Abstract
One-dimensional PbTiO3 (PTO) nanotubes (NTs) were fabricated via a hydrothermal method based on an anodized TiO2 template. Anatase-structured TiO2 NTs were converted into tetragonal-structured PTO NTs by the entry of Pb2+ ions from high pressure generated during the hydrothermal process. This was confirmed through structural analysis using X-ray diffraction and Raman scattering; increase in the wall thickness of the NTs was observed. However, it was observed that when the conversion process from TiO2 to PTO NTs exceeds the saturation state, the surface morphology of PTO NTs is changed, and PbO particles are formed on the PTO NTs. This constitutes an inhibitory factor in forming PTO NT arrays with clear surface morphology. To prevent this phenomenon, we successfully fabricated PTO NT arrays by controlling the reaction temperature, reaction time, and molar concentration of lead acetate solutions in a hydrothermal process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Because nanoscale structures have novel properties that depend on their size, they continue to receive much attention. Particularly, one-dimensional (1D) structures such as nanotubes (NTs) and nanowires (NWs) are the smallest-dimensional structures that can be used for efficient electron transport and optical excitations. Therefore, they are expected to play an essential role in the function and integration of components in nanoscale electronic devices [1,2,3].
Ferroelectrics have been widely applied in various fields owing to their unique physical properties such as piezoelectric, ferroelectric, ferroelastic, and pyroelectric behaviors. Because nanostructures have relatively high specific surface areas compared to the bulk, they present a greater amount of surface atoms and higher level of surface energy [4]. Accordingly, the characteristics of the piezoelectric response, including spontaneous polarization, dielectric properties, and phase transitions, which are intrinsic properties of ferroelectrics, are modified [5]. Additionally, a reduction in size and dimensionality can promote the formation of single domain structures, thereby significantly improving ferroelectric properties [4]. Moreover, NT geometries can lead to novel properties that enable various applications such as piezoelectric nanogenerators, terahertz emitters, high-density memory, high-density dielectric energy storage, and fluidic devices [6]. Therefore, theoretical and experimental studies of ferroelectric domain-wall nanoelectronics on polarization domains and complex interactions such as geometry, surface effect, and crystal symmetry of nanostructures have been recently conducted [6, 7]. In this sense, developing and applying various methods to fabricate high-quality 1D ferroelectric nanostructures for related research and applications is essential.
Hydrothermal, sol–gel template, nanosolid-state reaction, molten salt, and electrospinning methods are used for manufacturing 1D ferroelectric nanostructures [4]. A universal method for manufacturing 1D ferroelectric nanostructures is the sol–gel template synthesis method, the reason for this is that the morphology of the 1D ferroelectric structures is inherited from the channel of templates.
Therefore, the diameters and lengths of the fabricated NTs or NWs are uniform. However, a relatively high temperature in calcination process for crystallization and removal of the used template are necessary [4].
On the other hand, the hydrothermal method is one of the most common methods for synthesizing nanomaterials, and is relatively easy and inexpensive. Also, the size, shape, and crystallinity of the resulting synthetic materials can be controlled through changing parameters such as reaction temperature, reaction time, solvent type, surfactant type, and pH of the solution [5, 8]. However, it is difficult to uniformly control the morphology in the fabrication of NTs or NWs. Therefore, we used a TiO2 template-assisted hydrothermal method for easy manufacturing process and uniform morphology control of the PbTiO3 (PTO) NT arrays.
We used a TiO2 template with anodized TiO2 NTs grown on Ti foil to fabricate ferroelectric PTO NTs. Previous studies reported that 1D nanostructures can be fabricated using a TiO2 template as both a precursor and template through a relatively simple low-temperature hydrothermal process [9, 10]. In this study, the TiO2 template formed an anatase phase through heat treatment; given that anatase TiO2 NTs have a similar structure to the final target (tetragonal PTO NTs), it was determined that they would be easy to synthesize via a hydrothermal process. Therefore, optimized PTO NTs were fabricated by applying a hydrothermal process characterized by various parameters.
2 Experiments
-
A.
