Abstract
Biodiesel is an alternative, clean and green fuel because of its non-toxic, biodegradable properties. The rationale behind this research is to examine the efficiency of biodiesel extracted from Glycine max oil by means of the heterogeneous catalyst method. The biodiesel was blended with mineral diesel at different percentages (%), before being applied to test the engine performance as a source of fuel. Variations of carbon monoxide, brake thermal efficiency, hydrocarbon, exhaust gas temperature, brake specific fuel consumption, NOX and brake thermal efficiency versus load for fuels were performed. At lower load BSFC and BTE of biodiesel are almost similar to diesel. The emission of CO and HC reduce appreciably as a result of biodiesel. It was obtained that at full load condition, there was a 2.38%, 4.76%, 7.61% and 10% reduction in CO emission, with B5, B10, B15 and B20, respectively. The characterization was also done by using spectroscopy like UV–Vis and Fourier transform infrared. The characteristic of diesel and fatty acid profile was observed at 1745/cm, showing the existence of strong ester carbonyl bond (C=O) indicating the presence of fatty acid methyl ester or biodiesel in FTIR. The variable concentration of blending was established by UV–Vis absorption spectra. The linearity of absorbance declined and linear line fitted for absorbance versus the percentage of biodiesel at 320 nm showed a high coefficient of determination (R2 = 0.9651).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing worldwide demand in energy production led to the reduction in use of fossil fuels and sustainable harness of biodiesel. Biodiesel is fatty acid esters, which may be obtained either from animal fat or vegetable.
During storage, biodiesel undergoes oxidative degradation due to the presence of reactive allylic and bisallylic sites. Oxidation stability is fuels reaction with oxygen at ambient temperature [1]. The degradation would lead to the development of unwanted products, which may affect the properties of biodiesel and also the performance of the engine [2]. The parameters like acid value and viscosity remain unchanged. The major change occur during storage is the peroxide value, measuring hydroperoxides, which decomposes to ketones, acids, etc. [3]. Saifuddin and Refal indicated that higher proportionate of polyunsaturated organic fuels is more reactive in terms of oxidation than monounsaturated, which are unstable [4]. Biodiesel produced from a broad range of feedstock varies in their characteristics. The production of biodiesel is also determined by thin layer chromatography [5]. The different methods like infrared spectroscopy, nuclear magnetic resonance (NMR) and chromatography are used to determine the content of biodiesel [6]. NMR is no doubt outstanding method, but it is costly in indicating biodiesel content with high coefficients of correlations [7]. FTIR technique is employed for examining oxidative degradation of biodiesel, which has their characteristic absorption in the region of 1745 per cm attributable to the presence of ester carbonyl group.
The attendance of conjugates in the oxidized biodiesel may be determined by UV–Vis spectroscopy. Vega-Lizama et al. [8] indicated that at a specific wavelength, a positive linear correlation existed between the peroxide value and the UV absorbance.
UV–Vis spectroscopy determines the content of heptanes-diluted biodiesel and diesel-blended concentration intervals. Zawadzki et al. [9] indicated two intensive absorption bands along 230–260 nm wavelengths. Nevertheless, UV–Vis spectroscopy provides faster results because of less time in analysis and also less reagent consumption.
Biodiesel has an advantage of lower emission rate and clean fuel [10, 11]. The main issue with biodiesel is it contains a lower energy rate and higher emission of NOx as compared to diesel. To overcome this issue various additives are added in biodiesel [12, 13]. The use of vegetable oil directly in the engine is prohibited as it leads to improper combustion, improper atomization, carbon deposition and injector chocking. This issue occurred, because the oil contained higher viscosity [14]. However, many advantages are there in using biodiesel such as it can be easily linked with the application of diesel engine with very low modification. It is also eco-friendly in nature and contains lower harmful substances. Biodiesel is also feasible at various blending rates with diesel [15]. Many researchers have found that the emission rates of HC, CO, smoke and CO2 were reduced up to maximum limit by the use of biodiesel. However, the emission rate of NOX increases with the increase in the percentage usage of biodiesel [16, 17]. The main reason behind the enhancement of the rate of NOX emissions is relative stoichiometry, aromatic content, heating value and biodiesel composition. The rate of production of NOx increases with the use of methyl soyate in the blend, and it varies from 3–19% to 10–100% blend, respectively. At full load condition (B100), the rate of NOX can be decreased by 4% (approx.) by reducing the heat obtained by exhaust temperature. The reduction in PM, HC and CO in biodiesel takes place due to advance combustion time (ACT), enhanced oxygen content, enhanced biodiesel saturation and higher rate of CN. This reduction was evaluated in comparison to diesel fossil fuel. The rate of reduction in emission of CO varies in the range of 7–42% with an increase in the rate of concentration of methyl soyate ranging from 10 to 100% [15,16,17].
In this paper, vegetable oil derived fuels produced from Glycine max is characterized by FTIR and UV–Vis spectroscopic method. The result would form the basis for controlling emission, performance and also optimization of the fuel.
Sample Preparation
Production of Glycine max Biodiesel
(a) Procurement of Oil
Glycine oil was purchased from the Ghaziabad market, U.P., India. The oil was filtered, and the filtered oil was trans-esterified.
(b) Transesterification of Glycine Oil
The filtered oil was heated on magnetic stirrer until the temperature of the oil reaches 60 °C. Methanol and heterogeneous catalyst Al2O3 (98%, Sigma Aldrich) were added to it. The heterogeneous catalyst Al2O3 was used for increasing the yield of biodiesel. It was trans-esterified for 1 h on magnetic stirrer at 1700 rpm around 60–65 °C (Fig. 1).
(c) Separation of Biodiesel
After transesterification, the produced biodiesel was kept at separating funnel for 24 h. The biodiesel being lighter settles at the top and glycerine at the bottom. The biodiesel collected is washed by means of distilled water 2–3 times to eliminate the glycerine. Washing of the biodiesel with distilled water turns milky, indicating the presence of traces of glycerine. The washed biodiesel was kept on the magnetic stirrer until the temperature of biodiesel reaches 100 °C. It is kept till the time until the first bubbles appear. The purpose of drying is to evaporate traces of distil water present on biodiesel.
(d) Sample Preparation
Fuel samples containing B5 (5% Glycine max biodiesel and 95% Pure Diesel), B10 (10% Glycine max biodiesel and 90% Pure Diesel), B15 (15% Glycine max biodiesel and 85% Pure Diesel) and B20 (20% Glycine max biodiesel and 80% Pure Diesel) of Glycine max biodiesel in diesel oil were used in the tests. Table 1 shows the physical and chemical properties of the base diesel oil, ASTM standard and pure biodiesel (B100).
Experimental Setup
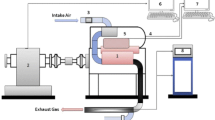
The performance was studied on a 5BHP single-cylinder four-stroke, direct-injection water-cooled diesel engine using diesel blends with glycimax biodiesel (B5, B10, B5 and B20) by varying the engine load (%) at constant speed 1500 rpm (Fig. 2). Details of the engine specification of the proposed engine are given in Table 2. AVL Di-Gas Analyzer was used to measure the exhausts gas of the engine. The inlet manifold of the analyzer is connected to the exhaust valve of the engine so that the emissions can be measured.
Results and Discussion
Fourier Transform Infrared Spectroscopy (FTIR) Studies of Biodiesel
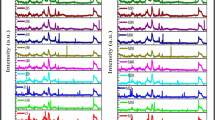
Characterization of Glycine max biodiesel for functional group determination was carried out by means of FTIR. This provides a mechanism of spectral changes ensuing from the reaction. Biodiesel spectrum patterns (5, 10, 15 and 20%) fall between the 500 and 3450/cm spectral range. Glycine max biodiesel infrared spectrum showed the triglyceride ester linkage at 1745/cm. The spectrum ranging from 725 to 1161/cm is for aromatic hydrocarbon, whereas 1160–1234/cm is for carbon–oxygen bond absorption (C–O bonds absorption) [18]. The absorption at 1373/cm designates the methyl group (CH3), whereas the peak at 1458/cm is indicated as the bending absorption of CH2. The peak at 1600/cm is missing in Glycine max biodiesel, where as it is present in all blended biodiesel as well as diesel. This indicates C=O signal area, absence of methyl in biodiesel diesel blends in Glycine max biodiesel. Again, the peak around 1886/cm is present in diesel, which is shifted to 1882–1878/cm in 5% and 10% blended biodiesel, respectively.
A peak at 1747.51/cm (5%), 1747.47/cm (10%), 1747.36 (15%) and 1751.36/cm (20% blended biodiesel) showed the occurrence of strong ester carbonyl bond (C=O), thus proving the presence of fatty acid methyl ester (FAME) or biodiesel. At higher blends of biodiesel, this peak is absent. This is observed as one of the most important differentiation of biodiesel from diesel, where the peak in the range of 1740–1755/cm is missing (Fig. 3). In the case of diesel, the absence of alcohol or un-reacted methanol has an absorption range of 3600–3500/cm.
IR spectrum of 10% and 15% blended Glycine max biodiesel indicated the strong peak of ester at 1234/cm (C–O vibrations). There is not any hindrance observed in the 1750/cm region, but the signals of diesel do meddle with the 1170–1200/cm region. The observed peaks at 2669/cm and 2727/cm as well as in the range of 3100–2700/cm is for the absorption of C–H bond. Table 3 illustrates peak point and AUC (area under the curve) of diesel, Glycine max biodiesel and its blends.
UV-Spectroscopy Analysis
Occurrence and absence of aromatics in diesel and biodiesel are differentiated by UV spectroscopy, respectively. Biodiesel has a negligible absorbance in comparison to aromatic compounds at the same frequency when diluted with n-heptane [18]. In the current study, biodiesel was produced from Glycine maximal and blended with diesel at 5, 10, 15 and 20%. With n-heptane, these samples of blended biodiesel and diesel were diluted to take the absorbance in the quantifiable range of spectrophotometer. The n-heptane in a quantity of 9.3 ml was mixed with 0.7 ml of the sample, and this process was replicated for 3 times to prepare the final sample. These samples of biodiesel were then measured with the help of UV–VIS spectrometer (Perker Elmer), and an absorbance of these samples was recorded. The absorbance of biodiesel at each wavelength decreases due to dilution with an aromatic compound like diesel (Fig. 4).
With both linoleic acid as well as methyl ester, fully saturated and monounsaturated FAAE in the UV region were not absorbed strongly while polyunsaturated samples absorbed strongly and having characteristic spectra. Diesel absorbs the least followed by 10% and 5% blended biodiesel samples showed the highest absorbance.
The linearity of absorbance declines with the augmentation in biodiesel blending with biodiesel and diesel due to decreased concentration of aromatic compound (Fig. 5). The linear line fitted for absorbance versus the percentage of biodiesel at 320 nm showed a high coefficient of determination (R2 = 0.9651).
Brake Specific Fuel Consumption
Figure 6 shows the variation in the proportion of biodiesel blends with the load on the BSFC, at the constant speed (1500 rpm). Generally, biodiesel blends have more specific fuel consumption as compared to that of diesel fuel, apart from having decreasing trend with increasing load. For all tested fuels BSFC is decreased with an increase in load percentage, because percentage in fuel consumption is less as compared to brake power. In the case of biodiesel, the primary reason for higher BSFC was the lower heating value of biodiesel as compared to that of diesel. Biodiesels have poor spray characteristics, and thus have differences in the mixture formation, owing to their higher kinematic viscosity and density, as compared to diesel fuel. Thus, it can be said that more biodiesel is required to produce the same torque. At a lower load, the percentage increase in BSFC was obtained as B5 (7.7%), B10 (5.69%), B15 (5.48%) and B20 (11.13%) compared with diesel. Similarly, at full load, the increase in BSFC was found to be B5 (2.7%), B10 (0.5%), B15 (2.53%) and B20 (3.79%).It can thus be observed that the variation of BSFC with increasing load at different blends of biodiesel is similar to the result obtained by other research work [19, 20].
Brake Thermal Efficiency
Figure 7 describes the alterations in the brake thermal efficiency as a function of load. It indicates that for a given set biodiesel blend, thermal efficiency is less, as compared to the thermal efficiency of diesel. Several factors are responsible for the observed lower thermal efficiency of biodiesel blends, such as lower calorific values, poor volatility, slow-burning rate and high viscosity. At a lower load percentage, decrease in BTE was obtained as B5 (0.04%), B10 (0.04%), B15 (0.02%) and B20 (0.16%) compared with diesel. Similarly, at full load, the decrease in BTE was B5 (1.20%), B10 (1.47%), B15 (1.76%) and B20 (2.52%). It has been observed that during the process of blending, the variation in BTE was less in full load condition as compared to the part load condition. This is because of the increase in temperature [21]. It can be observed that the variation of BTE with increasing load at different blends of biodiesel is similar to the result obtained by other research work [22, 23].
Exhaust Gas Temperature
Figure 8 shows the exhaust gas temperature (EGT) variation in comparison with load, for the diesel and biodiesel blends. Exhaust gas temperature is observed to be lower for diesel than that for biodiesel blends. At a lower load percentage, the increase in EGT was obtained as B5 (2.23%), B10 (4.07%), B15 (8.85%) and B20 (12.5%) compared with diesel. Similarly, at full load, the reported increase in EGT was B5 (1.13%), B10 (2.64%), B15 (1.88%) and B20 (3.77%). It augments with the increase in biodiesel proportion in the blend, since, as seen, B20 exhibits higher exhaust temperature than other blends. The larger proportion of biodiesel may lead to the augmentation of the cetane number of the fuel blend. The higher cetane number fuel exhibits lower ignition delay, and consequently, shorter premixed combustion period. It leads to higher exhaust temperature for biodiesel blend. However, biodiesel blends and diesel fuel were observed to have comparable EGT at higher loads. The higher loads require more fuel to be injected. Thus, the amount of heat release and hence consequently, the exhaust temperature, is found to be comparable for both, biodiesel as well as diesel. It can be observed that the variation of EGT with increasing load at different blends of biodiesel is similar to the result obtained by other research work [22, 23].
Carbon Monoxide (CO)
Figure 9 shows the carbon monoxide (CO) variation in comparison to load, for the diesel and biodiesel blends. As the rate of the proportion of biodiesel blend increases, the rate of CO emission is decreased. This is because blend in biodiesel produces more appropriate oxidation in comparison to diesel.
It has been observed that the rate of emission of CO decreases initially with load, and later increases rapidly up to full load. Also, it has been observed that cylinder temperature was low initially in the low load condition, and it increased rapidly as the load was increased. This is because of the enhancement of fuel injection inside the engine cylinder. As the temperature of the engine also increases due to enhancement in the rate of burning of fuel, it results in the decrease in CO. However, CO emission increases rapidly on further loading, as it requires an excessive amount of fuel, which leads to the formation of huge smoke [24, 25]. It was thus obtained that at full load, there was a 2.38%, 4.76%, 7.61% and 10% reduction in CO emission with B5, B10, B15 and B20, respectively [26, 27]. It can be observed that the variation of CO of with increasing load at different blends of biodiesel is similar to the result obtained by other research work [28, 29].
Hydrocarbon (HC)
It was observed that the emission rate of HC decreases with the increase in the proportionate rate of biodiesel (Fig. 10). The emission of HC decreases due to higher cetane number of biodiesel, which results in delayed combustion [19]. It was obtained that at full load of 1.66%, 2.5%, 4.16% and 6.25%, there is a reduction in HC emission with B5, B10, B15 and B20, respectively. It can be observed that the variation in HC with increasing load at different blends of biodiesel is similar to the result obtained by other research work [24,25,26,27,28,29].
Nitrous Oxide (NOX)
NOX emissions are found to be increasing with the increase in proportion of blend (Fig. 11). The main reasons for this are EGT, more oxygen content and faster reaction rate. At a lower temperature, atmospheric nitrogen exists as a stable diatomic molecule, with a small amount of NOX, but at a higher temperature, in the combustion chamber of an engine, some diatomic nitrogen breaks down to monoatomic nitrogen, which is reactive. It was obtained that at full load, there was a 1.42%, 3.57%, 5.71% and 7.32% increase in NOX emission with B5, B10, B15 and B20, respectively. It can be observed that the variation of NOX with increasing load at different blends of biodiesel is similar to the result obtained by other research work [19, 20].
Conclusion
The characterization of biodiesel was done by spectroscopic method (FTIR and UV–Vis spectroscopy). The results indicate that biodiesel retains the properties of diesel. The diesel characteristics of Glycine biodiesel are almost like those of conventional diesel. Glycine max biodiesel infrared spectrum showed the triglyceride ester linkage at 1745/cm. The occurrence of strong ester carbonyl bond (C=O.) indicates the presence of Fatty Acid Methyl Ester (FAME) and is present in all blended biodiesel. The linear line fitted for absorbance versus the percentage of biodiesel at 320 nm showed a high coefficient of determination (R2 = 0.9651).
The performance analysis of the engine has been done using biodiesel at different blending ratios. The BSFC of the engine on blends is higher than of neat diesel operation. The increase in BSFC at full load condition gives the report as B5 (2.7%), B10 (0.5%), B15 (2.53%) and B20 (3.79%). At a lower load percentage, decrease in BTE was obtained as B5 (0.04%), B10 (0.04%), B15 (0.02%) and B20 (0.16%) compared with diesel. Similarly, at full load, the decrease in BTE was B5 (1.20%), B10 (1.47%), B15 (1.76%) and B20 (2.52%). The EGT increased as the function of the concentration of blend. The higher the percentage of biodiesel in the blend, the higher was the temperature. Similarly, at full load condition increase in EGT was reported as B5 (1.13%), B10 (2.64%), B15 (1.88%) and B20 (3.77%). Increase in the NOX emission of biodiesel fuelled engine at full load condition was 1.42%, 3.57%, 5.71% and 7.32% with B5, B10, B15 and B20, respectively. The emission of CO and HC reduce appreciably as a result of biodiesel. It was obtained that at full load condition, there was a 2.38%, 4.76%, 7.61% and 10% reduction in CO emission, with B5, B10, B15 and B20, respectively. It was obtained that at full load condition, there was a 1.66%, 2.5%, 4.16% and 6.25% reduction in HC emission with B5, B10, B15 and B20, respectively
References
J. Pullen, K. Saeed, An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 16(8), 5924–5950 (2012)
G.G. Pereira, S. Marmesat, D. Barrera-Arellano, M.C. Dobarganes, Evolution of oxidation in soybean oil and its biodiesel under the conditions of the oxidation stability test. Grasas Aceites 64(5), 482–488 (2013)
P. Bondioli, A. Gasparoli, L. Della Bella, S. Tagliabue, G. Toso, Biodiesel stability under commercial storage conditions over one year. Eur. J. Lipid Sci. Technol. 105(12), 735–741 (2003)
N. Saifuddin, H. Refal, Spectroscopic analysis of structural transformation in biodiesel degradation. J. Appl. Sci. Eng. Technol 8(9), 1149–1159 (2014)
A.M. Khan, N. Fatima, Synthesis of biodiesel from the oily content of marine green alga Ulva fasciata. J. Chem. Soc. Pak. 37(05), 1040–1045 (2015)
W. Tiyapongpattana, P. Wilairat, P.J. Marriott, Characterization of biodiesel and biodiesel blends using comprehensive two-dimensional gas chromatography. J. Sep. Sci. 31(14), 2640–2649 (2008)
D.M. Pinho, V.O. Santos Jr., V.M. dos Santos, M.C. Oliveira, M.T. da Silva, P.G. Piza, A.C. Pinto, M.J. Rezende, P.A. Suarez, Evaluating the use of EN 14078 for determination of biodiesel in diesel blends sold in the Brazilian market. Fuel 136, 136–142 (2014)
T. Vega-Lizama, L. Díaz-Ballote, E. Hernández-Mézquita, F. May-Crespo, P. Castro-Borges, A. Castillo-Atoche, L. Maldonado, Thermogravimetric analysis as a rapid and simple method to determine the degradation degree of soy biodiesel. Fuel 156, 158–162 (2015)
A. Zawadzki, D.S. Shrestha, B. He, Biodiesel blend level detection using ultraviolet absorption spectra. Trans. ASABE 50(4), 1349–1353 (2007)
M. Mofijur, H.H. Masjuki, M.A. Kalam, A.E. Atabani, M. Shahabuddin, S.M. Palash, M.A. Hazrat, Effect of biodiesel from various feedstocks on combustion characteristics, engine durability and materials compatibility: a review. Renew. Sustain. Energy Rev. 28, 441–455 (2013)
S.M. Hasnain, R.P. Sharma, Evaluation of the performance and emission and spectroscopic analysis of an improved soy methyl ester. RSC Adv. 9(46), 26880–26893 (2019)
H.K. Rashedul, H.H. Masjuki, M.A. Kalam, A.M. Ashraful, S.A. Rahman, S.A. Shahir, The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers. Manag. 88, 348–364 (2014)
R.D. Misra, M.S. Murthy, Blending of additives with biodiesels to improve the cold flow properties, combustion and emission performance in a compression ignition engine—a review. Renew. Sustain. Energy Rev. 15(5), 2413–2422 (2011)
P.K. Sahoo, L.M. Das, M.K.G. Babu, S.N. Naik, Biodiesel development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel 86(3), 448–454 (2007)
M. Mofijur, H.H. Masjuki, M.A. Kalam, A.E. Atabani, Evaluation of biodiesel blending, engine performance and emissions characteristics of Jatropha curcas methyl ester: Malaysian perspective. Energy 55, 879–887 (2013)
G. Dwivedi, S. Jain, M.P. Sharma, Impact analysis of biodiesel on engine performance—a review. Renew. Sustain. Energy Rev. 15(9), 4633–4641 (2011)
Y. Devarajan, A. Mahalingam, D.B. Munuswamy, T. Arunkumar, Combustion, performance, and emission study of a research diesel engine fueled with palm oil biodiesel and its additive. Energy Fuels 32(8), 8447–8452 (2018)
R. Chatterjee, S.K. Mukherjee, Spectroscopic analysis and performance studies of jatropha extracted bio-diesel. Waste Biomass Valoriz. 9(9), 1579–1585 (2018)
H. