Abstract
The present study investigated the effects of heat treatment by low-temperature annealing and temperature change on the electrochemical properties of aluminium (Al) alloy A6061-T6 in 0.5 M sulphuric acid (H2SO4) solution. Subsequent to heat treatment at five different temperatures of 250 °C, 300 °C, 350 °C, 400 °C, and 500 °C (constant time of 1 h.), the heat-treated Al alloy A6061-T6 samples were subjected to corrosion tests via potentiodynamic polarization techniques at room temperature. Optical microscopy was used for the characterization of the corroded areas for both the control and heat-treated samples. Compared to the control Al alloy A6061-T6 sample with a higher corrosion current density of 391.38 µA/cm2, the Al alloy A6061-T6 samples annealed at 250 °C, 300 °C, 350 °C, 400 °C, and 450 °C possessed a lower corrosion current density of 390.62 µA/cm2, 191.66 µA/cm2, 113.89 µA/cm2, 64.23 µA/cm2, and 60.99 µA/cm2, respectively. The annealed samples are characterized by lower corrosion density as well as the presence of little or no corrosion products and pits. Heat treatment by low-temperature annealing improves the electrochemical properties of Al alloy A6061-T6, and the corrosion resistance increases with increasing annealing temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminium (Al) alloys mostly find applications in aerospace, automotive, and building industries [1, 2] due to their exceptional properties including high mechanical strength, light weight, low cost, high ductility, low density [3, 4], and formability [5], and moderate corrosion resistance. As a form of aluminium alloy, Al 6061 T6 is a promising material used in transportation, aerospace, and other industries due to its interesting properties such as good weldability and hardenability [6,7,8].
Generally, the Al alloys are readily oxidized to form a protective thin and fragile oxide layer when exposed to the atmosphere. However, their continuous exposure to atmosphere especially in aggressive environments results in corrosion which causes a serious setback to their mechanical properties. In addition, several factors could influence the corrosion resistance behaviour of Al 6061 T6 alloy including the presence of stray electric current, temperature of the solution, presence of other ions in solution, differences in electrical potentials in solutions, and instability of metals in their refined form [2, 6, 7]. The corrosion resistance behaviour of Al 6061 T6 alloy can be further enhanced in aggressive environments via surface engineering technologies including surface nanocrystallization [9,10,11,12,13,14,15,16], coatings/electrodepositions [17,18,19], machining/moulding [20], heat treatments [21,22,23,24], etc. It is important to know that heat treatment is presently gaining more recognition as an important technique for improving the mechanical properties as well as enhancing the corrosion resistance property.
The influence of heat treatments on the mechanical properties and microstructure of Al 6061 T6 alloy has been investigated in the past [7, 8, 25,26,27,28,29]. As stated by Li et al. [7], heat treatment reportedly improves the mechanical properties of Al 6061 alloy as a result of the precipitation hardening mechanism. A similar enhancement in properties of Al 6061 was reported by Maisonnette et al. [8] which was mainly attributed to the weldability and inherent hardenability nature of the sample. In another investigation, the corrosion behaviour of Al 6061 alloy under heat treatment at different temperatures and oxidation states was examined by Wang et al. [29]. The corrosion rate of Al 6061 alloy showed an increasing trend with the increase of temperature. Samsu et al. [30] presented a detailed study on the corrosion behaviour of Al 6061 T6 immersed in reactor pool water containing about 0.1 NaCl content. It was reported that the corrosion rate value of the Al 6061 T6 increased with the presence of 0.1% ion chloride content in the demineralized water reactor pool as compared to normal demineralized water. As described by Zeid [31], Al alloy 6061 can also be used in corrosive media after artificial aging at the optimum temperature of 175 °C for 30 min.
However, the influence of heat treatment via low-temperature annealing on the corrosion resistance behaviour of Al alloy A6061-T6 has not been fully studied in 0.5 molar H2SO4 solution. In addition, the electrochemical properties of heat-treated Al alloy A6061-T6 over five different temperatures with the range 250–450 °C have not been investigated in-depth, hence the motivation behind this study.
In the present study, the electrochemical properties of heat-treated Al alloy A6061-T6 in 0.5 M H2SO4 solution were determined through low-temperature annealing process, potentiodynamic polarization techniques, and optical microscopy. Most importantly, the influence on temperature change during annealing treatment on the corrosion resistance behaviour of Al alloy A6061-T6 was investigated.
Experimental
The commercial Al alloy A6061-T6 sample with chemical compositions in Table 1 was used in the present work. The samples were first cut into sizes suitable for different operations required. The samples with the dimension, 100 × 100 × 5 mm3 were then subjected to heat treatment by low-temperature annealing at five different temperatures: 250 °C, 300 °C, 350 °C, 400 °C, and 500 °C using a Detachable Muffle Furnace with a constant time of 1 h.
The electrochemical properties of the unannealed (control) and annealed Al alloy A6061-T6 samples (20 × 20 × 5 mm3) was carried out using Princeton Applied Research Electrochemical workstation (model-VersaSTAT 4) at room temperature. The test was carried out at a scan rate of 1 mV/s, scanning potential range of − 250 to + 250 mV, in 0.5 molar sulphuric acid (H2SO4) solution. The corrosion test was performed in a tradition three-electrode system using Ag/AgCl as the refence electrode, platinum rod as the counter electrode and the sample as the working electrode, with sample exposure diameter of 10 mm.
The corrosion potential and current densities values were obtained from the corrosion graph via Tafel extrapolation, and the corrosion rates were obtained from the software embedded in the electrochemical system. All experiments were repeated for at least three times to ensure reproducibility and accuracy of results.
After slight polishing and etching using Keller’s reagent [27], the optical microstructure of the unannealed (control) and annealed Al alloy A6061-T6 samples (15 mm × 15 mm) before and after corrosion was examined on Omax 40X–2500X Optical Metallurgical Microscope at room temperature, and micrographs taken via bright-field illumination technique. The samples were cleaned with acetones and adequately polished for effective microstructure.
Results
Figure 1 shows the images of the control Al alloy A6061-T6 sample and those subjected to heat treatment by annealing at temperatures of 250 °C, 300 °C, 350 °C, 400 °C, and 450 °C in 0.5 molar sulphuric acid (H2SO4) solution. Compared to the control Al alloy A6061-T6 sample (Fig. 1a), the samples annealed for 250 °C (Fig. 1b), 300 °C (Fig. 1c), 350 °C (Fig. 1d), 400 °C (Fig. 1e), and 450 °C (Fig. 1f) exhibited shiny dark surfaces, indicating the extent of heat treatment. The surfaces get more shiny and darker with increasing temperature.
The electrochemical properties of as-received Al alloy A6061-T6 (control) and those subjected to annealing treatment at temperatures of 250 °C, 300 °C, 350 °C, 400 °C, and 450 °C in 0.5 molar sulphuric acid (H2SO4) solution were shown in Fig. 2. The open circuit potentials (Fig. 2a) of samples immersed for 600 s at room temperature revealed that it took around 300 s for the control Al alloy A6061-T6 sample to reach stability in the solution. The A6061-T6 sample annealed at 350 °C got stabilized at around 250 s, whereas it only took around 100 s for the Al alloy A6061-T6 sample annealed at 450 °C to be stable in the sulphuric solution. It is generally believed the faster the rate at which a sample reach a stability in a solution, the more the reduction in electrochemical activities hence reduced rate of corrosion. As observed in Fig. 2a, the continuous step like nature observed in the present work may be due to some side reaction during the open circuit potential measurement.
The electrochemical properties of as-received Al alloy A6061-T6 (control) and those subjected to annealing treatment at temperatures of 250 °C, 300 °C, 350 °C, 400 °C, and 450 °C in 0.5 molar sulphuric acid (H2SO4) solution; a open circuit potentials of samples immersed for 600 s at room temperature, and b potentiodynamic polarization curves of the unannealed and annealed samples from − 250 to + 250 mV
The corrosion current density and potential are the two main parameters for determining the corrosion resistance behaviour of a material. As shown in Fig. 2b and summarized in Table 2, the potentiodynamic polarization curves revealed that the control A6061-T6 sample exhibited a corrosion current density and potential of 391.38 µA/cm2 and − 707.17 V, respectively (Table 2). The Al alloy A6061-T6 sample annealed at 250 °C possessed a lower corrosion current density of 390.62 µA/cm2, as compared to the control sample. In addition, annealed samples at higher temperatures experienced a drastic reduction in corrosion current densities (Table 2). For instance, the Al alloy A6061-T6 sample annealed at 300 °C, 350 °C, 400 °C and 450 °C possessed corrosion current densities of 191.66 µA/cm2, 113.89 µA/cm2, 64.23 µA/cm2, and 60.99 µA/cm2, respectively (Fig. 2b). From the potentiodynamic polarizations, it can be deduced that the corrosion current densities of the annealed Al alloy A6061-T6 samples reduced as compared to that of the control counterpart. Similarly, the corrosion current density reduces with increasing annealing temperatures. Attributed to the artificial age hardening, a similar improvement in electrochemical properties of AA 6061 alloy after heat treatment at 175 °C [31] was achieved.
In contrary, the corrosion rate of Al 6061 alloy showed an increasing trend with the increase of temperature [29]. It is generally believed that a reduction in the corrosion current density signifies lesser electrochemical activities, more tendency for passivation, and hence an improved corrosion resistance behaviour [32,33,34].
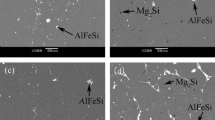
Figure 3 shows the optical microstructure properties of as-received Al alloy A6061-T6 (control) and those subjected to annealing treatment at temperatures of 250 °C, 300 °C, 350 °C, 400 °C, and 450 °C in 0.5 molar sulphuric acid (H2SO4) solution. More corrosion products and pits were observed on the control Al alloy A6061-T6 sample (Fig. 3a) indicating more corrosion attacks and susceptibility of the sample in the presence of sulphuric acid. A similar formation of pits was observed on the sample subjected to heat treatment by annealing at 250 °C (Fig. 3b) and 300 °C (Fig. 3c), but the pits and corrosion products reduced with increasing annealing temperature.
This was evident on the annealed Al alloy A6061-T6 at 350 °C (Fig. 3d), 400 °C (Fig. 3e) with less corrosion products. As evident in Fig. 3f, the corrosion products and pits formation reduced drastically after heat treatment at 450 °C as compared to the control sample which exhibited more corrosion products and pits.
Discussion
From the potentiodynamic polarization curves, the current density reduces with an increasing annealing temperature signifying an increase in the corrosion resistance behaviour. i.e., the corrosion resistance increases with increasing annealing temperatures. The heat treatment by low-temperature annealing reduces the formation of pits and corrosion attacks for Al alloy A6061-T6 sample, and the corrosion resistance behaviour improves with increasing annealing temperature. The reduction in pits and corrosion attacks may be attributed to the relaxation of the sample surface which possibly reduces the surface roughness and the electrochemical activity on the sample [28], hence reducing the rate of corrosion on the sample.
In the present study, the physical appearance of the samples before and after low-temperature annealing treatment at different temperatures depicts the extent of treatment. As it is, the colour change is the main factor to differentiate the samples after annealing treatment. As observed in Fig. 1, the sample colour gets darker with increasing annealing temperature. Increasing the annealing temperature is directly proportional to the treatment time. The higher the annealing temperature, the longer the time the sample is treated. Hence, the effect of the annealing treatment is more felt on the sample treated at higher temperature with darker colour change as the main indicator. There is a direct relationship between the annealing process and microstructures of Al alloys. Annealing could cause a change in the microstructure of Al alloy due to possible rearrangement in microstructural constituents and reduction in residual internal stress.
The results obtained in the present study is quite different with that of Wang et al. [29]. Investigating the influence of temperature treatment on the corrosion resistance behaviour of 6061 Al alloy in sulphuric acid [29], the corrosion resistance reduces with increasing treatment temperature after oxidation. However, subjecting the 6061 Al alloy to low-temperature annealing in the present study reduces the corrosion current density which means an improved corrosion resistance. An enhancement in the corrosion resistance of AA 6061 was also achieved after heat treatment [31]. In sulphuric acid solution, the improvement in the corrosion resistance behaviour experienced in the present study is similar to that of AA 7075 Al alloy [2] after heat treatment. The corrosion resistance behaviour of aluminium alloy [6] was also improved after heat treatment in NaOH and HCL acid. The interesting fact here is that heat treatment by low-temperature annealing or other treatments such as anodization is capable of improving the corrosion resistance behaviour of 6061 aluminium alloy. The reverse is the case for heat-treated 1060 Al alloy [3] where a reduction in corrosion resistance in dilute solutions was observed after annealing. A possible rearrangement in microstructural constituent which incapacitated the protective passive film to combat the corrosion reaction could be a reason behind the reduction in corrosion resistance as observed for 1060 Al alloy.
Conclusion
In the present study, the influence of heat treatment by low-temperature annealing as well as the annealing temperature change on the electrochemical properties of Al alloy A6061-T6 was investigated. Compared to the control Al alloy A6061-T6 sample with the corrosion current density of 391.38 µA/cm2, the Al alloy A6061-T6 sample annealed at 250 °C and 300 °C possessed a lower corrosion current density of 390.62 µA/cm2 and 191.66 µA/cm2, respectively. A further reduction in corrosion current density was observed for the Al alloy A6061-T6 sample annealed at 350 °C, 400 °C, and 450 °C with current density values of 113.89 µA/cm2, 64.23 µA/cm2, and 60.99 µA/cm2, respectively.
The optical micrographs revealed the reduction in pits and corrosion attacks on the Al alloy A6061-T6 sample subjected to low-temperature treatment, as compared to the control sample which exhibited more corrosion products and pits, indicating more corrosion attack and low corrosion resistance. Heat treatment by low-temperature annealing enhances the corrosion resistance behaviour of Al alloy A6061-T6, and the corrosion resistance increases with increasing annealing temperatures. This may be attributed to the reduction in electrochemical activity on the sample, hence reducing the rate of corrosion rate.
References
N.C.T. Martins, T. Moura e Silva, M.F. Montemor, J.C.S. Fernandes, M.G.S. Ferreira, Electrodeposition and characterization of polypyrrole films on aluminium alloy 6061-T6. Electrochim. Acta 53, 4754–4763 (2008)
M. Abdullahi, L.S. Kuburib, P.T. Zubairua, U. Jaboc, A.A. Yahayad, Y. James, Effect of heat treatment and sulfuric acid anodization on corrosion resistance of aluminum alloy (AA7075). Niger. J. Technol. 40(1), 56–62 (2021)
R.T. Loto, E.A. Igbogbo, Corrosion behaviour of heat treated 1060 aluminium in dilute acid solutions. Rev. Téc. Ing. Univ. Zulia. 39, 35–40 (2016)
S. Tsai, W.C. Jean, P. Chao, S. Fu, S. Tsai, Whole pH range anti-corrosion property of aluminium alloy coated with MFI zeolite film. Corros. Eng. Sci. Technol. 53, 34–38 (2018)
D. Loganathan, A. Gnanavelbabu, Formability analysis of AA6061 aluminium alloy at room temperature. Appl. Mech. Mater. 591, 55–59 (2014)
P. Madakson, I. Malik, S. Laminu, I. Bashir, Effect of anodization on the corrosion behavior of aluminium alloy in HCl acid and NaOH. Int. J. Mater. Eng. 2, 38–42 (2012)
L. Li, E.A. Flores-Johnson, L. Shen, G. Proust, Effects of heat treatment and strain rate on the microstructure and mechanical properties of 6061 Al alloy. Int. J. Damage Mech 25, 26–41 (2015)
D. Maisonnette, M. Suery, D. Nelias, P. Chaudet, T. Epicier, Effects of heat treatments on the microstructure and mechanical properties of a 6061 aluminium alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 528, 2718–2724 (2011)
T.O. Olugbade, J. Lu, Enhanced corrosion properties of nanostructured 316 stainless steel in 0.6 M NaCl solution. J Bio Tribo Corros 5, 38 (2019)
T.O. Olugbade, J. Lu, Literature review on the mechanical properties of materials after surface mechanical attrition treatment (SMAT). Nano Mater. Sci. 2, 3–31 (2020)
T. Olugbade, C. Liu, J. Lu, Enhanced passivation layer by Cr diffusion of 301 stainless steel facilitated by SMAT. Adv. Eng. Mater. 21, 1900125 (2019)
T.O. Olugbade, J. Lu, Characterization of the corrosion of nanostructured 17-4 PH stainless steel by surface mechanical attrition treatment (SMAT). Anal. Lett. 52, 2454–2471 (2019)
T. Olugbade, Datasets on the corrosion behaviour of nanostructured AISI 316 stainless steel treated by SMAT. Data-in-brief 25, 104033 (2019)
T.O. Olugbade, Electrochemical characterization of the corrosion of mild steel in saline following mechanical deformation. Anal. Lett. 54, 1055–1067 (2021)
T. Olugbade, J. Lu, Effects of materials modification on the mechanical and corrosion properties of AISI 316 stainless steel, in Twelfth international conference on fatigue damage of structural materials, (Cape Cod, Hyannis, USA, 2018)
T. Olugbade, J. Lu, Improving the passivity and corrosion behaviour of mechanically surface-treated 301 stainless steel, in International Conference on Nanostructured Materials (NANO 2020), (Australia, 2020), p. 117
C. Dang, Y. Yao, T.O. Olugbade, J. Li, L. Wang, Effect of multi-interfacial structure on fracture resistance of composite TiSiN/Ag/TiSiN multilayer coating. Thin Solid Films 653, 107–112 (2018)
T.O. Olugbade, T.E. Abioye, P.K. Farayibi, N.G. Olaiya, B.O. Omiyale, T.I. Ogedengbe, Electrochemical properties of MgZnCa-based thin film metallic glasses fabricated via magnetron sputtering deposition coated on a stainless steel substrate. Anal. Lett. 54, 1588–1602 (2021)
C. Dang, T.O. Olugbade, S. Fan, H. Zhang, L.L. Gao, J. Li, Y. Lu, Direct quantification of mechanical responses of TiSiN/Ag multilayer coatings through uniaxial compression of micropillars. Vacuum 156, 310–316 (2018)
H. Zu, K. Chau, T.O. Olugbade, L. Pan, D.H. Chow, L. Huang, L. Zheng, W. Tong, X. Li, Z. Chen, X. He, R. Zhang, J. Mi, Y. Li, B. Dai, J. Wang, J. Xu, K. Liu, J. Lu, L. Qin, Comparison of modified injection molding and conventional machining in biodegradable behavior of perforated cannulated magnesium hip stents. J. Mater. Sci. Technol. 63, 145–160 (2021)
T.E. Abioye, I.S. Omotehinse, I.O. Oladele, T.O. Olugbade, T.I. Ogedengbe, Effects of post-weld heat treatments on the microstructure, mechanical and corrosion properties of gas metal arc welded 304 stainless steel. World J. Eng. 17, 87–96 (2020)
T.E. Abioye, T.O. Olugbade, T.I. Ogedengbe, Welding of dissimilar metals using gas metal arc and laser welding techniques: a review. J. Emerg. Trends Eng. Appl. Sci. 8, 225–228 (2017)
T. Mohammed, T.O. Olugbade, I. Nwankwo, Determination of the effect of oil exploration on galvanized steel in Niger Delta, Nigeria. J. Sci. Res. Rep. 10, 1–9 (2016)
T.O. Olugbade, Stress corrosion cracking and precipitation strengthening mechanism in TWIP steels: progress and prospects. Corros. Rev. 38, 473–488 (2020)
A.G. Odeshi, G.M. Owolabi, M.N.K. Singh, M.N. Bassim, Deformation and fracture behavior of alumina particle-reinforced Al 6061-T6 composite during dynamic mechanical loading. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 38A, 2674–2680 (2007)
F. Ozturk, A. Sisman, S. Toros, S. Kilic, R.C. Picu, Influence of aging treatment on mechanical properties of 6061 aluminum alloy. Mater. Des. 31, 972–975 (2010)
E.B. Hilty, C.C. Menzemer, K. Manigandan, T.S. Srivatsan, Influence of welding and heat treatment on microstructure, properties and fracture behaviour of a wrought aluminium alloy. Emerg. Mater. Res. 3, 230–242 (2014)
Z. Zhou, X. Qi, S. Zhenxi, X. Jing, Z. Zhengfeng, C. Shuying, K.L. Peter, W.S. Yan, Effects of annealing on the microstructure, corrosion resistance, and mechanical properties of RE65Co25Al10 (RE = Ce, La, Pr, Sm, and Gd) bulk metallic glasses. Mater. Sci. Eng. A 626, 467–473 (2015)
W. Zhisheng, F. Runhua, C. Kun, H.L. Qing, Y. Yansheng, Effect of temperature treatment on microstructure and electrochemical properties of 6061 aluminum alloy. Int. J. Electrochem. Sci. 16, 1–8 (2021)
S. Zaifol, D. Muhamad, R.M. Siti, R.R. MohdSa’ariRipin, S.S. Mohd, Electrochemical corrosion characteristic of aluminium alloy 6061 T6 in the demineralized water containing 0.1% chloride ion. J. Nucl. Relat. Technol. 10, 1 (2013)
E.F.A. Zeid, Mechanical and electrochemical characteristics of solutionized AA 6061, AA6013 and AA 5086 aluminum alloys. J. Mater. Res. Technol. 8, 1870–1877 (2019)
T.O. Olugbade, E.O. Olutomilola, B.J. Olorunfemi, Review - passivity and electrochemical properties of nanostructured stainless steels: trend and progress. Corros. Rev. (2021)
T.O. Olugbade, B.O. Omiyale, O.T. Ojo, Corrosion, corrosion fatigue and protection of magnesium alloys: mechanisms, measurements, and mitigation. J. Mater. Eng. Perform. (2021)
T.O. Olugbade, O.T. Ojo, B.O. Omiyale, E.O. Olutomilola, B.J. Olorunfemi, A review on the corrosion fatigue strength of surface-modified stainless steels. J. Braz. Soc. Mech. Sci. Eng. 43, 421 (2021)
Funding
The present work was carried out without financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olugbade, T.O., Omoniyi, O.O. & Omiyale, B.O. Electrochemical Properties of Heat-Treated Al Alloy A6061-T6 in 0.5 M H2SO4 Solution. J. Inst. Eng. India Ser. D 103, 141–147 (2022). https://doi.org/10.1007/s40033-021-00313-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40033-021-00313-x