapidly reverses the physiological increase in marrow fat content seen with aging [22••].
Although androgen and estrogen levels both decline physiologically in later years, estrogen levels fall more sharply in peri- and post-menopausal females leading to a higher circulating androgen: estrogen ratio. This and other factors promote an ‘android’ pattern of fat deposition in post-menopausal females with greater intra-abdominal (visceral) fat deposition [23, 24] as opposed to the gynoid-pattern of fat deposition targeting the thighs and gluteal regions of pre-menopausal women [23, 24]. Visceral fat deposition (i.e., the android pattern) correlates positively with marrow fat deposition [25] such that age-related marrow fat deposition in females can be considered the bone-equivalent of android fat deposition. Female marrow fat content remains consistently about 10% higher than males from 65 years to extreme old age. This remains true even after the error of increasing fat on BMD estimation by DXA is taken into account [19]. Similar trends are seen in the sacrum [26] and hip region [10••]. The hip region is interesting to study as it allows areas of predominantly fatty marrow and predominantly red marrow to be directly compared. In young people, aged 20–30 years, the marrow fat content is highest in the greater trochanter (92%) >femoral head (86%) >proximal femoral neck (74%) >distal femoral neck, and proximal diaphysis (69%) >acetabulum (54%) [10••]. These young males have about 2% more marrow fat on average than females in the predominantly fatty marrow areas (greater trochanter, femoral head, and proximal femoral neck), while in the predominantly red marrow areas (distal femoral neck, proximal diaphysis, acetabulum), they have about 10–15% more fatty marrow than females. For both areas, these gender differences gradually lessen up to 60 years of age [10••]. Cessation of menstruation is another potential reason for increasing marrow fat in elderly females, though this is not likely to be the dominant cause given that menstrual blood loss is generally quite low (median 43 ml per menstrual cycle) [27].
Overall, there is at least a 40–50% increase in fat cell content with increasing age. This increase in fat cell volume occurs primarily at the expense of functioning marrow volume. Although the trabecular volume decreases with age, the overall percentage decrease in trabecular volume is small at about 5% in volume. Since the marrow cavity is a defined space and vascular sinusoids and other marrow structures do not seem to expand with age, one can infer that for any increase in fat cell content, there is almost a corresponding decrease in functioning or red marrow content [17, 18]. In other words, marrow fat content is a good surrogate marker for hematopoietic marrow content (Fig. 5) [17, 18].
The bone marrow is a confined space. As marrow fat content increases with age, red marrow content decreases since any age-related and/or osteoporotic-related change in trabecular bone volume is relatively small. These physiological changes in the bone marrow are all exaggerated in patients with osteoporosis [17, 18]
An increase in marrow fat is well recognized to be associated with a reduction in bone mineral density. There is also a recognized increase in marrow fat in conditions known to be associated with reduced bone mineral density such as anorexia nervosa, alcohol abuse, spinal cord injury, and prolonged bed rest [28•]. Medications associated with reduced bone mineral density such as glucocorticoid and thiazolidinediones (oral antidiabetic drug) are also associated with increased marrow fat [28•]. Higher prevalent marrow fat also predicts future increased bone loss. Post-menopausal women with a marrow fat content above the median experienced average bone loss of 4.7% over 4 years, while those with a marrow fat content below the median only experienced a bone loss of 1.6% [29]. Increased marrow fat may also increase fracture risk. Mean vertebral marrow fat was 55 and 45% (P < 0.001) in those with and without a prevalent vertebral fracture, respectively, even after adjusting for the effect of marrow fat on DXA measurements [30].
MR spectroscopy also has the ability to evaluate the type as well as the quantity of fat. Young normal controls have a higher (0.127) portion of unsaturated fat in the bone marrow than post-menopausal females with osteoporosis (0.091) [31]. This finding has also been shown by high-resolution spectroscopic study of iliac crest aspirates [32], though an earlier study using gas chromatography showed no difference in marrow fat composition with changing BMD [12]. Vertebral insufficiency fracture patients were shown to have 1.7% lower unsaturation levels and 2.9% higher vertebral marrow fat saturation levels [33••]. In the same study, diabetics had 1.3% lower unsaturation and 3.3% higher vertebral marrow fat saturation levels. Diabetics with fractures had the lowest marrow unsaturation and highest saturation. [33••]. Although changing marrow fat composition was associated with diabetes and insufficiency fracture, the marrow fat content (%) per se did not change with either diabetes or fracture [33••].
