Abstract
In this study, electrospun polyvinylidene fluoride (PVDF) nanofibers containing filler materials composed of copper oxide and calcium carbonate with different contents were prepared to enhance the electroactive β phase content in the PVDF matrix. The fillers were synthesized using the plasma–liquid electrochemical synthesis method, where copper, a tungsten electrode, and seawater were used as the anode, cathode, and electrolyte, respectively. The atmospheric plasma was generated by applying a high voltage between the electrolyte surface and the tungsten electrode 1 mm above. The electrochemical reactions for particle synthesis were promoted by plasma irradiation, with the charged particles entering the electrolyte. The PVDF solution for the electrospun nanofiber was mixed with the synthesized particles that were a mixture of copper oxide with a dominant fraction of calcium carbonate, as determined using the X-ray diffraction patterns. The structural and the electrical properties of the as-prepared electrospun nanofibers were characterized using microscopy, spectroscopy, and electrical testing. Small loadings of the synthesized particles into in the PVDF matrix induced clear changes in terms of crystallization, morphology, and electrical properties of the prepared materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Miniaturization technology has allowed more widespread use of microelectronics. Particularly, innovative developments in the field of semiconductor manufacturing and architecture technologies have played a key role in reducing component size and power consumption. However, on-board batteries have limited their further development and miniaturization, as they require constant recharging and/or replacing because of the finite amount of energy and limited life, despite the rapid development of battery technology [1]. Thus, developing a power unit with a long lifetime and high long-term stability that does not require periodic maintenance is important. Recently, energy harvesting from ambient waste energy has attracted significant attention due to its potential applications in wireless electronic devices such as portable/wearable devices, environment/structure health monitoring systems, and internet of things (IoT) [2], and is expected to be utilized in self-powered devices, which can be operated in remote places without external electric power [3]. Among the various types of ambient energy sources, mechanical energy sources, such as a rotating engines/structures, flow turbulence, and human motion, have emerged as attractive energy sources due to their restrictive accessibility and abundance. Furthermore, the design of mechanical-to-electrical energy transducers that generate voltage and currents in a useful range is simple compared to electrostatic/electromagnetic methods, because their energy conversion efficiency heavily depends on the electroactive materials [4, 5].
Piezoelectric materials are capable of converting mechanical energy to electrical power and are important for determining energy conversion efficiency and device capability in energy harvesters. To date, ceramic-based piezoelectric materials, including BaTiO3, BaZrO2, PZT, and ZnO [6], have been used as piezoelectric constituents due to their excellent piezoelectric constant and electromechanical coupling factor. Although they exhibit excellent piezoelectric material properties by virtue of their ferroelectric structure, they remain brittle and require well-ordered processing conditions with high manufacturing costs. In contrast, piezoelectric polymers, such as polyvinylidene fluoride (PVDF) and related co-polymers (PVDF-TrFE, PVDF-HFP), can offer a high piezoelectric performance while maintaining high flexibility with device compatibility and cost effectiveness. Thus, polymeric piezoelectric materials are considered to be attractive alternatives to fragile ceramics. However, the desired piezoelectric performance of PVDF is mainly obtained through the β crystalline phase of the PVDF matrix. PVDF is well known to be a semicrystalline polymer with five different polymorphs: α, β, γ, δ, and ε phases. Both the β and the γ phases are crystalline with piezoelectric properties, but the β phase is better than the γ phase due to its TTTT (all-trans) conformation [7]. Many strategies have been adopted, including electrical poling under high voltages, stretching the non-polar α phase, thermal annealing, and loading nano materials, to obtain the polar β crystalline phase. Recently, a number of papers have reported that aligned or randomly orientated nanofibers fabricated via electrospinning produce an electroactive polar β phase due to the electrical field-induced strong elongation flow between the spinneret and the collector [8]. Furthermore, the electrospun nanocomposite nanofibers fabricated from a nanoparticle-incorporated PVDF solution showed distinct advantages over pure homopolymers, including improved thermal and electrical properties [9,10,11,12]. While nanoparticle filler loading is capable of enhancing the content of the polar β phase in the PVDF matrix without additional treatments, such as electrical polling, we should note that only electrospinning or simply loading filler materials results in the coexistence of a non-polar phase with the polar β/γ phase, making the achievement of a pure polar phase difficult.
