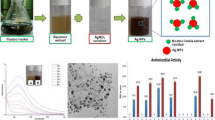
Abstract
In this study, the silver nanoparticles (AgNPs) were extracellularly synthesized using a bioluminescent bacterium, Vibrio campbellii, and characterized their functional properties and morphological nature by UV–Vis spectroscopy, X-ray diffraction (XRD), Fourier transformed infrared spectroscopy, scanning electron microscopy coupled with energy dispersive X-ray spectroscopy (SEM–EDS), and atomic force microscopy (AFM). Further, the synthesized AgNPs were analyzed for their antibacterial and antioxidant activity (2,2-diphenyl-1-picrylhydrazyl (DPPH), and hydrogen peroxide) in in vitro method. The antibacterial activity of AgNPs was tested against pathogenic bacteria such as Aeromonas hydrophila MTCC 1739, Klebsiella pneumoniae MTCC 4030, Klebsiella oxytoca MTCC 3030, and Pseudomonas aeruginosa MTCC 1934. Characterization studies revealed that the synthesized AgNPs were poly-dispersed, spherical shaped with various size ranges, and exhibited as crystalline in nature. The assay of antibacterial activity showed the synthesized AgNPs strongly inhibited the tested pathogenic bacterial growth. Also, the AgNPs showed good antioxidant activity by strong scavenging actions on DPPH (61.88%) and hydrogen peroxide (53.48%) free radicals. Overall results demonstrated that AgNPs could be used in the pharmaceutical field due to their good antibacterial and antioxidant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, nanotechnology has increased attention due to its attractive and diversified applications in various fields of medicine, environmental control, agriculture, cosmetics, solar cells, food, and the textile industry [1,2,3,4]. Presently, the materials of various types at the nanoscale level are produced by nanotechnology, in particular, materials with less than 100 nm of dimensions. Nano-sized materials have attracted a lot of attention in the fields of electronics and biotechnology because of their unique physicochemical and electrical properties. In medicine, metal nanoparticles are being used in the delivery system for drugs, proteins, DNA, and monoclonal antibodies [5]. Although the metal nanoparticles have several therapeutic benefits, some disadvantages have been noted i.e., they can be toxic to both diseased and healthy cells even at low concentrations. Some metal nanoparticles have toxicity at high doses over a long period [6]. However, metal nanoparticles synthesis is important due to its extensive use in various fields. Various methods such as physical, chemical, biological, and hybrid systems have been employed in nanotechnology to synthesize nanoparticles. The synthesis of nanoparticles by physical, chemical, and hybrid systems is costly and generates undesirable environmentally hazardous byproducts [7]. Biological-based metal nanoparticles synthesis has several unique advantages over the physical and chemical-based synthesis approach. The nanoparticles synthesized by the biological method are eco-friendly, less cost, and very efficient and also alternatives to physical and chemical methods [8]. Different metal nanoparticles (Au, Ag, Fe, Cu, Zn, Ti, Co, and Ni) are efficiently synthesized by biological methods in recent years. In the biological methods, the various parts of plants and microbes (bacteria, fungi, algae, and actinomycetes), as well as their derivatives, have been successfully employed to synthesize nanoparticles [9]. For instance, the plant parts include extracts of bark, leaves, fruits, stem, root, seed, and flower are used to syntheses of metallic nanoparticles [10]. For microbes, the cells and their derivatives are used for nanoparticle synthesis. In general, the synthesis of metal nanoparticles by microbes is performed extracellularly or intracellularly. Various species of bacteria and their derivatives have been studied for their metal nanoparticle synthesizing capability [11].
Multidrug-resistant bacteria have become a serious public health problem and the treatment of infection caused by those bacteria is very difficult using conventional antibiotics. Widespread overuse and abuse of conventional antibiotics can lead to the emergence of multidrug-resistant bacteria in the environment. Therefore, the development of bioactive metal nanoparticles could be a new strategy to combat multi-drug-resistant bacteria [12]. AgNPs have been experimentally proved as effective bioactive materials against multidrug-resistance bacteria and have been vastly used as antimicrobial, anticancer, and anti-inflammatory agents in medicine [2, 13]. In addition, the AgNPs have potential larvicidal, and nematicidal properties [14, 15]. Bioluminescent bacteria are light-emitting organisms and they are found as free-living organisms or symbionts which diversely exist in both terrestrial and marine environment, surface, and gut of marine animals [16]. They are classified into four major genera such as Vibrio, Photobacterium, Alteromonas, and Xenorhabdus [17] and are used in various applications include biosensors for contaminants detection, and pollutant toxicity measurement [18, 19]. Amongst, the Photobacterium and Vibrio species predominantly exist in the marine environment. Some of the luminescent bacteria cause diseases in the aquaculture industry [20]. Further, the research on bioactive compounds isolation from bioluminescent bacterial species has already shown their promising antibiotic characteristics. Bioactive substances produced by bioluminescent bacteria include polysaccharides, proteins (bacteriocins), enzymes (proteinase, l-asparaginase), and organic acids, which may be responsible for metal ions reduction [21]. To our knowledge, this is the first study to investigate the AgNPs synthesizing ability of bioluminescent bacteria isolated from coastal water samples of Thondi, Palk Strait region, India. The study aimed to investigate the AgNPs synthesizing capability of bioluminescent bacteria, in particular, belongs to Vibrio species which were isolated in the earlier study [22, 23]. Further, the structural characterization and investigation of the antibacterial and antioxidant potential of AgNPs synthesized by Vibrio campbellii were performed.
Materials and methods
Bacterial strains and screening of AgNPs synthesis ability
In this study, the bioluminescent bacteria, in particular, belongs to Vibrio species were screened for their AgNPs synthesizing ability. In total, 8 Vibrio species (Vibrio sp. GSAU-14, Vibrio campbellii GSAU-15, Vibrio sp. GSAU-17, Vibrio owensii, Vibrio harveyi, Vibrio campbellii, Vibrio sp. GSAU-22, and Vibrio rotiferianus) were screened in this study. The Vibrio species and their GenBank Accession number are shown in Table 1. For screening, all bacterial strains were inoculated separately in 20 ml of prepared luminescence (LM) medium and incubated on a shaker (200 rpm) at room temperature for 24 h. The LM medium contained 3 g yeast extract; 3 g glycerol; 1 g CaCO3; 3 g trypton; dissolved in aged seawater (1000 ml); pH 7.2 [24]. After 24 h, the cultures were centrifuged (10,000 rpm for 10 min) to collect the supernatant for AgNPs synthesis and the rest of the biomass was discarded. The collected supernatant was mixed with 100 ml of freshly prepared 1 mM AgNO3 (1:4) solution and incubated at room temperature and 200 rpm for 72 h. The synthesis of AgNO3 in the reaction mixture was checked periodically by monitoring the color changes and spectral analysis in the range of 200–800 nm by UV–Vis spectrophotometer.
Synthesis and characterization of AgNPs
From the screening assay, Four Vibrio species such as Vibrio sp. GSAU-17, V. harveyi, V. campbellii, and V. rotiferianus showed strong AgNPs synthesizing capability. Amongst, V. campbellii were selected for further studies due to their strong AgNPs synthesizing capability within the shorter period of incubation. The AgNPs synthesis by V. campbellii was done as the method described above and collected after 48 h. To collect the synthesized AgNPs, the reaction mixture was centrifuged (10,000 rpm for 10 min) and the collected pellet was purified by washing with 50 mM Tris buffer (pH 7.0). The purified pellet was dried (80 °C for 12 h) and powdered for characterization studies [9]. The characterization of powdered AgNPs was characterized using Fourier transformed infrared (FT-IR), X-ray diffraction (XRD), scanning electron microscope coupled with energy dispersive X-ray spectroscopy (SEM–EDS), and atomic force microscopy (AFM) analysis. The functional groups of AgNPs were examined through FT-IR (Thermo scientific Nicolet iS5) in the range between 4000 and 400 cm−1. XRD analysis was performed to determine the crystallinity nature of AgNPs. For this, the spectrum for AgNPs was scanned at 15 kV and 25 mA in the range between 10 and 80º counts (2θ) using PANalytical X'PERT-PRO powder X-ray diffractometer. The AgNPs size and morphology were studied using SEM–EDS analysis (Joel JSM-56010 with INSA-EDS) and surface topography was analyzed by AFM analysis (A100 SGS, A.P.E. Research-Italy).
Antibacterial activity assay
The antibacterial activity of AgNPs was evaluated by the agar well diffusion method against pathogenic bacteria. The pathogenic bacterial strains such as A. hydrophila MTCC 1739, K. oxytoca MTCC 3030, K. pneumoniae MTCC-4030, and P. aeruginosa MTCC 1934 were obtained from Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh, India. Briefly, 12 h grown pathogenic bacterial cultures were swabbed on previously well punched Mueller Hinton agar plates, and then, AgNPs solution at different concentrations (10, 25, 50, and 100 µl) were loaded in each well and incubated at 37 °C for 24 h. After incubation, the zone of inhibition was measured and expressed as mm in diameter. The experiment was conducted in triplicate.
Antioxidant activity assay
The antioxidant activity of AgNPs was determined by evaluating their potential free radical scavenging properties. The scavenging of free radicals by different concentrations (10, 20, 40, 60, 80, 100 µg/ml) of AgNPs solution was investigated using two in vitro assays such as DPPH (2,2-diphenyl-1-picrylhydrazyl) and hydrogen peroxide scavenging assay. The DPPH and hydrogen peroxide scavenging assay was conducted according to the method [25]. For each assay, l-ascorbic acid and double distilled water were used as the reference and blank, respectively. Each assay was conducted in triplicate.
Statistical analysis
All the experiments were done in triplicate. The experimental data were analyzed by calculating mean ± SD and analysis of variance (ANOVA) with Bonferroni test using GraphPad Prism 5.0 for windows.
Results and discussion
Screening of AgNPs synthesizing bacteria
The photograph of AgNPs synthesis by selected eight Vibrio species is shown in Fig. S1. Amongst, four strains such as Vibrio sp. GSAU-17, V. harveyi, V. campbellii, and V. rotiferianus synthesized the AgNPs effectively in the reaction mixture after 48 h incubation. The rest of the four strains (Vibrio sp. GSAU-22, V. campbellii GSAU-15, V. owensii, and Vibrio sp. GSAU-14) did synthesize the AgNPs moderately even after 48 h. Generally, the colour changes of the reaction mixture from yellow into brown colour might be due to Ag+ reduction [26]. The UV spectra for the reaction mixture of Vibrio sp. GSAU-17, V. harveyi, V. campbellii, and V. rotiferianus are shown in Fig. 1a–d. A strong absorption peak in the UV spectra of Vibrio sp. GSAU-17, V. harveyi, V. campbellii, and V. rotiferianus were observed in 400 nm, 420 nm, 430 nm, and 423 nm, respectively. The UV spectral results confirmed the strains such as Vibrio sp. GSAU-17, V. harveyi, V. campbellii, and V. rotiferianus were able to synthesize AgNPs extracellularly. Amongst, the strain V. campbellii showed a strong AgNPs synthesizing ability within the short period of incubation. In general, the AgNPs characteristics peak can be detected between 400 and 450 nm in the UV–Vis region [27]. Also, the existence of absorption peaks at various positions between 400 and 450 nm in the UV–Vis absorption spectra for AgNPs is generally determined by nanoparticle size. Hong and Li [28] reported that the absorption peak for gold nanoparticles was found to increase between 510 and 550 nm in the UV-spectra when analyzed various sizes of nanoparticles (17–80 nm in size). When 17 nm, 30 nm, 40 nm, 50 nm, 60 nm, and 80 nm size gold nanoparticles were examined, the absorption peak was observed at 510 nm, 525 nm, 530 nm, 536 nm, 540 nm, and 550 nm in the UV spectra, respectively.