Bone Marrow Perfusion
As the two main constituents of bone marrow are red marrow and fatty marrow, fatty marrow content is a surrogate marker for red marrow content. In other words, the higher the fatty marrow content, the lower the red marrow content. This strongly affects bone marrow perfusion as the more metabolically active red marrow is the main driver of bone marrow perfusion [17, 18, 34]. As a result, changes in bone marrow perfusion coincide well with changes in the marrow content.
Cortical bone receives most (approximately two-thirds) of its blood supply from the medullary cavity and the remainder from the periosteal arteries [35]. The medullary cavity is supplied by larger nutrient arteries that pass through the bone cortex and run along the center of the marrow space as ascending and descending nutrient arteries [1]. These arteries give rise to small thin-walled arterioles that extend to the endosteal margin of the cortex. These arterioles anastomose with a plexus of large thin-walled venous sinuses composed of flat endothelial cells with a discontinuous highly permeable basement membrane [1, 36]. The venous sinuses drain via collecting venules back to the nutrient or emissary veins. This structural arrangement leads to a higher concentration of vascular channels and also slower flow at the periphery of the marrow cavity. Bone trabeculae do not contain vessels and are dependent on osmosis from the adjacent marrow for their nutrients. Lymphatic drainage seems to be absent in the bone marrow [37].
Blood flow is a flow within vessels i.e., intravascular flow. Perfusion, on the other hand, is a much more encompassing parameter reliant on a host of variables beyond blood flow such as capillary surface area, capillary exchange, interstitial space, interstitial pressure, interstitial diffusion, and venous flow [17, 18]. In a study of 10 young patients, lumbar vertebral body blood flow assessed by PET and a 15O-labeled CO2 steady-state technique was estimated to be 15 ml/min/100 g of healthy bone marrow [38].
MRI is the most widely used method of assessing tissue perfusion. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) (or MR perfusion imaging) involves the rapid acquisition of serial MRI images before, during, and after the administration of an MR contrast agent. Gadolinium chelate contrast agents rapidly diffuse into the extravascular interstitial space (i.e., the ‘leakage space’), at a rate determined by blood flow, capillary permeability, capillary surface area, interstitial pressure, interstitial space, and interstitial flow. T1-signal intensity increases proportionate to the concentration of gadolinium chelate. Pharmacokinetic modeling ideally requires a nearly linear relationship between gadolinium chelate concentration and signal intensity. Exact linearity is never achieved and can only be approximated with low doses of gadolinium chelate [39]. DCE-MRI permits a depiction of the wash-in and wash-out contrast kinetics. DCE-MRI or perfusion imaging is a very robust imaging technique which can be applied to bone marrow and extra-osseous tissues. Cortical bone blood flow is too slow to be measured. Empirical measures such as maximal signal intensity enhancement (Emax) and enhancement slope (Eslope) can be readily obtained. Reproducibility of bone marrow DCE-MRI perfusion parameters is sufficiently high, ranging from 0.59 to 0.98 and highest in those areas with better marrow perfusion [15].
Eslope and Emax are derived from the first-pass phase of signal intensity enhancement and represent arrival of contrast material into the arteries and capillaries and its diffusion into the extracellular space. Although Eslope and Emax are indices of perfusion rather than a direct measure of perfusion, they are strongly predictive of tissue vascularity, microvessel density, and tissue necrosis [40, 41]. Essentially, Eslope equates more with the ability to deliver gadolinium to the tissues (vascular supply, capillary network size, permeability), while Emax equates more perfusion demands of the tissue being investigated (metabolic rate). Perfusion data acquired from dynamic contrast-enhanced MR imaging are also amenable to two-compartment pharmacokinetic modeling (such as the Tufts or Brix models) though the test–retest reliability of the pharmacokinetic parameters analyzed is only modest while comprehension of what factors contribute to each parameter is also largely theoretical.
