Abstract
Introduction
To assess changes in choriocapillaris (CC) vascular density surrounding macular neovascularization (MNV) in treatment-naïve age-related macular degeneration (AMD) after faricimab application using optical coherence tomography angiography (OCTA).
Methods
Twenty-five eyes of 25 treatment-naïve individuals who underwent intravitreal faricimab injections for neovascular AMD (nAMD) with type 1 MNV were included. Spectral-domain optical coherence tomography (SD-OCT) images and en-face swept-source OCTA images were analyzed, and the percentage of CC flow deficit (FD%), FD average area (FDa) and FD number (FDn) in five progressive 20.0-μm-wide concentric rings (R1, R2, R3, R4 and R5) surrounding the dark halo around the MNV were calculated. Image acquisition was carried out prior to the first faricimab injection (T0) and 1 month after the injection (T1).
Results
The topographical sub-analysis revealed noteworthy changes in all rings at T1 compared to T0. There was a notable progressive reduction in FD% at T1 compared to T0 values across all rings, indicating a gradual CC reperfusion following anti-VEGF treatment. Additionally, the average size of FD decreased after the loading phase. Although not reaching statistical significance, there was a progressive reduction in the FDa across all rings.
Conclusion
Our study highlights a CC FD reduction following the administration of three consecutive faricimab injections. This effect was detected in all rings surrounding the dark halo. These observations suggest a partial CC reperfusion surrounding the MNV, potentially serving as an indicator for disease regression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
There is convincing evidence emphasizing the role of the choriocapillaris in the setting of age-related macular degeneration. |
The aim of the study was to analyze the influence of faricimab treatment on choriocapillaris perfusion. |
What was learned from the study? |
Analysis of optical coherence tomography angiography images demonstrated a significant reduction in flow deficit within the choriocapillaris surrounding the dark halo around the macular neovascularization following faricimab treatment. |
Faricimab treatment seems to initiate re-perfusion of the choriocapillaris surrounding the macular neovascularization membrane in the setting of neovascular age-related macular degeneration. |
Introduction
Age-related macular degeneration (AMD) is a significant cause of blindness affecting > 6 million individuals globally [1, 2]. Compelling evidence points to the crucial role of impaired choroidal flow signal in the development of AMD. Recent histopathologic examinations have revealed instances of choroidal dysregulation even in the early and intermediate stages of AMD, promoting angiogenesis and continuous vascular remodeling [3,4,5,6]. Notably, a heightened impairment in choroidal and particularly choriocapillaris (CC) flow is significantly associated with eyes affected by AMD compared to eyes without AMD [7]. This impairment is particularly pronounced in neovascular AMD (nAMD), where irregularities in the spatial distribution of the CC align with the development of abnormal blood vessels in the sub-retinal pigment epithelium (RPE) cell spaces, leading to the occurrence of macular neovascularization (MNV) [5, 8]. The spatial distribution of CC impairment surrounding MNV can be quantified by delineating the MNV and measuring the flow deficit (FD) at specific distances from the MNV outline [5, 9].
The RPE secretes vascular endothelial growth factor (VEGF), which plays a crucial role in the formation of MNV. Historically, VEGF has been the primary protein targeted in various diseases associated with MNV formation [10, 11]. However, our understanding of the effects of anti-VEGF drugs on the choroid is limited and subject to controversy. Some studies have suggested that prolonged use of anti-VEGF agents may lead to a reduction in vascular density, particularly in the CC [12, 13]. Utilizing optical coherence tomography (OCT) and OCT angiography (OCTA), previous research has demonstrated that intravitreal administration of anti-VEGF agents does influence the choroid, affecting the CC flow signal [14,15,16]. This impact was investigated by Viggiano et al., revealing a decrease in FD and reperfusion of the CC surrounding MNV following anti-VEGF therapy [16].
In 2022, faricimab was approved by the Food and Drug Administration (FDA) for the treatment of nAMD and diabetic macular edema [17]. It is the first intraocular drug approved by the FDA to target both VEGF and angiopoietin2 (Ang2). Ang2, a crucial component of the Ang/Tie pathway, plays a multifaceted role in vascular homeostasis, influencing vascular permeability and participating in neoangiogenic and proinflammatory processes [18, 19]. Despite its recognized impact on these processes, the specific influence of Ang2 on choroidal flow signal remains unexplored in vivo. This aspect is particularly intriguing, given Ang2’s role in regulating vascular homeostasis. As a result, this research aims to assess the choroidal flow signal both before and after the intravitreal injection of faricimab, utilizing OCTA.
Methods
Study Participants
In this retrospective study, we assessed 25 eyes from 25 individuals who received intravitreal faricimab injections for neovascular AMD and type 1 MNV at the Department of Ophthalmology, Klinikum Klagenfurt, Austria. The period of investigation spanned from April 2023 to January 2024. None of the study eyes had previously received any intravitreal injection. The study adhered to the principles outlined in the Declaration of Helsinki and received approval from the local review board (Ethikkommission Kärnten, S2023-13). All subjects provided informed consent prior to treatment. As data were deidentified, consent for publication was not required. Each patient underwent a comprehensive ophthalmologic assessment, encompassing the measurement of best-corrected visual acuity (BCVA), intraocular pressure (IOP) and dilated ophthalmoscopy. Only individuals with IOP levels falling within the normal range (10–21 mmHg) were considered for inclusion. Exclusion criteria comprised: (1) significant cataract; (2) myopia > 3.00 diopters; (3) occurrence of myocardial infarction or cerebrovascular disease within the preceding 6 months; (4) presence of infection or inflammation in both eyes; (5) existence of other concurrent retinal and/or macular conditions (e.g., diabetic retinopathy and retinal venous occlusion); (6) any form of optic neuropathy, including glaucoma; (7) presence of type 2 or type 3 MNV; (8) previous intravitreal medical treatment.
Imaging Acquisition
All imaging was performed by well-trained ophthalmologists (M.B. and T.M.). High-resolution SD-OCT images (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany) taken before and after a series of three faricimab injections were analyzed. For each OCT scan, a pattern of B scans (n = 19), horizontally oriented and centered over the fovea in a 20° × 15° (5.7 × 4.2 mm) area, were acquired. The central retinal thickness (CRT) was determined using the macular thickness map tool in the Heidelberg software (Heidelberg Eye Explorer, Heidelberg Engineering, Heidelberg, Germany).
Zeiss PLEX Elite 9000 Swept-Source OCTA (Carl Zeiss AC, Jena, Germany) was used to capture imaging data for all patients. OCTA imaging occurred at two specific time points: 0 to 3 days before the initial injection of faricimab (T0) and 1 month after completion of a series of three consecutive faricimab injections (T1). Time span between the injections was 6 weeks.
En-face OCTA 6 × 6-mm volume scans were obtained. Each imaging session comprised OCTA volumetric scans of the posterior pole. The device’s follow-up mode ensured consistent measurements at identical locations across all time points. OCTA scans with a strength index below eight out of ten or exhibiting significant motion artifacts or shadowing effects were excluded from the analysis [20]. As part of our clinic’s protocol, all medical retina patients underwent routine assessments during a specified time frame (between 08:00 and 12:00 a.m.), mitigating potential bias arising from physiologic diurnal changes in ocular flow signal [21, 22]. Manufacturer default settings were employed for the automatic segmentation of OCTA images in all B scans [23]. Automated segmentation facilitated the extraction of 10-µm slabs of the CC. CC areas beneath major superficial retinal vessels were excluded from the analysis to prevent potential shadows or projection artifacts [24] (Fig. 1). Additionally, for inclusion in the study, all edges of the MNV were required to be localized at least 1 mm away from the scan edge.