Various nano materials, including ceramic particles (BaTiO3 [13], BaTi(1 − x)ZrxO3, CaCO3 [14,15,16]), metals and metal salts (copper, cerium(III)/yttrium(III) nitrate hexahydrate, Fe2O3–Co3O4, SiO2 [17], ZrO2 [18]), nanoclay, and carbon nanotubes (SWCNT and MWCNT), have attracted significant interest due to their interaction with the positively charged –CH2 or the negatively charged –CF2 groups of PVDF, resulting in large-scale polar β phase formation [19]. However, the synergetic effect of ceramic particles and metallic oxide particles, such as copper oxide and calcium carbonate has not been fully studied yet, even though some studies on carbon-based hybrid particles have been conducted [20, 21]. In particular, copper oxide has attracted interest due to its capability to enhance β phase formation and its cost effectiveness [22], but conventional synthesis methods, including the hydrothermal [23, 24], electrochemical [25, 26], and solution synthesis [17] methods, require long reaction times, high processing temperatures, and further modification of the copper-oxide nanoparticles. Recently, plasma–liquid electrochemistry technology has received significant attention as it offers simplicity of experimental design, mild reaction conditions, and cost effectiveness. The plasma–liquid interaction, in which charged particles such as electrons and excited radicals interact with the material, differs radically from traditional solution-based reactions [27,28,29].
In this study, copper-oxide nanoparticles were prepared based on calcium carbonate (CuNPs-CaCO3) using the plasma–liquid electrochemical synthesis method. This is a very simple and inexpensive way to prepare a composite of CuNP and CaCO3 and observe their synergistic effect. The prepared CuNPs-CaCO3 samples were incorporated into the PVDF matrix to investigate their effect on the electroactive phase. Detailed analyses of the structural and the electrical properties of the particle-loaded PVDF films were performed, and that the electrically spun PVDF matrix and inorganic filler were demonstrated to increase the β-crystalline phase and enhance the electric responses under applied periodic forces.
2 Materials and methods
2.1 Materials
The PVDF materials used herein were purchased from Sigma Aldrich (St. Louis, MO, USA) and had an average molecular weight (Mw) of 540,000 g/mol. N,N-dimethylacetamide and acetone were used as the main solvents for the preparation of the polymer solutions (Samchun Chemical Co., Yeosu-city, South Korea). The fluorosurfactant (Capstone, FS-66) was purchased from Sigma Aldrich. Copper oxide was synthesized using the liquid–plasma interaction method. The chemicals used for the preparation of copper oxide, i.e., NaOH, NaNO3, and ethylene glycol, were purchased from Samchun Chemical Co.
2.1.1 Preparation of CuNPs-CaCO3 via liquid–plasma electrochemical synthesis
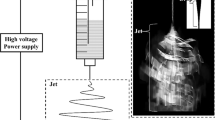
The experimental setup of the liquid–plasma electrochemical synthesis method for fabricating filler particles is shown in Fig. 1a. First, a 100-mL reaction vessel with a polytetrafluoroethylene (PTFE) cap was cleaned, and two gas tubes and two anodic/cathodic electrodes were inserted into the container. The cathode (tungsten, 1-mm outside diameter) was placed 2 cm away from the anode (copper, 1 mm outside diameter) with a space of 1 mm between end of the rod and the surface of liquid. Both electrodes were connected to a bipolar pulsed DC power supply (PEKURIS, Model MPP-HV04) to generate the plasma. A continuous argon flow at a rate of 50 sccm was introduced thorough a gas inlet tube to generate an argon plasma between the liquid and the cathode. The electrolyte solution was prepared by dissolving 1 g/L of NaOH and 1.3 g/L of NaNO3 in 50 mL of a seawater water–ethylene glycol solution. The resulting colloidal samples were washed by centrifugation (3000 rpm for 1 h) in deionized water 3 times and in ethanol 3 times. Afterwards, the samples were dried in a vacuum oven at 70 °C for 10 h. Here, we used acetone to obtain a higher specific surface and intrafiber pores [30, 31]. A change in the ratio of DMF/ACETONE is known to be related to the fiber’s diameter, density and bead size. According to the literature [32, 33], an increase in the acetone fraction causes a decrease in the surface tension and viscosity of the nanofiber. The high volatility and low viscosity of acetone are believed to have an effect on increasing the porosity and the surface roughness of the fiber. For this experiment, various reports in the literature were reviewed so that we could stably produce nanofibers with high porosity. As the volume ratio of acetone was increased, the nanofiber performance was improved due to the specific surface area of the nanofibers. However, when the volume ratio increased to over 6:4, fibers were not stably formed due to the low surface tension.
2.2 Preparation of CuNPs-CaCO 3 /PVDF composites
The CuNPs-CaCO3/PVDF composites were prepared by mixing a solution of CuNPs/acetone with PVDF/DMF, as shown in Fig. 1b. The CuNPs were dispersed in acetone using an ultrasonicator for 1 h. Separately, PVDF was dissolved in a DMF solution under magnetic stirring at 70 °C for 2 h. Afterwards, the PVDF/DMF solution was mixed with the prepared CuNPs/acetone solution (PVDF/DMF and CuNPs-CaCO3/acetone volume ratios = 6:4) by stirring at 70 °C for 3 h. The prepared solutions were electrically spun into nanofibers using an electrospinning apparatus, as depicted in Fig. 1c. The PVDF solution containing CuNPs-CaCO3 was placed in a 10-mL syringe fitted with a 21 G blunt end needle and injected at a volume flow rate of 1.0 mL/h. A 15-kV voltage was applied across a distance of 15 cm between the needle tip and the drum collector rotating at 500 rpm.
2.3 Characterization
For phase confirmation of the prepared nanocomposites and determination of the crystallographic structure, the resulting nanocomposites were examined using X-ray diffraction (XRD; EMPYREAN, PANalytical, The Netherlands) and Fourier transform infrared spectroscopy (FTIR; Frontier, Perkin Elmer, USA). Morphological analyses were performed using field-emission scanning electron microscopy (FE-SEM; S-4800, Hitachi, Japan). The electric characteristics such as the output voltage and the power density were tested to evaluate the potential as an energy harvester, in which 2 cm × 2 cm PVDF nanofibers were sandwiched between two ITO films, as shown in Fig. 1d.
3 Results and discussion
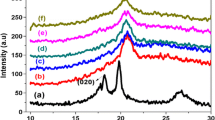
Figure 2 shows the XRD pattern of the copper oxide as-synthesized using the liquid–plasma interaction method and reveals the crystalline phases of the sample. The peaks at 2θ values of 23.1, 29.4, 31.5, 36.0, 39.4, 43.2, 47.2, 47.5, and 57.4° correspond to the (012), (104), (006), (110), (113), (202), (024), (018), and (122) planes of calcium carbonate (CaCO3, calcite; JCPDS#47-1743). The diffraction peaks at 2θ values of 35.3, 38.5, 52.3, 61.2, 66.0, and 67.9° correspond to the (1–11), (111), (020), (1–13), (3–11), (113), and (311) planes of the monoclinic cupric-oxide (CuO) crystalline phase in accordance with JCPDS no. 45-0937. The peaks at 2θ values of 13.7, 16.8, 21.3, 27.6, and 32.3° were assigned to the (110), (111), (220), and (311) planes of the cubic cuprous-oxide crystalline phase (Cu2O; JCPDS no. 05-0667). The XRD analysis indicates that the as-synthesized nanoparticles contained mixed phases of CuO and Cu2O (CuNPs) with a dominant fraction of the CaCO3 phase.
Figure 3 shows SEM images of the electrospun PVDF nanofibers with different contents of CuNPs-CaCO3 additives. As shown in Fig. 3a, well-aligned nanofibers without any fine branch fibers were formed with a mean diameter of 2.33 µm. With increasing CuNPs-CaCO3 content from 0.01 to 0.03 wt%, the mean nanofiber diameter decreased from 1.18 to 0.72 µm. For a polymer with a filler loading over 0.03%, fibers were not formed due to the low surface energy and tensile strength [34]. The fibers broke while landing on the drum collector, resulting in samples in the form of a mat rather than a fiber. The reduced diameter was attributed to the fine branch fibers that can be observed in Fig. 3b–d as a result of uneven elongation that did not extend over the entire fibers.
The crystallinity and the fraction of electroactive polarized β and γ-phases in the CuNPs-CaCO3/PVDF composites were calculated from the corresponding deconvoluted XRD patterns, as shown in Fig. 4. With increasing CuNPs-CaCO3 content, the area of the diffraction peak at 2θ ≈ 20.1° corresponding to the γ-phase decreased, and the intensity of the β crystal peak at 2θ ≈ 20.7° corresponding to the (200/110) plane was enhanced [35, 36]. The calculated β-crystallinity (χc(β)) indicated that addition of filler materials slightly increased χc(β) from 70% in PVDF to 85% in the CuNPs-CaCO3/PVDF nanocomposite. This suggested that the incorporation of filler particles may further enhance the formation of the β-phase. As shown in Fig. 4a, the application of high voltages in the electrospinning process aligned the electric dipoles in the PVDF solution in proportion to the magnitude of the applied electric field. The subsequent increase observed in Fig. 4b–d was attributed to the enhanced nucleation of the β crystalline phase caused by the increased filler density or the capability of CuNPs-CaCO3 particles to reduce the mobility of the polymer chain during crystallization, resulting in the formation of more extended β crystal chains [37]. Thus, the electrospinning process incorporating the fillers was effectively utilized to form β-phase crystals and achieve improved piezoelectric properties in the prepared nanofiber mats.
To further clarify the formation of crystalline phases in the CuNPs-CaCO3/PVDF nanofibers, we performed FT-IR spectral analysis, and the results are as shown in Fig. 5a. The FTIR spectrum of the nanofibers showed vibration peaks at 764 cm−1 (CF2 bending and skeletal bending) and 840 cm−1 (CH2 rocking), which are typical of the α and the β crystalline phase, respectively [38, 39]. To determine the fraction of the β crystalline phase in each sample, we evaluated the absorption peaks of the α and the β-phases [40]. The fraction of the β crystalline phase was calculated as follows [41]:
where Aα and Aβ are the absorbances at 764 and 840 cm−1, respectively; Kα and Kβ are the absorption coefficients at the respective wavenumbers, with values of 6.1 × 104 and 7.7 × 104 cm2 mol−1, respectively; and Xα and Xβ indicate the respective crystallization rates of the α- and the β-phases. The results of the calculations based on the above formula are presented in Fig. 5b. Electrospun PVDF without filler loading was 57% β-phase and increased with filler addition. The enhanced β-phase fraction reached a maximum (68%) at a filler loading of 0.03 wt%, indicating that the nanoparticle surfaces form the β-phase via preferential nucleation of the chains.