MR-based studies have shown that marrow perfusion reduces as one grows older [42–44]. Chen et al. were the first to investigate MR perfusion imaging of bone marrow perfusion in normal subjects [39]. They showed a decrease of 62% in Emax (P < 0.005) between subjects aged less 50 years (Emax 58.21 ± 44.65) and those aged more than 50 years (Emax 21.88 ± 14.77) [42]. When analyzed according to sex, a greater age-related difference was observed. For females, aged old than 50 years Eslope was 80% lower (from 87.17 ± 54.13 to 17.98 ± 13.80) in those older than 50 years (P < 0.005) [42]. For male subjects, Eslope was 33% lower (from 38.16 ± 21.69 to 25.38 ± 15.43) in subjects older than 50 years though this did not reach statistical significance (P > 0.05) [42]. Vertebral bone marrow perfusion was higher in young females than young males [42]. However, the rate of perfusion decline was slower in males, such that vertebral bone marrow perfusion was higher in elderly males than elderly females [42]. This pattern closely matches the changes that occur in red:fatty marrow composition with aging.
Similarly, Emax values were found to be significantly higher in patients less than 40 years of age compared to those aged 40 years or older (P < 0.001). Perfusion parameters decreased with increasing age in a logarithmic relationship (r = 0.71) paralleling increases in marrow fat content [43]. Bone perfusion in elderly subjects is related to bone mineral density (BMD). Even in elderly patients of normal BMD, Emax is lower in elderly females (32.3 ± 8.5%) than elderly males (34.5 ± 13%), while Eslope is higher in elderly females (1.70 ± 5.2%/s) than elderly males (1.48 ± 0.7%/s) [45, 46]. This may relate to the presence of arterial disease, red marrow volume, and the metabolic requirements of bone [34]. In other words, elderly females probably have less atherosclerosis and hence better bone blood flow (leading to better Eslope), but since their red marrow volume is lower, they have reduced Emax reflecting a reduced overall metabolic requirement. The lumbar marrow fat content of elderly females is higher so their functioning marrow volume and metabolic requirement is lower. Also in line with changing marrow fat content, the upper (L1, L2) lumbar vertebral bodies are better perfused than the lower (L3–L5) vertebral bodies [47].
Bone perfusion is a critical element in bone fracture healing, including microfracture healing [17, 18]. This is particularly relevant to osteoporosis. Bone perfusion decreases as marrow fat content increases, even to a greater degree than that expected with normal physiological aging, in patients with osteopenia and osteoporosis. Patients with osteoporosis have lower bone marrow perfusion than patients with osteopenia, while patients with osteopenia have lower bone marrow perfusion than those with normal bone mineral density [45–48]. This applies to the hip region as well as the lumbar spine and reflects the reduction in red marrow volume/increase in marrow fat volume that accompanies a reduction in bone mass [34, 48]. This reduction in bone marrow perfusion can preferentially affect bone areas at high fracture risk such as the femoral neck [49]. Reduced perfusion may lead to poor healing of microfractures, microfracture accumulation, and ultimately clinical fracture [17, 18]. Non-union of clinical fractures is also a feature of impaired bone perfusion.
To summarize, vertebral marrow perfusion is higher in young females than young males. However, perfusion decreases to a greater degree in females than males. Elderly female have reduced Emax but not Eslope compared to elderly males [45, 46]. These changes in marrow perfusion with increasing age tally with lifelong changes observed in marrow fat content which serves as a reciprocal marker of hematopoietic marrow content which is turn seems to be the main driving force behind the perfusion requirements of bone marrow [34]. Histological studies have also shown how red marrow volume decreases significantly throughout life accompanied by a corresponding increase in fat cells and a decrease in arterial capillary and sinus numbers [15, 50]. Bone marrow perfusion is also related to atherosclerosis. A weak negative correlation (r = −0.33, P = 0.0018) was found between vertebral body Emax and carotid artery intima media thickness (IMT) age after adjusting for the effect of sex, age, blood pressure, BMI, total cholesterol, high density lipoprotein, and triglycerol level in a linear regression model (P = 0.008). Emax was significantly lower in those subjects with intimal thickening group compared to those with normal intima thickness (73 ± 23 vs. 90 ± 27, P = 0.0023) [51].