Characterization studies
FTIR analysis
The FTIR spectrum obtained for AgNPs synthesized by V. campbellii is illustrated in Fig. 2. The wavenumber of peaks detected in the IR spectra and their corresponding functional groups are shown in Table 2. As shown in Table 2, the functional groups such as O–H, N–H, C–H, C=O, and C–O groups had existed in the IR spectra of AgNPs and these functional groups containing biomolecules (polysaccharides, proteins, and other constituents) might be responsible for Ag+ reduction [29]. Sayed Ahmed et al. [30] stated that functional groups such as amide, hydroxyl, and carboxylate can be involved in the reduction of Ag+ to Ag0. Whereas, the functional groups such as amine and carbonyl groups belong to protein molecules present in the culture supernatant of Bacillus sp. possessed silver ions reducing capability [31]. Sing et al. [32] stated that hydroxyl groups present in the polysaccharides produced by brown macroalga Padina gymnospora took part in the reduction of gold ions. From this, the biomolecules having functional groups such as O–H, N–H, C–H, C=O, and C–O are involved in metal ions reduction and also acted as stabilization agents. Mathivanan et al. [9] stated that the biomolecules present in the reaction mixture might have acted as a capping/stabilization agent for AgNPs.
XRD spectral analysis
XRD spectrum of AgNPs synthesized by V. campbellii is shown in Fig. 3. The spectrum showed the diffraction peaks at 2θ values of 27.75°, 32.15°, 46.15°, 54.75°,, and 76.65°, corresponded to plans of (101), (111), (200), (220), and (311), respectively (Fig. 3). Further, the diffraction data of AgNPs were compared with the powder diffraction data files of known compounds (ICDD/JCPDS, PDF Nos. 04-0783 and 84-0713). The XRD spectral analysis confirmed the presence of AgNPs in the sample and revealed the AgNPs were face-centered, cubic, and crystalline in nature [34].
SEM–EDS and AFM analysis
The structure, size, and morphology of the synthesized AgNPs were studied by scanning electron microscope (SEM) are shown in Fig. 4a. SEM analysis showed that the synthesized AgNPs by V. campbellii predominantly were polydispersed, spherical in shape, and existed uniformly. The size of AgNPs was varied and observed in the range between 10 and 250 nm. The EDS analysis showed strong peaks at 2–3 keV regions, which confirms the presence of elemental silver. Further, the EDS results revealed the formation of Ag0 by reduction of Ag+ through biomolecules present in the culture supernatant (Fig. 4b). The AFM images of AgNPs synthesized V. campbellii is shown in Fig. 5a, b. Results showed that the synthesized AgNPs existed as aggregates with various heights and roughness. The roughness and height of the AgNPs are 0.43 nm and 16 nm.
Antibacterial activity of AgNPs
The growth inhibition activity of AgNPs synthesized by V. campbellii against pathogenic bacteria such as A. hydrophila MTCC 1739, K. pneumoniae MTCC 4030, K. oxytoca MTCC 3030, and P. aeruginosa MTCC 1934 are shown in Table 3 and Supplementary Fig. S2. The agar well diffusion assay results showed that AgNPs strongly inhibited the growth of tested pathogenic bacteria. The maximum inhibition activity of AgNPs against tested pathogenic bacteria was observed at a higher concentration (100 µl). The inhibitory zone of AgNPs (100 µl) observed for tested pathogenic bacteria is as follows; 8 ± 0.1 mm for A. hydrophila MTCC 1739; 6.8 ± 0.4 mm for K. pneumoniae MTCC-4030; 9.4 ± 0.5 mm for K. oxytoca MTCC 3030; 7.2 ± 0.3 mm for P. aeruginosa MTCC 1934. Loo et al. [12] reported that the AgNPs synthesized using pu-erh tea leaves extracts inhibited the growth of K. pneumoniae (10 mm) in the disk diffusion method. Singh et al. [35] reported that the AgNPs synthesized using culture supernatant of Pseudomonas sp. THG-LS1.4 had good growth inhibition against pathogenic bacteria such as Bacillus cereus, Staphylococcus aureus, Candida tropicalis, Vibrio parahaemolyticus, Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica.