Periodic impact force experiments were performed using different force conditions to investigate the electric responses, such as characteristic voltage, generated by external stimulations (Fig. 6). Compressive impacts were applied to the sample using a vibrator (Modal shop, 2007E), as shown in the inset of Fig. 6, and the applied forces were measured using a force sensor (PCB piezotronics, 208C01) attached to the end of the rod connected to the vibrator. With increasing applied force, the output voltage of the samples with filler loading increased to approximately 5 V at 15 N, whereas the sample without filler showed lower output voltages. These results indicate that the synthesized CuNPs-CaCO3 fillers in the PVDF fibers work well and sensitize the PVDF harvester to external stimulation more than the unmodified matrix.
To convert the harvested alternative output voltage obtained from the periodic impact force shown in Fig. 6 to a DC voltage, we used a full bridge rectifier, as shown in the inset of Fig. 7. The power was obtained using the RMS value of the AC voltage in Fig. 6 and the resistance applied at that time, and the power density was obtained by dividing the obtained power by the volume of the polymer to which the force was applied. The output power density increased with increasing load resistance before reaching the optimized output power density of 35 µW/cm3 at a matched resistance of 10 MΩ.
4 Conclusion
In summary, electrospun nanofibers were prepared for use in an energy harvester composed of CuNPs-CaCO3 to improve the fraction of the electroactive phase in a PVDF matrix. The effects of the CuNPs-CaCO3 nanoparticles synthesized using the plasma–liquid electrochemical method were evaluated. The FTIR and the XRD results showed that small amounts of CuNPs-CaCO3 loading in the PVDF matrix could induce significant changes in the crystallization, morphology, and electrical properties. Outstanding piezoelectric properties were obtained with an open circuit voltage (Voc) as high as 4.5 V under a compressive force of 15 N. The maximum output power reached 35 µWcm3 with an external resistance of 10 MΩ. This study describes promising particles synthesized using a plasma–liquid interaction for the development of next-generation renewable energy harvesters that can create usable electricity from mechanical energy.
References
Z.L. Wang, Adv. Mater. 24, 280 (2012)
W. Seung, M.K. Gupta, K.Y. Lee, K.S. Shin, J.H. Lee, T.Y. Kim, S. Kim, J. Lin, J.H. Kim, S.W. Kim, ACS Nano 9, 3501 (2015)
S. Chandrasekaran, C. Bowen, J. Roscow, Y. Zhang, D.K. Dang, E.J. Kim, R.D.K. Misra, L. Deng, J.S. Chung, S.H. Hur, Phys. Rep. 792, 1 (2019)
K.J. Kim, F. Cottone, S. Goyal, J. Punch, Bell Labs Tech. J. 15, 7 (2010)
S. Meninger, J.O. Mur-Miranda, R. Amirtharajah, A. Chandrakasan, J.H. Lang, IEEE Trans. Very Large Scale Integr. VLSI Syst. 9, 64 (2001)
Z.L. Wang, J. Song, Science 312, 242 (2006)
P. Thakur, A. Kool, B. Bagchi, N.A. Hoque, S. Das, P. Nandy, RSC Adv 5, 62819 (2015)