Younger females have more functioning marrow than males and thereby require more perfusion. Older females have less functioning marrow than males and therefore have lower perfusion requirements. However, elderly females, possibly as a result of less atherosclerosis, are able to main Eslope but have decreased Emax.
Bone Marrow Diffusion
The free movement of water molecules within the extracellular fluid of the bone marrow is affected by the degree of marrow compaction and amount of interstitial fluid [17, 18]. The more closely packed the cells, the more restricted the water motion. Although cell packing is likely to be one of the main modulators, extracellular water motion is also dependent on other factors such as blood flow, capillary permeability, interstitial pressure, temperature, and the viscosity of interstitial fluid. Overall, bone marrow viscosity in the proximal femur is several times greater (123–400 cP) than in more peripheral bones such as the distal radius or calcaneus (37.5–44 cP) [52]. These figures can be compared to the viscosity of water which is 1cP.
Diffusion-weighted MR imaging is able to measure water diffusivity by applying ‘diffusion sensitizing gradients’ to T2-weighted spin echo sequences using echoplanar readouts [53]. The strength and duration of diffusion sensitizing gradients are indicated by their ‘b-value’ with a range of ‘b-values’ being used in diffusion-weighted imaging (DWI) to interrogate the water diffusion property of tissues. The ‘b-values’ applied to clinical studies are such that only the extracellular, and not the intracellular component, of water diffusivity is being measured.
The most commonly applied measure of water diffusivity in tissues is ‘apparent diffusion coefficient’ or ADC. The ADC of water is 3 × 10–3 mm2/s [54]. The ADC of fat is close to zero with values of 0.011–0.012 × 10−3 for subcutaneous fat [55]. ADC values of 0.2 × 10−3 and 0.1 × 10−3 have been reported for red and yellow marrow respectively [56].
Age-Related Changes in Bone Marrow Diffusion
Just as marrow content affects marrow perfusion, it also affects marrow diffusivity. In a study of 36 healthy subjects (16 men, 14 women), mean age 56 years, no relationship was found between vertebral marrow ADC and age applying b values of 400 and 750 s/mm2 [57]. Yeung et al. studied changes in bone marrow diffusion in young (mean age 28 years) and elderly females (mean age 70 years) [58]. They found that elderly female subjects (n = 20) with normal BMD have significantly (P = 0.029) lower ADC values in the bone marrow (0.43 ± 0.08) than young female subjects (0.49 ± 0.08). The lower water diffusion in the vertebral bodies among elderly female subjects compared to younger females is most likely due to age-related increase in marrow fat. Bone marrow ADC values are reduced when fat marrow accumulation occurs as judged by iliac crest bone marrow biopsy [59]. Increased fat packing of bone marrow in the elderly and, especially those with reduced BMD, are reflected in lower ADC and diffusion values. The accumulation of fat cells within the bony trabeculae restricts extracellular diffusion and leads to a fall in ADC and diffusivity. ADC values in females aged less than 50 years were overall significantly higher than males (presumably reflecting reduced fat packing), while ADC values of post-menopausal females were lower than pre-menopausal females (reflecting increased fat packing) [60•]. Particularly among females, ADC values reduced with increasing age [60•]. These are expected results given the known changes that occur in the lumbar bone marrow with age, especially in females [60•].
Bone Marrow Metabolism
Since trabecular bone and marrow fat have relatively low metabolic rates, bone marrow metabolism is predominantly a feature of functioning or red marrow content.
18F-flurodeoxyglucose (FDG) is a glucose analog and its whole body distribution can be imaged and quantified using positron emission tomography (PET). Following intravenous injection, FDG is transported into cells by glucose transporters where it is trapped through phosphorylation by hexokinase to produce FDG-6-phosphate [61]. Based on the degree of glucose utilization, FDG accumulation provides a measure of tissue metabolic activity. Although FDG-PET imaging is not a bone marrow specific tracer, it can be used as a useful measure of bone marrow metabolic activity [61].
Age-Related Changes in Bone Marrow Metabolism
Positron emission imaging allows assessment of bone marrow metabolism using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) imaging. Combining volumetric data from magnetic resonance imaging and quantitative metabolic information from positron emission tomography (PET) has been used to calculate the relative metabolic activity of red and yellow marrow in the L1, L3, and L5 vertebral bodies [62]. The mean volume of the lumbar vertebral bodies in five middle aged subjects (age 55 ± 6 years) was 15.6 ± 1.4 cm3 of which about 50% was red marrow and 50% fatty marrow. The mean SUV (standardized uptake value) max of fatty marrow and hematopoietic marrow was 0.38 ± 0.1 and 2.6 ± 0.6, respectively. In other words, the metabolic activity of red marrow was deemed to be about 7 times that of fatty marrow [62]. In a related PET imaging study, bone marrow metabolic activity (also as judged by SUV max) tended to decrease with advancing years more in the proximal femora and humeri (correlation coefficient −0.60 to −0.67, P < 0.01) than the axial skeletal (sternal manubrium, 12th thoracic, 5th lumbar vertebra, and iliac crests) correlation coefficient −0.28 to −0.48, P < 0.05) with advancing age [63].
Conclusion
There exists in the bone marrow pluripotent stem cells with the ability to differentiate along hematopoietic or adipocytic/osteoblastic cell lines. Marrow fat increases throughout life. As marrow fat increases, there is a reciprocal decrease in red marrow content, bone mass, bone perfusion, and marrow diffusivity. There is a dramatic increase in marrow fat in females around the time of menopause, as bone mass decreases. Marrow composition also changes thoughout life with a decrease in the amount of unsaturated marrow fats. Marrow fat content inversely reflects the red marrow content of bone. The more metabolically active red marrow drives bone blood flow which is critical to bone healing.
It is clear that the hard component of bone cannot be studied in isolation. One clearly needs to encompass a more encompassing paradigm encompassing the closely intertwined bony and marrow elements to more fully understand bone physiology and pathology. MRI is an exceptional imaging modality for determining age- and other related changes in marrow content and composition.
References
Papers of particular interest, published recently, have been 33409 highlighted as: • Of importance •• Of major importance
Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicol Pathol. 2006;34:548–65.
Hwang S, Panicek DM. Magnetic resonance imaging of bone marrow in oncology, Part 1. Skeletal Radiol. 2007;36:913–20.
Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–24.
•• Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, Collins TJ, Shapovalova Z, Xenocostas A, Bhatia M. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell. 2013;13:175–89. Human haematopoetic stem cells localizing to the trabecular bone areas show enhanced regenerative and self-renewal capacity and are molecularly distinct from those localizing to other bone areas. Osteoblasts may regulate these haematopoetic stem cells.
Köhler A, Schmithorst V, Filippi MD, Ryan MA, Daria D, Gunzer M, Geiger H. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009;114:290–8.
•• Pang WW, Schrier SL, Weissman IL. Age-associated changes in human hematopoietic stem cells. Semin Hematol. 2017;54:39–42. Possibly due to changes in aging bone marrow microenvironment, even though human HSCs increase in number, they have decreased self-renewal capacity, decreased reconstitution potential and are more myeloid-biased in their differentiation potential conferring an increased risk of developing into an age-associated diseases, such as myelodysplastic syndrome and myeloproliferative disorders.
Emery JL, Follett GF. Regression of bone-marrow haemopoiesis from the terminal digits in the foetus and infant. Br J Haematol. 1964;10:485–9.