Preferred anatase TiO2 NTs (TiO2 template)
To obtain anatase TiO2 NTs, pure Ti foil (99.5%, 250-µm thickness, Alfa Aesar) was prepared with a size of 1.0 × 1.5 cm2. Anodization was performed using ethylene glycol (99.0%, Daejung Chemical) dissolved in 0.3 wt% NH4F (≥ 98.0%, Sigma Aldrich) and 10 wt% deionized (DI) water as electrolyte solutions at 25 °C and applied electric voltage with 50 V for 1 h [11]. Then, a TiO2 template of the anatase phase was prepared by heat treatment at a temperature of 450 °C for 2 h.
Figure 1a shows the X-ray diffraction (XRD) results of the Ti foil and TiO2 template before and after heat treatment. Only Ti foil peaks were observed, indicating that the anodized TiO2 NTs had an amorphous structure. Then, TiO2 NTs with anatase phase (JCPDS No. 21-1272) were formed through heat treatment [11, 12]. It can be concluded from the field-emission scanning electron microscope (FE-SEM) results that the TiO2 NTs produced in the form of a tube had an average wall thickness of 14 nm and an average length of 2.2 µm (Fig. 1b, c).
-
B.
Fabrication of ferroelectric PTO NTs by hydrothermal method
Lead acetate solutions were prepared by dissolving 0.005 M and 0.05 M lead acetate trihydrate (≥ 99.99%, Sigma Aldrich) in DI water. Vessels were filled with the lead acetate solutions up to 140 mL (approximately 70% of the total capacity of the vessels). Then, the prepared TiO2 template was placed in the vessels. Finally, the hydrothermal vessels were placed in an oven at 200 °C for 6–36 h to form PTO NTs [13].
The prepared TiO2 and PTO NTs were characterized by a FE-SEM (S-4800, Hitachi) and XRD (D/MAX-2500, Rigaku) analyses. Energy-dispersive X-ray spectroscopy (EDS) was used to analyze the Pb, Ti, and O spectra. In addition, the local crystal symmetry was evaluated through room-temperature Raman scattering analysis (Raman, Confotec NR500, SOL).
3 Results and discussion
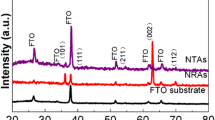
Figure 2a shows the XRD results of PTO NTs prepared using 0.005 M or 0.05 M lead acetate solutions with a reaction time of 24 h. In both cases, the peaks related to PTO of the tetragonal phase (space group P4mm) can be identified; the lattice parameters calculated through the (100) and (001) peaks are a = 3.926 and 3.921 Å, c = 4.172 and 4.191 Å, respectively. These results are similar to those previously reported [14, 15]. Also, peaks related to the TiO2 template (Fig. 1a) were also observed. These results suggest that polycrystalline PTO (JCPDS No. 78-0298) NTs were properly synthesized and TiO2 NTs grown on Ti foil by the hydrothermal method were successfully converted into PTO NTs. However, the PTO NTs do not show the expected preferential orientation. The reason is that the anatase TiO2 NTs are not continuous tubes but an agglomerated form of anatase nanoparticles. This is known to be a typical feature of anodized TiO2 NTs [16]. In this regard, it has been reported that single crystal PTO NWs can be fabricated using high-quality regular and uniform TiO2 NTs [16]. These results suggest that the structural properties of the TiO2 template have a great influence on the structure of PTO NTs.
E(1TO), A(1TO), E(2TO), and B1 + E modes related to tetragonal PTO were also confirmed from the Raman results (Fig. 2b) [14, 15, 17]. Moreover, Eg (symmetric stretching vibration of O–Ti–O) and B1g (symmetric bending vibration of O–Ti–O) modes were found near 144 and 395 cm−1 due to the TiO2 template [14]. These results are consistent with XRD results.