Antioxidant activity of AgNPs by V. campbellii
The antioxidant activity of AgNPs synthesized by V. campbellii was evaluated through their scavenging potential of DPPH and nitric oxide free radicals. Results showed that the scavenging of DPPH free radicals by AgNPs increased with an increasing concentration from 10 to 100 µg/ml. The scavenging free radicals of DPPH by AgNPs of V. campbellii was 61.88% at 100 µg/ml concentration (Fig. 6a). Keshari et al. [36] reported that the free radicals of DPPH were scavenged by AgNPs synthesized by Cestrum nocturnum was 29.55% at 100 µg/ml concentration. Saravanakumar et al. [37] stated the scavenging of DPPH free radicals by AgNPs synthesized by Prunus japonica (Rosaceae) leaf extract was 55% at 200 µg/ml concentration. A similar trend of DPPH free radicals scavenging activity was reported for AgNPs synthesized by Streptomyces olivaceus MSU3 [37] and EPS of Streptomyces violaceus MM72 [25].
The hydrogen peroxide scavenging activity of AgNPs increased (%) with increasing the concentration of AgNPs from 10 to 100 µg/ml (Fig. 6b). The maximum scavenging of hydrogen peroxide by AgNPs of V. campbellii was observed as 53.48% at 100 µg/ml concentration. Keshari et al. [36] reported the AgNPs of Cestrum nocturnum scavenged 45.41% of hydrogen peroxide radicals at 100 µg/ml concentration. Sanjivkumar et al. [38] reported that AgNPs synthesized by Streptomyces olivaceus MSU3 have strong antioxidant activity. Further, the DPPH and hydrogen peroxide scavenging activity observed for AgNPs of V. campbellii was considerably lower than that of the reference standard ascorbic acid.
Conclusions
In the present study, the AgNPs synthesizing capability of bioluminescent bacteria, in particular, belongs to Vibrio species were screened. Amongst, four strains such as Vibrio sp. GSAU-17, V. harveyi, V. campbellii, and V. rotiferianus showed strong AgNPs synthesizing capability. The characterization studies revealed that AgNp synthesized by V. campbellii were polydispersed, spherical shaped with sizes ranging from 10 to 250 nm. Further, the synthesized AgNPs were crystalline in nature and the bioactive compounds present in the solution were acted as a capping/stabilizing agent. The antibacterial activity assay showed the synthesized AgNPs by V. campbellii strongly inhibited the growth of tested pathogenic bacteria and showed a maximum inhibition on K. oxytoca MTCC 3030. Also, the AgNPs synthesized by V. campbellii effectively scavenged the DPPH (61.88%) and hydrogen peroxide (53.48%) free radicals. Overall experimental results suggested that AgNPs could be used in the pharmaceutical field due to their strong antibacterial and good antioxidant activity. Also, future work will be focused on understanding the inhibitory action of AgNPs on pathogenic bacteria.
References
Wong, K.V, Perilla, N., Paddon, A.: Nanoscience and nanotechnology in solar cells. J. Energy Resour. Technol. 136 (2014). https://doi.org/10.1115/1.4024715
Ramalingam, V., Rajaram, R., Premkumar, C., Santhanam, P., Dhinesh, P., Vinothkumar, S., Kaleshkumar, K.: Biosynthesis of silver nanoparticles from deep sea bacterium Pseudomonas aeruginosa JQ989348 for antimicrobial, antibiofilm, and cytotoxic activity. J. Basic Microbiol. 54, 928–936 (2014)
Vasantharaj, S., Sathiyavimal, S., Saravanan, M., Senthilkumar, P., Gnanasekaran, K., Shanmugavel, M., Manikandan, E., Pugazhendhi, A.: Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B Biol. 191, 143–149 (2019)
Singh, T., Shukla, S., Kumar, P., Wahla, V., Bajpai, V.K., Rather, I.A.: Application of nanotechnology in food science: perception and overview. Front. Microbiol. 8, 1501 (2017)
Bahadar, H., Maqbool, F., Niaz, K., Abdollahi, M.: Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 20, 1 (2016)
Mortezaee, K., Najafi, M., Samadian, H., Barabadi, H., Azarnezhad, A., Ahmadi, A.: Redox interactions and genotoxicity of metal-based nanoparticles: a comprehensive review. Chem. Biol. Interact. 312, 108814 (2019)
Patra, J.K., Baek, K.-H.: Green nanobiotechnology: factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 219 (2014)
Oves, M., Aslam, M., Rauf, M.A., Qayyum, S., Qari, H.A., Khan, M.S., Alam, M.Z., Tabrez, S., Pugazhendhi, A., Ismail, I.M.I.: Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C 89, 429–443 (2018). https://doi.org/10.1016/j.msec.2018.03.035
Mathivanan, K., Selva, R., Chandirika, J.U., Govindarajan, R.K., Srinivasan, R., Annadurai, G., Duc, P.A.: Biologically synthesized silver nanoparticles against pathogenic bacteria: synthesis, calcination and characterization. Biocatal. Agric. Biotechnol. 22, 101373 (2019)
Kuppusamy, P., Yusoff, M.M., Maniam, G.P., Govindan, N.: Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—an updated report. Saudi Pharm. J. 24, 473–484 (2016)
Deljou, A., Goudarzi, S.: Green extracellular synthesis of the silver nanoparticles using thermophilic Bacillus sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iran. J. Biotechnol. 14, 25 (2016)
Loo, Y.Y., Rukayadi, Y., Nor-Khaizura, M.-A.-R., Kuan, C.H., Chieng, B.W., Nishibuchi, M., Radu, S.: In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 9, 1555 (2018)
Wong, K.K.Y., Cheung, S.O.F., Huang, L., Niu, J., Tao, C., Ho, C., Che, C., Tam, P.K.H.: Further evidence of the anti-inflammatory effects of silver nanoparticles. ChemMedChem Chem. Enabling Drug Discov. 4, 1129–1135 (2009)
Sutthanont, N., Attrapadung, S., Nuchprayoon, S.: Larvicidal activity of synthesized silver nanoparticles from Curcuma zedoaria essential oil against Culex quinquefasciatus. Insects 10, 27 (2019)
Barbosa, A.C.M.S., Silva, L.P.C., Ferraz, C.M., Tobias, F.L., de Araújo, J.V., Loureiro, B., Braga, G.M.A.M., Veloso, F.B.R., de Freitas Soares, F.E., Fronza, M.: Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 14, 2341 (2019)
Iqbal, B.M.M., Rajendran, S., Vasudevan, S.: Isolation, identification and characterization of the bioluminescent bacteria isolated from the blue swimmer crab Portunus pelagicus along Thondi Coast and virulence studies at high temperatures. Microb. Pathog. 117, 232–236 (2018)
Baker, J.M., Griffiths, M.W., Collins-Thompson, D.L.: Bacterial bioluminescence: applications in food microbiology. J. Food Prot. 55, 62–70 (1992)
Frischer, M.E., Danforth, J.M., Foy, T.F., Juraske, R.: Bioluminescent bacteria as indicators of chemical contamination of coastal waters. J. Environ. Qual. 34, 1328–1336 (2005)
Gellert, G.: Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicol. Environ. Saf. 45, 87–91 (2000)
Ramesh, C.H., Mohanraju, R.: Antibacterial activity of marine bioluminescent bacteria. Indian J Geo-Mar Sci. 46(10), 2063–2074 (2017)
Iravani, S., Korbekandi, H., Mirmohammadi, S.V., Zolfaghari, B.: Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci. 9, 385 (2014)
Srinivasan, R.: Phylogenetic characterization of bioluminescent bacteria in Palk strait southeast coast of India. Ph.D thesis, Alagappa University (2014)
Govindasamy, C., Srinivasan, R.: Association of bioluminescent bacteria from blue swimmer crab Portunus pelagicus (Linneaus, 1758). Asian Pacific J. Trop. Dis. 2, S699–S702 (2012)
Danyluk, B., Uchman, W., Konieczny, P., Bilska, A.: An objective method to assess bioluminescent properties of selected bacterial strains. Acta Sci. Pol. Technol. Aliment. 6, 5–16 (2007)
Sivasankar, P., Seedevi, P., Poongodi, S., Sivakumar, M., Murugan, T., Sivakumar, L., Sivakumar, K., Balasubramanian, T.: Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr. Polym. 181, 752–759 (2018)
Augustine, R., Kalarikkal, N., Thomas, S.: A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Appl. Nanosci. 4, 809–818 (2014)
Kheybari, S., Samadi, N., Hosseini, S.V., Fazeli, A., Fazeli, M.R.: Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. Daru 18, 168–172 (2010)
Hong, S., Li, X.: Optimal size of gold nanoparticles for surface-enhanced raman spectroscopy under different conditions. J. Nanomater. 2013, 790323 (2013). https://doi.org/10.1155/2013/790323
Jyoti, K., Baunthiyal, M., Singh, A.: Characterization of silver nanoparticles synthesized using Urtica dioica Linn. Leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. (2016). https://doi.org/10.1016/j.jrras.2015.10.002
Sayed Ahmed, H.I., Elsherif, D.E., El-Shanshory, A.R., Haider, A.S., Gaafar, R.M.: Silver nanoparticles and Chlorella treatments induced glucosinolates and kaempferol key biosynthetic genes in Eruca sativa. Beni-Suef Univ. J. Basic Appl. Sci. 10, 1–15 (2021)
Gopinath, V., Velusamy, P.: Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 106, 170–174 (2013)
Singh, M., Kalaivani, R., Manikandan, S., Sangeetha, N., Kumaraguru, A.K.: Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl. Nanosci. 3, 145–151 (2013)
Monowar, T., Rahman, M.S., Bhore, S.J., Raju, G., Sathasivam, K.V.: Silver nanoparticles synthesized by using the endophytic bacterium Pantoea ananatis are promising antimicrobial agents against multidrug resistant bacteria. Molecules 23, 3220 (2018). https://doi.org/10.3390/molecules23123220
Raut, R.W., Kolekar, N.S., Lakkakula, J.R., Mendhulkar, V.D., Kashid, S.B.: Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L.) pierre. Nano-Micro Lett. 2, 106–113 (2010)
Singh, H., Du, J., Singh, P., Yi, T.H.: Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1. 4 and their antimicrobial application. J. Pharm. Anal. 8, 258–264 (2018)
Keshari, A.K., Srivastava, R., Singh, P., Yadav, V.B., Nath, G.: Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 11, 37–44 (2020)
Saravanakumar, A., Peng, M.M., Ganesh, M., Jayaprakash, J., Mohankumar, M., Jang, H.T.: Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif. Cells Nanomed. Biotechnol. 45, 1165–1171 (2017)
Sanjivkumar, M., Vaishnavi, R., Neelakannan, M., Kannan, D., Silambarasan, T., Immanuel, G.: Investigation on characterization and biomedical properties of silver nanoparticles synthesized by an actinobacterium Streptomyces olivaceus (MSU3). Biocatal. Agric. Biotechnol. 17, 151–159 (2019)
Acknowledgements
We thank the authorities of Alagappa University for providing facilities and encouragement. We also thank the Department of Science and Technology (DST) Promotion of University Research and Scientific Excellence (PURSE) Rc.A13 Dt/29.08.11 sponsored research project, Government of India, New Delhi, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of supporting data
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Srinivasan, R., Mathivanan, K., Govindarajan, R. et al. Extracellular synthesis of silver nanoparticles by bioluminescent bacteria: characterization and evaluation of its antibacterial and antioxidant properties. Int Nano Lett 12, 169–177 (2022). https://doi.org/10.1007/s40089-021-00360-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-021-00360-y