Hartsock RJ, Smith EB, Petty CS. Normal variations with aging of the amount of hematopoetic tissue in bone marrow from the anterior iliac crest. A study made from 177 cases of sudden death examined by necropsy. Am J Clin Pathol. 1965;43:326–31.
Poulton TB, Murphy WD, Duerk JL, Chapek CC, Feiglin DH. Bone marrow reconversion in adults who are smokers: MR Imaging findings. Am J Roentgenol. 1993;161:1217–21.
•• Pansini V, Monnet A, Salleron J, Hardouin P, Cortet B, Cotten A. 3 Tesla (1) H MR spectroscopy of hip bone marrow in a healthy population, assessment of normal fat content values and influence of age and sex. J Magn Reson Imaging. 2014;39:369–76. Shows how the proximal femur is a good area to compare chronological changes in marrow fat content being relatively fat rich areas and red marrow rich areas. Fat content increased in all areas with increasing years.
Steiner RM, Mitchell DG, Rao VM, Schweitzer ME. Magnetic resonance imaging of diffuse bone marrow disease. Radiol Clin North Am. 1993;31:383–409.
Griffith JF, Yeung DK, Ahuja AT, Choy CW, Mei WY, Lam SS, Lam TP, Chen ZY, Leung PC. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone. 2009;44:1092–6.
Bernard C, Liney G, Manton D, Turnbull L, Langton C. Comparison of fat quantification methods: a phantom study at 3.0T. J Magn Reson Imaging. 2008;27:192–7.
Griffith JF, Yeung DK, Chow SK, Leung JC, Leung PC. Reproducibility of MR perfusion and (1)H spectroscopy of bone marrow. J Magn Reson Imaging. 2009;29:1438–42.
Dunnill MS, Anderson JA, Whitehead R. Quantitative histological studies on age changes in bone. J Pathol Bacteriol. 1967;94:275–91.
Duda SH, Laniado M, Schick F, Strayle M, Claussen CD. Normal bone marrow in the sacrum of young adults: differences between the sexes seen on chemical-shift MR imaging. AJR Am J Roentgenol. 1995;164:935–40.
Griffith JF. Bone marrow changes in osteoporosis. In: Guglielmi G editor. Medical radiology, osteoporosis and bone densitometry measurements. New York: Springer; 2013.
Griffith JF. Age-related physiological changes of the bone marrow and immune system. In: Guglielmi G, Peh WCG, Guermazi A, editors. New York: Geriatric imaging. Springer; 2013. p. 891–904.
Griffith JF, Yeung DKW, Ma HT, Leung JSC, Kwok TCY, Leung PC. Bone marrow fat content in the elderly: a reversal of trend seen in younger subjects. J Magn Reson Imaging. 2012;36:225–30.
Liney GP, Bernard CP, Manton DJ, Turnbull LW, Langton CM. Age, gender, and skeletal variation in bone marrow composition: a preliminary study at 3.0 Tesla. J Magn Reson Imaging. 2007;26:787–93.
•• Wáng YX, Griffith JF, Deng M, Yeung DK, Yuan J. Rapid increase in marrow fat content and decrease in marrow perfusion in lumbar vertebra following bilateral oophorectomy: an MR imaging-based prospective longitudinal study. Korean J Radiol. 2015;16:154–9. Shows how bone density decreases and marrow fat increases dramatically after surgical oophorectomy.
•• Limonard EJ, Veldhuis-Vlug AG, van Dussen L, Runge JH, Tanck MW, Endert E, Heijboer AC, Fliers E, Hollak CE, Akkerman EM, Bisschop PH. Short-term effect of estrogen on human bone marrow fat. J Bone Miner Res. 2015;30:2058–66. Vertebral bone marrow fat fraction increased by 2% during the follicular phase (P = 0.033), and decrease by a comparable degree during the luteal phase. Bone marrow fat fraction decreased by 5% during hormone replacement in post-menopausal women and increased after cessation. Estrogen seems to regulate bone marrow fat independent of bone mass.
Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann NY Acad Sci. 2000;904:502–6.
Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108:272–80.
Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity. 2011;19:49–53.
Shih TT, Chang CJ, Hsu CY, Wei SY, Su KC, Chung HW. Correlation of bone marrow lipid water content with bone mineral density on the lumbar spine. Spine (Phila Pa 1976). 2004;29:2844–50 (15).
Gao J, Zeng S, Sun BL, Fan HM, Han LH. Menstrual blood loss and hematologic indices in healthy Chinese women. J Reprod Med. 1987;32:822–6.
•Schwartz AV. Marrow fat and bone: review of clinical findings. Front Endocrinol (Lausanne). 2015;6:40. Good review of the current knowledge between marrow fat and bone.
Griffith JF, Yeung DK, Leung JC, Kwok TC, Leung PC. Prediction of bone loss in elderly female subjects by MR perfusion imaging and spectroscopy. Eur Radiol. 2011;21:1160–9.
Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–38.
Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22:279–85.
Li X, Shet K, Rodriguez JP, Pino AM, Kurhanewicz J, Schwartz A, Rosen CJ. Unsaturation level decreased in bone marrow lipids of postmenopausal women with low bone density using high resolution HRMAS NMR. J Bone Miner Res. 2012;27(Suppl):1.
•• Patsch JM, Li X, Baum T, Yap SP, Karampinos JDC, Schwartz AV, Link Tm. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28:1721–8. Insufficiency fracture patients had 1.7% lower unsaturation levels and 2.9% higher saturation levels. Diabetics had 1.3% lower unsaturation and 3.3% higher saturation levels. Diabetics with fractures had the lowest marrow unsaturation and highest saturation. Although marrow fat composition was associated with diabetes and fracture, marrow fat content did not change with diabetes or fracture.
Griffith JF, Wang YX, Zhou H, Kwong WH, Wong WT, Sun YL, Huang Y, Yeung DK, Qin L, Ahuja AT. Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology. 2010;254:739–46.
Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–17.
Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9:391–410.
Munka V, Gregor A. Lymphatics and bone marrow. Folia Morphol (Praha). 1965;13:404–12.
Kahn D, Weiner GJ, Ben-Haim S, Ponto LL, Madsen MT, Bushnell DL, Watkins GL, Argenyi EA, Hichwa RD. Positron emission tomographic measurement of bone marrow blood flow to the pelvis and lumbar vertebrae in young normal adults. Blood. 1994;83(4):958–63 (erratum in: Blood 1994; 15;84:3602).
Donahue MJ, Lu H, Jones CK, Pekar JJ, van Zijl PC. An account of the discrepancy between MRI and PET cerebral blood flow measures. A high-field MRI investigation. NMR Biomed. 2006;19:1043–54.
Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2003;17:509–20.
Knopp EA, Cowper SE. Nephrogenic systemic fibrosis: early recognition and treatment. Semin Dial. 2008;21:123–8.
Chen WT, Shih T, Chen RC, et al. Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology. 2001;220:213–8.
Montazel JL, Divine M, Lepage E, Kobeiter H, Breil S, Rahmouni A. Normal spinal bone marrow in adults: dynamic gadolinium-enhanced MR imaging. Radiology. 2003;229:703–9.
Baur A, Stabler A, Bartl R, Lamerz R, Scheidler J, Reiser M. MRI gadolinium enhancement of bone marrow: age-related changes in normals and in diffuse neoplastic infiltration. Skeletal Radiol. 1997;26:414–8.
Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–8.
Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–51.
Savvopoulou V, Maris TG, Vlahos L, Moulopoulos LA. Differences in perfusion parameters between upper and lower lumbar vertebral segments with dynamic contrast-enhanced MRI (DCE MRI). Eur Radiol. 2008;18:1876–83.
Griffith JF, Yeung DK, Kwok TC, Ahuja AT, Leung PC. Compromised bone perfusion in osteoporosis. J Bone Miner Res. 2008;23:1068–75.