The formation mechanism of PTO NTs that can be inferred from these results is as follows. In the initial stage of hydrothermal synthesis, Pb2+ ions dissolved in a hydrothermal solution have a high diffusion rate under high-pressure conditions generated in hydrothermal vessels. These Pb2+ ions are concentrated around the TiO2 template, resulting in local supersaturation of Pb2+ ions. Then, supersaturated Pb2+ ions are inserted into the crystal lattice of TiO2 NTs and gradually converted into tetragonal PTO NTs [9, 15]. The in situ reaction of these Pb2+ ions with the TiO2 template is known to depend on the reaction time of the hydrothermal process [15]. Therefore, the shape and structure transitions as a function of the reaction time in each solution were investigated.
In the case of 0.005 M lead acetate solution, in observing the transitions in morphology with the lapse of reaction time, there was no significant difference from the initial TiO2 template for a reaction time of 6 h (Fig. 3a). For a reaction time of 12 h, the conversion of TiO2 NTs into PTO NTs was induced, and the wall thickness increased owing to crystal cell expansion (Fig. 3b). The wall thickness of the initial TiO2 NTs had an average value of ~ 14 nm and increased to ~ 31 nm after 12 h (Fig. 3b) and ~ 42 nm after 24 h (Fig. 3c). Then, after 36 h, it became saturated, and the wall thickness was maintained at ~ 41 nm (Fig. 3d).
Considering the XRD (Fig. 4a) and Raman (Fig. 4b) measurements together, it can be expected that the conversion from the initial anatase TiO2 structure to the PTO structure started after a reaction time of 6 h. When the reaction time reached 12 h, the conversion of TiO2 NTs into PTO NTs was accelerated, and a distinct PTO structure was formed with an increase in the wall thickness. Then, after 24 h, TiO2 NTs were mostly converted to PTO NTs, and the wall thickness reached its maximum. However, when the synthesis time exceed 36 h, Ti4+ partially dissolved in the Ti foil and PTO NTs formed PTO particles on their surface as a result of a dissolution–precipitation process by reacting with excess Pb2+, thereby changing the surface morphology of NTs (Fig. 3d) [15].
In the case of 0.05 M solution, characteristic new types of particles were formed on PTO NTs for a reaction time of 24 h (Fig. 5a, b). These particles were subjected to elemental analysis through an EDS. The results indicate that these new types of particles (indicated by yellow dotted lines) are mainly composed of Pb and O, unlike PTO NTs (Fig. 5c–e). These results also suggest that in the presence of a large amount of excess Pb2+ ions, as the reaction time increases, PbO particles form on the already formed PTO NTs in the in situ reaction and dissolution–precipitation process.
Therefore, when it comes to fabricating PTO NTs using a TiO2 template and a hydrothermal process, the reaction time of the hydrothermal process and the molar concentration control of lead acetate solutions are crucial parameters to form PTO NTs with clear surface morphology.
4 Conclusion
To fabricate 1D-structured PTO NTs, a TiO2 template was prepared through anodization, and various hydrothermal parameters were adjusted. As a result, TiO2 NTs were converted into PTO NTs by reflecting the shape of the TiO2 template and the wall thickness was increased. However, they were not completely converted to PTO NTs, and showed a polycrystalline structure. This is expected to be due to the structural properties of the TiO2 template.
The surface change of PTO NTs and the formation of PbO particles were investigated as a function of the molar concentration and reaction time of the lead acetate solutions. In the case of 0.05 M solution, a large amount of excess Pb2+ ions formed PbO particles on the PTO NTs surface.
These results are expected to provide information on important experimental parameters and their effects in the fabrication of high-quality ferroelectric nanotubes through TiO2 template-assisted hydrothermal method.
References
J. Hu, T.W. Odom, C.M. Lieber, Chemistry and physics in one dimension: synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 32, 435 (1999). https://doi.org/10.1021/ar9700365
Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim, H. Yan, One-dimensional nanostructures: synthesis, characterization, and applications. Adv. Mater. 15, 353 (2003). https://doi.org/10.1002/adma.200390087
P.M. Rorvik, T. Grande, M.-A. Einarsrud, One-dimensional nanostructures of ferroelectric perovskites. Adv. Mater. 23, 4007 (2011). https://doi.org/10.1002/adma.201004676
L. Liang, X. Kang, Y. Sang, H. Liu, One-dimensional ferroelectric nanostructures: synthesis, properties, and applications. Adv. Sci. 3, 1500358 (2016). https://doi.org/10.1002/advs.201500358
J. Varghese, R.W. Whatmore, J.D. Holmes, Ferroelectric nanoparticles, wires and tubes: synthesis, characterisation and applications. J. Mater. Chem. C 1, 2618 (2013). https://doi.org/10.1039/C3TC00597F
A. Ross, S. Zhuang, M. Zhang, J.-M. Hu, Geometric control of domain structure stability in ferroelectric nanotubes. Adv. Electron. Mater. 8, 2200132 (2022). https://doi.org/10.1002/aelm.202200132
D. Meier, S.M. Selbach, Ferroelectric domain walls for nanotechnology. Nat. Rev. Mater. 7, 157 (2022). https://doi.org/10.1038/s41578-021-00375-z
A. Rabenau, The role of hydrothermal synthesis in preparative chemistry. Angew. Chem. Int. Ed. Engl. 24, 1026 (1985). https://doi.org/10.1002/anie.198510261
N.P. Padture, X. Wei, Hydrothermal synthesis of thin films of barium titanate ceramic nano-tubes at 200 °C. J. Am. Ceram. Soc. 86, 2215 (2003). https://doi.org/10.1111/j.1151-2916.2003.tb03636.x
Y. Mao, S. Banerjee, S.S. Wong, Hydrothermal synthesis of perovskite nanotubes. Chem. Commun. (2003). https://doi.org/10.1039/B210633G
S. Noothongkaew, J.K. Han, Y.B. Lee, O. Thumthan, K.S. An, Au NPs decorated TiO2 nanotubes array candidate for UV photodetectors. Prog. Nat. Sci. 27, 641 (2017). https://doi.org/10.1016/j.pnsc.2017.10.001
S.A. Ansari, M.M. Khan, M.O. Ansari, M.H. Cho, Gold nanoparticles-sensitized wide and narrow band gap TiO2 for visible light applications: a comparative study. New J. Chem. 39, 4708 (2015). https://doi.org/10.1039/C5NJ00556F
B.H. Kim, A study on the crystal structure and shape change of PbTiO3 nanotubes fabricated by hydrothermal synthesis, MS Thesis, Jeonbuk National University (2022)
Y.B. Lee, J.K. Han, S. Noothongkaew, S.K. Kim, W. Song, S. Myung, S.S. Lee, J. Lim, S.D. Bu, K.-S. An, Toward arbitrary-direction energy harvesting through flexible piezoelectric nanogenerators using perovskite PbTiO3 nanotube arrays. Adv. Mater. 29, 1604500 (2017). https://doi.org/10.1002/adma.201604500
Y. Yang, X. Wang, C. Zhong, C. Sun, L. Li, Ferroelectric PbTiO3 nanotube arrays synthesized by hydrothermal method. Appl. Phys. Lett. 92, 122907 (2008). https://doi.org/10.1063/1.2903497
B. Im, H. Jun, K.H. Lee, S.-H. Lee, I.K. Yang, Y.H. Jeong, J.S. Lee, Fabrication of a vertically aligned ferroelectric perovskite nanowire array on conducting substrate. Chem. Mater. 22, 4806 (2010). https://doi.org/10.1021/cm101412d
E. Ching-Prado, A. Reynes-Figueroa, R.S. Katiyar, Raman spectroscopy and x-ray diffraction of PbTiO3 thin film. J. Appl. Phys. 78, 1920 (1995). https://doi.org/10.1063/1.360229
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2021R1A2C2011350) and by "Research Base Construction Fund Support Program" funded by Jeonbuk National University in 2022.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, B.H., Cho, S.Y., Kim, EY. et al. Synthesis and structural properties of PbTiO3 nanotube arrays using a TiO2 template-assisted hydrothermal method. J. Korean Phys. Soc. 82, 40–45 (2023). https://doi.org/10.1007/s40042-022-00640-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40042-022-00640-6