E. Zdraveva, J. Fang, B. Mijovic, T. Lin, “Electrospun Nanofibers”, Structure and Properties of High-Performance Fibers (Woodhead Publishing, Duxford, 2017), p. 267
Y.L. Liu, Y. Li, J.T. Xu, Z.Q. Fan, A.C.S. Appl, Mater. Interfaces 2, 1759 (2010)
J.S. Andrew, D.R. Clarke, Langmuir 24, 8435 (2008)
S. Huang, W.A. Yee, W.C. Tjiu, Y. Liu, M. Kotaki, Y.C.F. Boey, J. Ma, T. Liu, X. Lu, Langmuir 24, 13621 (2008)
L. Yu, P. Cebe, Polymer 50, 2133 (2009)
Z.M. Dang, D. Xie, C.Y. Shi, Appl. Phys. Lett 91, 222902 (2007)
N. Jahan, F. Mighri, D. Rodrigue, A. Ajji, J. Appl. Polym. Sci. 134, 44940 (2017)
J.S.D. Campos, A.A. Ribeiro, C.X. Cardoso, Mater. Sci. Eng. B 136, 123 (2007)
W. Ma, X. Wang, J. Zhang, J. Polym. Sci. B Polym. Phys. 48, 2154 (2010)
E. Kar, N. Bose, S. Das, Phys. Chem. Chem. Phys. 17, 22784 (2015)
J. Li, P. Khanchaitit, K. Han, Q. Wang, Chem. Mater. 22, 5350 (2010)
Z. Cui, N.T. Hassankiadeh, Y. Zhuang, E. Drioli, Y.M. Lee, Prog. Polym. Sci. 51, 94 (2015)
A. Samadi, S.M. Hosseini, R. Ahmadi, Org. Electron. 59, 149 (2018)
A. Samadi, R. Ahmadi, S.M. Hosseini, Org. Electron. 75, 105405 (2019)
B. Dutta, E. Kar, N. Bose, S. Mukherjee, RSC Adv. 5, 105422 (2015)
W. Jia, E. Reitz, P. Shimpi, E.G. Rodriguez, P.X. Gao, Y. Lei, Mater. Res. Bull. 44, 1681 (2009)
J.G. Zhao, S.J. Liu, S.H. Yang, S.G. Yang, Appl. Surf. Sci. 257, 9678 (2011)
G. Yuan, J. Zhu, F. Xie, X. Chang, J. Nanosci. Nanotechnol. 10, 5258 (2010)
S.O. Kang, S. Hong, J. Choi, J.S. Kim, I. Hwang, I.S. Byun, K.S. Yun, B.H. Park, Appl. Phys. Lett. 95, 092108 (2009)
D. Mariotti, R.M. Sankaran, J. Phys. D Appl. Phys. 43, 323001 (2010)
L. Lin, S.A. Starostin, S. Li, V. Hessel, Phys. Sci. Rev. 3, 1 (2018)
C. Du, M. Xiao, Sci. Rep. 4, 7339 (2014)
P. Lu, Y. Xia, Langmuir 29, 7070 (2013)
D. Chen, T. Sharma, J.X.J. Zhang, Sens. Actuators A 216, 196 (2014)
S.W. Won, S.M. Jo, W.S. Lee, Y.R. Kim, Adv. Mater. 15, 2027 (2003)
Z. Zhao, J. Li, X. Yuan, X. Li, Y. Zhang, J. Sheng, J. Appl. Polym. Sci. 97, 466 (2005)
X. Li, X. Lu, J. Appl. Polym. 101(5), 2944 (2006)
M. Kanik, O. Aktas, H.S. Sen, E. Durgun, M. Bayindir, ACS Nano 8, 9311 (2014)
S. Jana, S. Garain, S. Sen, D. Mandal, Phys. Chem. Chem. Phys. 17, 17429 (2015)
J. Buckley, P. Cebe, D. Cherdack, J. Crawford, B.S. Ince, M. Jenkins, J. Pan, M. Reveley, N. Washington, N. Wolchover, Polymer 47, 2411 (2006)
R. Gregorio, M. Cestari, J. Polym. Sci. B 32, 859 (1994)
F. Khatun, N.A. Hoque, P. Thakur, N. Sepay, S. Roy, B. Bagchi, A. Kool, S. Das, Energy Technol. 5, 2205 (2017)
X. Cai, T. Lei, D. Sun, L. Lin, RSC Adv. 7, 15382 (2017)
P. Martins, A.C. Lopes, Prog. Polym. Sci. 39, 683 (2014)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y., Yang, J. Enhancement of the electroactive β phase in electrospun PVDF fibers by incorporation of CaCO3-based Cu hybrid particles prepared using plasma–liquid electrochemical synthesis. J. Korean Phys. Soc. 78, 27–33 (2021). https://doi.org/10.1007/s40042-020-00025-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40042-020-00025-7