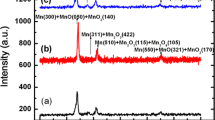
Abstract
In this work the corrosion resistance of stainless steel 304L coated with Mn-based thin film and post annealed with flow of nitrogen at 723 K in 0.6 M NaCl solution is reported. The latter was performed at three different solution temperatures of 293 K, 313 K and 333 K. X-ray diffraction analysis was used to determine the crystallographical structure and phases of the annealed samples. Atomic force microscope and field emission scanning electron microscope were employed to determine the morphology of the surface of the samples. Corrosion behavior of the samples in the corroding media was studied by means of electrochemical impedance spectroscopy (EIS) and polarization analysis. Results showed that the sample investigated in the 0.6 M NaCl solution at 293 K temperature has the highest corrosion resistance than those studied at higher temperatures. The correctness of the EIS results was confirmed by Kramers–Kronig transformation, while fitting of the data (Nyquist and Bode diagrams) to suitable equivalent electrical circuits showed that the highest corrosion enhancement is achieved for the Mn-based/SS304L sample in the 0.6 M NaCl solution at 293 K temperature, resulting in a 90.57% corrosion inhibition enhancement factor (η%). Polarization measurements also showed that this sample has the lowest corrosion current density, lowest corrosion rate and highest corrosion potential with a 96% corrosion inhibition efficiency factor (PE%). Consistent results are achieved for EIS and polarization measurements which are then correlated with the nanostructure of the films using X-ray diffraction and atomic force microscope analyses.










Similar content being viewed by others
References
Elanchezhian, C., Vijaya Ramnath, B., Ramakrishnan, G., Sripada Raghavendra, K.N., Muralidharan, M., Kishore, V.: Review on metal matrix composites for marine applications. Mater. Today Proc. 5, 1211–1218 (2018)
Dirisu, J.O., Fayomi, O.S.I., Oyedepo, S.O., Jolayemi, K.J., Moboluwarin, D.M.: Critical evaluation of aluminium dross composites and other potential building ceiling materials. Procedia Manuf. 35, 1205–1210 (2019)
Saravanan, C., Subramanian, K., Krishnan, V.A., Sankara Narayanan, R.: Effect of particulate reinforced aluminium metal matrix composite—a review. Mech. Mech. Eng. 19, 23–30 (2015)
Abrahami, S.T., de Kok, J.M.M., Terryn, H., Mol, J.M.C.: Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: a review. Front. Chem. Sci. Eng. 11, 465–482 (2017)
Javidparvar, A.A., Ramezanzadeh, B., Ghasemi, E.: Effect of various spinel ferrite nanopigments modified by amino propyl trimethoxy silane on the corrosion inhibition properties of the epoxy nanocomposites. Corrosion 72, 761–774 (2016)
Canepa, E., Stifanese, R., Merotto, L., Traverso, P.: Corrosion behaviour of aluminium alloys in deep-sea environment: a review and the KM3NeT test results. Mar. Struct. 59, 271–284 (2018)
Fateh, A., Aliofkhazraei, M., Rezvanian, A.R.: Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 13, 481–544 (2017)
Bodunrin, M.O., Alaneme, K.K., Chown, L.H.: Aluminium matrix hybrid composites: a review of reinforcement philosophies; mechanical, corrosion and tribological characteristics. J. Mater. Res. Technol. 4, 434–445 (2015)
Javidparvar, A.A., Naderi, R., Ramezanzadeh, B.: Epoxy-polyamide nanocomposite coating with graphene oxide as cerium nanocontainer generating effective dual active/barrier corrosion protection. Compos. B Eng. 172, 363–375 (2019)
Manam, N.S., Harun, W.S.W., Shri, D.N.A., Ghani, S.A.C., Kurniawan, T., Ismail, M.H., et al.: Study of corrosion in biocompatible metals for implants: a review. J. Alloy Compd. 701, 698–715 (2017)
Corradi, M., Di Schino, A., Borri, A., Rufini, R.: A review of the use of stainless steel for masonry repair and reinforcement. Constr. Build. Mater. 181, 335–346 (2018)
Lai, J.K.L.: A review of precipitation behaviour in AISI type 316 stainless steel. Mater. Sci. Eng. 61, 101–109 (1983)
Davison, R.M., Laurin, T.R., Redmond, J.D., Watanabe, H., Semchyshen, M.: A review of worldwide developments in stainless steels. Mater. Des. 7, 111–119 (1986)
Loto, R.T.: Pitting corrosion evaluation of austenitic stainless steel type 304 in acid chloride media (PDF Download Available). J. Mater. Environ. 4, 448–459 (2013)
Sun, G.F., Zhang, Y.K., Zhang, M.K., Zhou, R., Wang, K., Liu, C.S., et al.: Microstructure and corrosion characteristics of 304 stainless steel laser-alloyed with Cr–CrB2. Appl. Surf. Sci. 295, 94–107 (2014)
Loto, R.T.: Study of the corrosion resistance of type 304L and 316 austenitic stainless steels in acid chloride solution. Orient. J. Chem. 33, 1090–1096 (2017)
Huang, C.A., Chang, Y.Z., Chen, S.C.: The electrochemical behavior of austenitic stainless steel with different degrees of sensitization in the transpassive potential region in 1 M H2SO4 containing chloride. Corros. Sci. 46, 1501–1513 (2004)
Bankiewicz, D.: Corrosion behaviour of boiler tube materials during combustion of fuels containing Zn and Pb [Academic Dissertation]. Process Chemistry Centre, Department of Chemical Engineering, Abo Akademi University, Turku, Finland, Laboratory of Inoganic Chemistry (2012)
Baddoo, N.R.: Stainless steel in construction: a review of research, applications, challenges and opportunities. J. Constr. Steel Res. 64, 1199–1206 (2008)
Agarwal, S., Suhane, A.: Study of boiler maintenance for enhanced reliability of system a review. Mater. Today Proc. 4, 1542–1549 (2017)
Zhang, H., Zhao, Y.L., Jiang, Z.D.: Effects of temperature on the corrosion behavior of 13Cr martensitic stainless steel during exposure to CO2 and Cl− environment. Mater. Lett. 59, 3370–3374 (2005)
Jian, L., Huanjun, Z., Ke, W., Xuehui, W.: Corrosion behavior of SS-304 in NaCl solution at different temperatures using electrochemical noise technique. Int. J. Electrochem. Sci. 10, 931–937 (2015)
Masalski, J., Gluszek, J., Zabrzeski, J., Nitsch, K., Gluszek, P.: Improvement in corrosion resistance of the 316L stainless steel by means of Al2O3 coatings deposited by the sol–gel method. Thin Solid Films 349, 186–190 (1999)
Shen, G.X., Chen, Y.C., Lin, C.J.: Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Films 489, 130–136 (2005)
Li, M., Luo, S., Zeng, C., Shen, J., Lin, H., Cao, C.: Corrosion behavior of TiN coated type 316 stainless steel in simulated PEMFC environments. Corros. Sci. 46, 1369–1380 (2004)
Javidparvar, A.A., Ramezanzade, B., Ghasemi, E.: The effect of oleic acid/silanetreatments of Fe3O4 nanoparticles on the mechanical properties of an epoxy coating, vol. 13, p. Dec. Institute for Color Scienceand Technology, Tehran (2015)
Zhang, X., Xiao, G., Jiang, C., Liu, B., Li, N., Zhu, R., et al.: Influence of process parameters on microstructure and corrosion properties of hopeite coating on stainless steel. Corros. Sci. 94, 428–437 (2015)
Mohammadloo, H.E., Sarabi, A.A., Alvani, A.A.S., Salimi, R., Sameie, H.: The effect of solution temperature and pH on corrosion performance and morphology of nanoceramic-based conversion thin film. Mater. Corros. 64, 535–543 (2013)
Chou, W.J., Yu, G.P., Huang, J.H.: Corrosion behavior of TiN-coated 304 stainless steel. Corros. Sci. 43, 2023–2035 (2001)
Ćurković, L., Ćurković, H.O., Salopek, S., Renjo, M.M., Šegota, S.: Enhancement of corrosion protection of AISI 304 stainless steel by nanostructured sol–gel TiO2 films. Corros. Sci. 77, 176–184 (2013)
Mahdi Salih, S., Shakir, I.K., Al-Sammarraie, A.M.A.: Comparison of aggressiveness behavior of chloride and iodide solutions on 304 and 304L stainless steel alloys. Mater. Sci. Appl. 8, 12 (2017)
Schönleber, M., Klotz, D., Ivers-Tiffée, E.: A method for improving the robustness of linear Kramers–Kronig validity tests. Electrochim. Acta 131, 20–27 (2014)
Boukamp, B.A.: Practical application of the Kramers–Kronig transformation on impedance measurements in solid state electrochemistry. Solid State Ionics 62, 131–141 (1993)
Khojier, K., Savaloni, H., Sadeghi, Z.: A comparative investigation on growth, nanostructure and electrical properties of copper oxide thin films as a function of annealing conditions. J Theor Appl Phys 8, 116 (2014)
Modiri, F., Savaloni, H.: A study of the corrosion of stainless steel 304L coated with a 190 nm-thick manganese layer and annealed with nitrogen flux in a 0.4-mole solution of H2SO4 at different temperatures. J. Theor. Appl. Phys. 14, 21–35 (2019)
Movchan, B.A., Demchishin, A.V.: Study of the structure and properties of thick vacuum condensates of nickel, titanium, tungsten, aluminium oxide and zirconium dioxide. Phys. Thin Film Met. Metall. 28, 83 (1969)
Savaloni, H., Player, M.A., Gu, E., Marr, G.V.: Influence of substrate temperature, deposition rate, surface texture and material on the structure of uhv deposited erbium films. Vacuum 43, 965–980 (1992)
Savaloni, H., Player, M.A.: Influence of deposition conditions and of substrate on the structure of uhv deposited erbium films. Vacuum 46, 167–179 (1995)
Savaloni, H., Bagheri Najmi, S.: Characteristics of Cu and Zn films deposited on glass and stainless steel substrates at different substrate temperatures and angle of incidence. Vacuum 66, 49–58 (2002)
Jonsson, T., Karlsson, S., Hooshyar, H., Sattari, M., Liske, J., Svensson, J.-E., Johansson, L.-G.: Oxidation after breakdown of the chromium-rich scale on stainless steels at high temperature: internal oxidation. Springer 85, 509–536 (2016)
Jankowski, J., Juchniewicz, R.: A four-point method for corrosion rate determination. Corros. Sci. 20, 841–851 (1980)
Rocchini, G.: Corrosion rate monitoring by the linear polarization method. Corros. Sci. 34, 2031–2044 (1993)
Escrivà-Cerdána, C., Blasco-Tamarita, E., García-Garcíaa, D.M., García-Antón, J., Guenbourb, A.: Temperature effect on the austenitic stainless steel UNS N08031 used in the wet method phosphoric acid production. Chem. Eng. Trans. 32, 1717–1722 (2013)
Blasco-Tamarit, E., Igual-Muñoz, A., García Antón, J., García-García, D.: Effect of temperature on the corrosion resistance and pitting behaviour of Alloy 31 in LiBr solutions. Corros. Sci. 50, 1848–1857 (2008)
Rahmouni, K., Keddam, M., Srhiri, A., Takenouti, H.: Corrosion of copper in 3% NaCl solution polluted by solphide ions. Corros. Sci. 47, 3249–3266 (2005)
Lei, Z., Zhanga, Q., Zhua, X., Maa, D., Maa, F., Songa, Z., Fub, Y.Q.: Corrosion performance of ZrN/ZrO2 multilayer coatings deposited on 304 stainless steel using multi-arc ion plating. Appl. Surf. Sci. 431, 170–176 (2018)
Al-Daraghmeha, M.Y., Hayajneha, M.T., Almomania, M.A.: Resistance of TiO2–ZrO2 nano-composite thin films spin coated on AISI3O4 stainless steel in 3.5 wt% NaCl solution. Mater. Res. 22, e20190014 (2019)
Carvalho, J.B.R., Silva, R.S., Cesarino, I., Machado, S.A.S., Eguiluz, K.I.B., Cavalcanti, E.B., Salazar-Banda, G.R.: Influence of the annealing temperature and metal salt precursor on the structural characteristics and anti-corrosion barrier effect of CeO2 sol–gel protective coatings of carbon steel. Ceram. Int. 40, 13437–13446 (2014)
Poorqasemi, E., Abootalebi, O., Peikari, M., Haqdar, F.: Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 51, 1043–1054 (2009)
Sorensen, P.A., Kiil, S., Dam-Johansen, K., Weinell, C.E.: Anticorrosive coatings: a review. J. Coat. Technol. Res. 6, 135–176 (2009)
Fattah-alhosseini, A., Farahani, H.: Electrochemical behavior of AISI 304 stainless steel in sulfuric solution. J. Mater. Sci. Eng. 10, 4 (2013)
Javidparvar, A.A., Ramezanzadeh, B., Ghasemi, E.: The effect of surface morphology and treatment of Fe3O4 nanoparticles on the corrosion resistance of epoxy coating. J. Taiwan Inst. Chem. Eng. 61, 356–366 (2015)
Palomino, L.E.M., Aoki, I.V., de Melo, H.G.: Microstructural and electrochemical characterization of Ce conversion layers formed on Al alloy 2024–T3 covered with Cu-rich smut. Electrochim. Acta 51, 5943–5953 (2006)
Yoganandan, G., Premkumar, K.P., Balaraju, J.: Evaluation of corrosion resistance and self-healing behavior of zirconium–cerium conversion coating developed on AA2024 alloy. Surf. Coat. Technol. 270, 249–258 (2015)
Mahdavian, M., Attar, M.M.: Another approach in analysis of paint coatings with EIS measurement: phase angle at high frequencies. Corros. Sci. 48, 4152–4157 (2006)
Hassanzadeh, A.: Validity of dynamic electrochemical impedance spectra of some amine corrosion inhibitors in petroleum/water corrosive mixtures by Kramers–Kronig transformation. Corros. Sci. 49, 1895–1906 (2007)
Abdeli, M., Parvini Ahmadi, N., Azari Khosroshahi, R.: Influence of bis-(2-benzothiazolyl)-disulfide on corrosion inhibition of mild steel in hydrochloric acid media. J. Solid State Electrochem. 15, 1867–1873 (2011)
Popkirov, G., Schindler, R.N.: A new approach to the problem of “good” and “bad” impedance data in electrochemical impedance spectroscopy. Electrochim. Acta 39, 2025–2030 (1994)
Allen, J., Bard, L.R.F.: Electrochemical methods, fundamentals and applications. 2nd Edition (2001). ISBN: 978-471-04372-0
Zhang, J., Monteiro, P.J.M.: Validation of resistivity spectra from reinforced concrete corrosion by Kramers–Kronig transformations. Cem. Concr. Res. 31, 603–607 (2001)
Achatz, G., Herzog, G.W., Plot, W.H.: Kramers–Kronig transformation of double-layer capacitances. Surf. Technol. 11, 431–441 (1980)
Javidparvar, A.A., Naderi, R., Ramezanzadeh, B.: Manipulating graphene oxide nanocontainer with benzimidazole and cerium ions: application in epoxy-based nanocomposite for active corrosion protection. Corros. Sci. 165, 108379 (2020)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Modiri, F., Savaloni, H. Anti-corrosion properties of stainless steel 304L coated with Mn-based thin film and annealed with nitrogen flux exposed to saline solution under various temperatures. J Theor Appl Phys 14, 223–236 (2020). https://doi.org/10.1007/s40094-020-00381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-020-00381-6