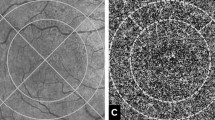
Analysis of choriocapillaris (CC) using optical coherence tomography angiography en-face imaging. Automated segmentation was employed to extract 10-µm slabs of the choriocapillaris, excluding CC areas beneath major superficial retinal vessels to mitigate potential shadows or projection artifacts. The 6 × 6-mm en-face CC image showcases subfoveal treatment-naïve macular neovascularization (MNV, highlighted in green). The perilesional dark halo, denoted by a low-flow area surrounding the MNV, is encircled in red
Image Analysis
A subsequent analysis was conducted following a pre-established protocol designed for the quantification of flow metrics [16, 25]. En-face OCTA images of the CC were imported into Fiji ImageJ (software version 2.0.0; National Institute of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Each CC image underwent compensation to eliminate retinal vessel projection artifacts and adjustment for shadowing artifacts (Fig. 1) [25, 26]. A low-flow signal area surrounding each MNV, known as the dark halo (DH), was identified [16]. The borders of the MNV lesion and the associated DH were manually outlined by two masked expert graders (P.V. and G.B.) in each en-face CC OCTA scan. Subsequently, five progressive 200-μm-wide concentric rings from the DH edge were generated using the “Distance Map” function in ImageJ, which automatically creates a border that follows the contour of the perilesional halo (Fig. 1) [27]. Each ring (R1, R2, R3, R4 and R5) was added to the region of interest (ROI) manager for CC flow analysis. The custom configuration, unique for each patient, consisting of these rings was applied to the CC en-face image at T0 and T1 at the same size and position (Fig. 2) [27]. Following this, the resulting CC images were binarized using the Phansalkar method for the quantitative measurement of the FD in each ring using a radius of 15 pixels (Fig. 2) [28]. The “Analyze Particles” command was utilized to calculate the CC FD, and the “Analyze Particles” tool provided by ImageJ was employed to quantify CC flow in each ring area (R1, R2, R3, R4 and R5). Specifically, the following metrics were quantified: (1) the FD percentage (FD%), representing the percentage of flow deficits within the analyzed area; (2) the FD average area (FDa), representing the average size of the flow deficits within the analyzed region; (3) the FD number (FDn), quantifying the number of flow deficits in the ROI. Histologic studies with direct measurements of intercapillary distances indicate that 76.8% are < 24 µm [29]. Thus, we did not enforce a minimum cutoff [30].
En-face choriocapillaris (CC) optical coherence tomography angiography images are employed for the manual delineation of borders for both the macular neovascularization type 1 lesion and its associated dark halo. Subsequently, utilizing the “Distance Map” function in ImageJ, five progressive concentric rings, each 200 μm wide, are generated from the periphery of the dark halo’s edge. This function in ImageJ automatically establishes a border that follows the contour of the perilesional halo. Every ring (R1, R2, R3, R4 and R5) was incorporated into the Region of Interest manager for CC flow analysis. This custom configuration, unique to each patient, was then implemented on the CC en-face images at T1, maintaining consistent size and position for comparative analysis. Subsequently, the obtained CC images underwent binarization for the quantitative assessment of the flow deficit within each ring. The Phansalkar method was employed for this purpose
Statistical Analysis
Statistical calculations were conducted using the Statistical Package for Social Sciences (SPSS IBM Statistic 25, Chicago, IL, USA). The data distribution was assessed using the Shapiro-Wilk test. Paired t-tests were used for analyzing quantitative data across the study visits. A significance level of p < 0.05 was chosen to determine statistical significance.
Results
After initial screening, 29 eyes which had received OCTA imaging at T0 and T1 were identified for this study. After further analysis of the OCTA images, four eyes were excluded because of poor imaging quality or presence of type 2 MNV. Twenty-five eyes of 25 patients of European descent receiving a series of three faricimab injections for the treatment of nAMD were finally included in the study. Seventeen patients were female; eight were male (Table 1). The cohort consisted of 11 right and 14 left eyes. The mean age was 76.6 ± 7.2 years (range = 64–88 years; ± SD, Table 1). Mean BCVA was 0.57 ± 0.34 (range 0.2–1.0 LogMAR, ± SD) at T0 and 0.55 ± 0.35 (range 0.2–1.0 LogMAR, ± SD) at T1, showing no significant change before and after treatment (p = 0.59, Table 1).
Mean CRT was 322.4 ± 102.1 µm before and 258.6 ± 50.0 µm after faricimab treatment (p = 0.002, Table 1). In the subjects included in the analysis, the MNV lesion displayed an average mean area of 0.68 ± 0.57 mm2 at T0 and 0.44 ± 0.47 mm2 at T1 (p = 0.007, Table 1). Interobserver agreement (average) was found to be excellent in the MNV assessment [0.90 (confidence interval, 0.86–0.93)]. Subretinal fluid (SRF) was detected in 14 eyes (56%), intraretinal fluid (IRF) in 15 eyes (60%) (Table 1). Pigment epithelial detachments (PEDs) were present in nine eyes (36%) (Table 1). Alle PEDs were located within a radius of 2000 µm from the foveal center.
The sub-analysis of topographic CC revealed statistically significant changes in all analyzed rings. Specifically, there was a notable progressive reduction in FD% at T1 compared to T0 values across all rings, indicating a gradual CC reperfusion following faricimab treatment (Table 2). Additionally, the average size of FD showed a decrease after the loading phase. Although not reaching statistical significance, there was a progressive reduction in the FDa across all rings (Table 3). Conversely, at T1, a significant increase in FDn was observed only in ring 1 and ring 2 compared to T0 (p < 0.034; Table 4).
Discussion
The primary objective of this study was to assess CC flow changes after faricimab treatment for nAMD in treatment-naïve eyes. Significant changes in choroidal perfusion following intravitreal injection therapy were observed across different regions, indicating a notable reperfusion of the CC. A significant decrease in perilesional CC FD was measured following faricimab treatment. At T1, there was a noteworthy increase in perilesional CC flow signal indicating reperfusion in all five rings. Specifically, our results demonstrate a significant reduction in CC FD and a reduction of FDa.
Prior research has underscored the significance of the choroid, particularly the CC, in the context of AMD [3, 6, 31, 32]. It is hypothesized that dysfunction of the CC plays a pivotal role in the initiation and progression of AMD [6, 32, 33]. Numerous studies have provided evidence supporting CC impairment in the context of AMD. Histologic findings indicate that CC degeneration has an impact on the viability of the RPE [32]. Biesemeier et al., using light and electron microscopy, observed that CC breakdown is a normal part of aging but becomes significantly more pronounced when AMD develops [3]. They further noted that CC alterations precede RPE loss, leading to the conclusion that AMD can be characterized as a vascular disease [3]. Spaide, in an examination involving 104 eyes of 80 patients with AMD using OCTA, described significant alterations in the flow pattern [34]. In a separate cohort of 42 eyes with intermediate AMD, Borrelli et al. later discovered that eyes with intermediate AMD in patients with nAMD in the fellow eye exhibited an increased average size of choriocapillaris signal voids compared to eyes without nAMD in the fellow eye [6]. Previous reports have documented a compromise in the CC adjacent to the dark halo and MNV. McLeod et al. substantiated this observation by demonstrating a 50% reduction in CC circulation surrounding MNV in postmortem nAMD eyes even in the presence of a structurally intact overlying RPE [32]. Recent advancements in OCTA have confirmed that CC hypoperfusion is most pronounced in the vicinity of the MNV membrane [5]. Treister et al., defining a 200-μm ring as the “halo” zone, showcased significantly greater CC non-perfusion adjacent to MNV [35]. In a prospective case series involving 80 eyes, Coscas et al. linked the presence of a dark halo to the shadowing effect caused by blood or intraretinal and subretinal fluids, interpreting it as a sign of active MNV that warrants treatment [36]. The true nature of the dark halo, whether it signifies genuine CC ischemia or is a result of a shadowing effect, remains uncertain [37]. To address this ambiguity, our investigation focused on CC flow outside the dark halo, excluding the halo itself.
Considering that faricimab is the first approved drug targeting not only VEGF but also Ang2, investigating its effects on CC flow signal holds particular interest. Ang2 is a component of the Ang/Tie pathway, participating in the regulation of vascular homeostasis, modulation of vascular permeability and involvement in neoangiogenic and proinflammatory processes [18]. The angiogenic or anti-angiogenic activity of Ang2 depends on the context, with one of the determining factors being the expression of other angiogenic growth factors, notably VEGF [38]. In vitro studies have indicated that Ang2 induces permeability and angiogenesis on the pupillary membrane when VEGF is present. However, in the absence of VEGF, Ang2 leads to vessel regression and endothelial cell death [39]. While caution is warranted in extrapolating these findings to clinical scenarios, it is conceivable that blocking Ang2, especially in conjunction with VEGF depletion, could impact the permeability of choroidal vessels, indirectly influencing CC perfusion. Razavi et al. have reported a similar observation, speculating that in neovascular AMD, anti-VEGF treatment may not only positively affect retinal exudation but also underlying choroidal exudation by reducing choroidal vascular hyperpermeability [40].
No notable change in BCVA was observed in our study. This lack of significant change is likely attributed to the small number of injections that had been applied. This constitutes a noteworthy limitation of our study.
As of our current understanding, this study represents the initial exploration into the influence of intravitreally administered faricimab on CC flow in treatment-naïve eyes. It is essential to acknowledge the limitations inherent in the retrospective design and the relatively small sample size of the study. Measuring CC flow and FD using OCTA is a challenging task especially in the setting of AMD. There is an ongoing discussion about potential errors caused by technical and anatomical conditions [41]. We have addressed this issue by applying an up-to-date acquisition and image processing method, which has proven to be robust with excellent repeatability in a number of published studies [25, 30]. This method has been shown to be effective in compensating for the signal loss caused by retinal vessels as well as the RPE/Bruch’s membrane complex. It was especially tested to account for the shadowing effect from the elevated RPE caused by drusen in the setting of AMD [25]. Adaptive local thresholding was performed to obviate small regional variations in image brightness, and the Phansalkar method was used because it was designed to select darker regions in potentially low-contrast images [28]. Moreover, we used a swept-source OCTA device that allowed for deeper light penetration into the choroid, thereby obtaining higher quality images with outstanding repeatability [25, 42]. Figure 3 displays three different eyes of three different individuals from our cohort. CC flow signal was clearly detectable despite presence of SRF/IRF as well as PEDs.
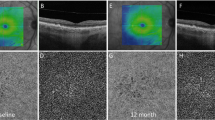
A and B Display central optical coherence tomography angiography (OCTA) B scans of three different eyes of three different individuals from our cohort. Detected flow signals are displayed as red dots according to the standard setting of the utilized OCTA machine. A Flow signal is clearly detectable at choriocapillaris (CC) layer (red dots between yellow arrowheads) despite presence of subretinal fluid (SRF, white arrowhead) and pigment epithelial detachment (PED, green arrowhead) containing the macular neovascularization (MNV) type I membrane. B Flow signal is clearly detectable at CC layer (red dots between yellow arrowheads) despite presence of SRF (white arrowhead), intraretinal fluid (pink arrowheads) and PED (green arrowhead) containing MNV type I membrane. C and D Corresponding en-face angiography images on the CC layer
Conclusions
In conclusion, our study reports a decrease of CC FD after the administration of a series of three faricimab injections. This effect was significant in all rings surrounding the dark halo around the MNV. This was accompanied by a decrease in CC FDa in all rings. These findings indicate reperfusion of the CC surrounding the MNV, which may be an indicator for disease regression. This is of particular interest as OCTA-based parameters gain prominence in the management of nAMD. In the future, larger prospective studies are needed to support our preliminary results.
Data Availability
The datasets generated and analysed during the current study are not publicly available due to patient confidentiality.
References
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602.
Arruabarrena C, Toro MD, Onen M, Malyugin BE, Rejdak R, Tognetto D, et al. Impact on visual acuity in neovascular age related macular degeneration (nAMD) in Europe due to COVID-19 pandemic lockdown. J Clin Med. 2021;10(15):3281.
Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35(11):2562–73.
Berlin A, Cabral D, Chen L, Messinger JD, Balaratnasingam C, Mendis R, et al. Correlation of optical coherence tomography angiography of type 3 macular neovascularization with corresponding histology. JAMA ophthalmology. 2022;140(6):628–33.
Moult EM, Alibhai AY, Rebhun C, Lee B, Ploner S, Schottenhamml J, et al. Spatial distribution of choriocapillaris impairment in eyes with choroidal neovascularization secondary to age-related macular degeneration: a quantitative OCT angiography study. Retina. 2020;40(3):428–45.
Borrelli E, Uji A, Sarraf D, Sadda SR. Alterations in the choriocapillaris in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(11):4792–8.
Borrelli E, Shi Y, Uji A, Balasubramanian S, Nassisi M, Sarraf D, et al. Topographic analysis of the choriocapillaris in intermediate age-related macular degeneration. Am J Ophthalmol. 2018;196:34–43.
Muth DR, Toro MD, Bajka A, Jonak K, Rieder R, Kohler MM, et al. Correlation between macular neovascularization (MNV) type and druse type in neovascular age-related macular degeneration (AMD) based on the CONAN classification. Biomedicines. 2022;10(10):2370.
Alagorie AR, Verma A, Nassisi M, Nittala M, Velaga S, Tiosano L, et al. Quantitative assessment of choriocapillaris flow deficits surrounding choroidal neovascular membranes. Retina. 2020;40(11):2106–12.
Dolar-Szczasny J, Bucolo C, Zweifel S, Carnevali A, Rejdak R, Zaluska W, et al. Evaluation of aqueous flare intensity in eyes undergoing intravitreal bevacizumab therapy to treat neovascular age-related macular degeneration. Front Pharmacol. 2021;12: 656774.
Plyukhova AA, Budzinskaya MV, Starostin KM, Rejdak R, Bucolo C, Reibaldi M, et al. Comparative safety of bevacizumab, ranibizumab, and aflibercept for treatment of neovascular age-related macular degeneration (AMD): a systematic review and network meta-analysis of direct comparative studies. J Clin Med. 2020;9(5):1522.
Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143(6):995–1002.
Hikichi T, Agarie M. Reduced vessel density of the choriocapillaris during anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60(4):1088–95.
Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012;119(8):1621–7.
Koizumi H, Kano M, Yamamoto A, Saito M, Maruko I, Kawasaki R, et al. Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(4):627–33.
Viggiano P, Grassi MO, Pignataro M, Boscia G, Borrelli E, Molfetta T, et al. Topographical analysis of the choriocapillaris reperfusion after loading anti-VEGF therapy in neovascular AMD. Transl Vis Sci Technol. 2022;11(9):18.
Ferro Desideri L, Traverso CE, Nicolo M, Munk MR. Faricimab for the treatment of diabetic macular edema and neovascular age-related macular degeneration. Pharmaceutics. 2023;15(5):1413.
Duran CL, Borriello L, Karagiannis GS, Entenberg D, Oktay MH, Condeelis JS. Targeting Tie2 in the tumor microenvironment: from angiogenesis to dissemination. Cancers (Basel). 2021;13(22):5730.
Kim J, Park JR, Choi J, Park I, Hwang Y, Bae H, et al. Tie2 activation promotes choriocapillary regeneration for alleviating neovascular age-related macular degeneration. Sci Adv. 2019;5(2): eaau6732.
Rommel F, Siegfried F, Kurz M, Brinkmann MP, Rothe M, Rudolf M, et al. Impact of correct anatomical slab segmentation on foveal avascular zone measurements by optical coherence tomography angiography in healthy adults. J Curr Ophthalmol. 2018;30(2):156–60.
Rommel F, Rothe M, Kurz M, Prasuhn M, Grisanti S, Ranjbar M. Evaluating diurnal variations in retinal perfusion using optical coherence tomography angiography. Int J Retina Vitreous. 2020;6:22.
Rommel F, Siegfried F, Sochurek JAM, Rothe M, Brinkmann MP, Kurz M, et al. Mapping diurnal variations in choroidal sublayer perfusion in patients with idiopathic epiretinal membrane: an optical coherence tomography angiography study. Int J Retina Vitreous. 2019;5:12.
Wiest MRJ, Bajka A, Hamann T, Foa N, Toro M, Barthelmes D, et al. Differences in mean values and variance in quantitative analyses of foveal OCTA imaging. Klin Monbl Augenheilkd. 2022;239(4):513–7.
Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(12):5548–55.
Zhang Q, Zheng F, Motulsky EH, Gregori G, Chu Z, Chen CL, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Invest Ophthalmol Vis Sci. 2018;59(1):203–11.
Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163–80.
Viggiano P, Miere A, Borrelli E, Boscia G, Grassi MO, Souied EH, et al. The impact of diabetic retinopathy on the choriocapillaris in neovascular AMD. Invest Ophthalmol Vis Sci. 2023;64(14):32.
Chu Z, Zhang Q, Gregori G, Rosenfeld PJ, Wang RK. Guidelines for imaging the choriocapillaris using OCT angiography. Am J Ophthalmol. 2021;222:92–101.
Chen L, Messinger JD, Sloan KR, Swain TA, Sugiura Y, Yannuzzi LA, et al. Nonexudative macular neovascularization supporting outer retina in age-related macular degeneration: a clinicopathologic correlation. Ophthalmology. 2020;127(7):931–47.
Kar D, Corradetti G, Swain TA, Clark ME, McGwin G Jr, Owsley C, et al. Choriocapillaris impairment is associated with delayed rod-mediated dark adaptation in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64(12):41.
Govetto A, Sarraf D, Figueroa MS, Pierro L, Ippolito M, Risser G, et al. Choroidal thickness in non-neovascular versus neovascular age-related macular degeneration: a fellow eye comparative study. Br J Ophthalmol. 2017;101(6):764–9.
McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–91.
Moreira-Neto CA, Moult EM, Fujimoto JG, Waheed NK, Ferrara D. Choriocapillaris loss in advanced age-related macular degeneration. J Ophthalmol. 2018;2018:8125267.
Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58–67.
Treister AD, Nesper PL, Fayed AE, Gill MK, Mirza RG, Fawzi AA. Prevalence of subclinical CNV and choriocapillaris nonperfusion in fellow eyes of unilateral exudative AMD on OCT angiography. Transl Vis Sci Technol. 2018;7(5):19.
Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina. 2015;35(11):2219–28.
Rispoli M, Savastano MC, Lumbroso B. Quantitative vascular density changes in choriocapillaris around CNV after anti-VEGF treatment: Dark Halo. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):918–24.
Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471.
Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99(17):11205–10.
Razavi S, Souied EH, Darvizeh F, Querques G. Assessment of choroidal topographic changes by swept-source optical coherence tomography after intravitreal ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2015;160(5):1006–13.
Borrelli E, Bandello F, Souied EH, Barresi C, Miere A, Querques L, et al. Neovascular age-related macular degeneration: advancement in retinal imaging builds a bridge between histopathology and clinical findings. Graefes Arch Clin Exp Ophthalmol. 2022;260(7):2087–93.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Acknowledgements
We thank the participants of the study and the staff of the Augenabteilung Klinikum Klagenfurt for their administrative and technical support.
Funding
We acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfond. The journal's Rapid Service Fee was funded by LKH Klagenfurt.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Max Brinkmann, Pasquale Viggiano, Giacomo Boscia and Tom Müller. The methodology was provided by Pasquale Viggiano, Giacomo Boscia and Francesco Boscia. Formal anaylsis was performed by Pasquale Viggiano and Giacomo Boscia. Investigation was performed by Tom Müller and Max Brinkmann. Statistical analysis was performed by Pasquale Viggiano and Max Brinkmann. Visualization was performed by Niccolò Castellino, Pasquale Viggiano and Max Brinkmann. The first draft of the manuscript was written by Max Brinkmann, Pasquale Viggiano and Jakob Schweighofer. Resources and supervision were provided by Francesco Boscia, Mario Damiano Toro and Yosuf El-Shabrawi. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Max Brinkmann, Pasquale Viggiano, Giacomo Boscia, Tom Müller, Niccolò Castellino, Jakob Schweighofer, Francesco Boscia, Mario Damiano Toro and Yosuf El-Shabrawi have nothing to disclose. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
The study adhered to the principles outlined in the Declaration of Helsinki and received approval from the local review board (Ethikkommission Kärnten, S2023-13). All subjects provided informed consent prior to treatment. As data were deidentified, consent for publication was not required.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brinkmann, M., Viggiano, P., Boscia, G. et al. Analysis of Choriocapillaris Reperfusion Topography Following Faricimab Treatment for Neovascular Age-Related Macular Degeneration in Therapy-Naïve Patients. Ophthalmol Ther 13, 1981–1992 (2024). https://doi.org/10.1007/s40123-024-00967-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00967-2