Wang YX, Griffith JF, Kwok AW, Leung JC, Yeung DK, Ahuja AT, Leung PC. Reduced bone perfusion in proximal femur of subjects with decreased bone mineral density preferentially affects the femoral neck. Bone. 2009;45:711–5.
Demmler K, Otte P, Bartl R, et al. Osteopenia, marrow atrophy and capillary circulation: comparative studies of the human iliac crest and 1st lumbar vertebra. Z Orthop. 1983;121:223–7.
Chen WT, Ting-Fang Shih T, Hu CJ, Chen RC, Tu HY. Relationship between vertebral bone marrow blood perfusion and common carotid intima-media thickness in aging adults. J Magn Reson Imaging. 2004;20:811–6.
Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978–91.
Khoo MM, Tyler PA, Saifuddin A, Padhani AR. Diffusion-weighted imaging (DWI) in musculoskeletal MRI: a critical review. Skeletal Radiol. 2011;40:665–81.
Mills R. Self-diffusion in normal and heavy water in the range 1-45deg. J Phy Chem. 1973;77:685–8.
Lehnert A, Machann J, Helms G, Claussen CD, Schick F. Diffusion characteristics of large molecules assessed by proton MRS on a whole-body MR system. Magn Reson Imaging. 2004;22:39–46.
Ward R, Caruthers S, Yablon C, Blake M, DiMasi M, Eustace S. Analysis of diffusion changes in posttraumatic bone marrow using navigator-corrected diffusion gradients. AJR Am J Roentgenol. 2000;174:731–4.
Hillengass J, Stieltjes B, Bäuerle T, McClanahan F, Heiss C, Hielscher T, Wagner-Gund B, Habetler V, Goldschmidt H, Schlemmer HP, Delorme S, Zechmann CM. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and diffusion-weighted imaging of bone marrow in healthy individuals. Acta Radiol. 2011;1(52):324–30.
Yeung DK, Wong SY, Griffith JF, Lau EM. Bone marrow diffusion in osteoporosis: evaluation with quantitative MR diffusion imaging. J Magn Reson Imaging. 2004;19:222–8.
Nonomura Y, Yasumoto M, Yoshimura R, Haraguchi K, Ito S, Akashi T, Ohashi I. Relationship between bone marrow cellularity and apparent diffusion coefficient. J Magn Reson Imaging. 2001;13:757–60.
•Jie H, Hao F, Na LX. Vertebral bone marrow diffusivity in healthy adults at 3T diffusion-weighted imaging. Acta Radiol. 2016;57:1238–43. ADC values of the female subjects were higher than those of males. ADC values of pre-menopausal females were higher than those of post-menopausal females. ADC value was also negatively correlated with age (r = −0.334, P = 0.001), particularly among women (r = −0.581, P < 0.001). In other words, higher ADC values reflect higher red marrow content and change in accordance with changes in the cellular composition of the lumbar bone marrow.
Blebea JS, Houseni M, Torigian DA, Fan C, Mavi A, Zhuge Y, Iwanaga T, Mishra S, Udupa J, Zhuang J, Gopal R, Alavi A. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med. 2007;37:185–94.
Basu S, Houseni M, Bural G, Chamroonat W, Udupa J, Mishra S, Alavi A. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18] fluoro-d-glucose-positron emission tomography: a novel application with significant implications for combined structure-function approach. Mol Imaging Biol. 2007;9:361–5.
Fan C, Hernandez-Pampaloni M, Houseni M, Chamroonrat W, Basu S, Kumar R, Dadparvar S, Torigian DA, Alavi A. Age-related changes in the metabolic activity and distribution of the red marrow as demonstrated by -deoxy-2-[F-18]fluoro-d-glucose-positron emission tomography. Imaging Biol. 2007;9:300–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
James F. Griffith declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Geriatrics.
Rights and permissions
About this article
Cite this article
Griffith, J.F. Age-Related Changes in the Bone Marrow. Curr Radiol Rep 5, 24 (2017). https://doi.org/10.1007/s40134-017-0218-8
Published:
DOI: https://doi.org/10.1007/s40134-017-0218-8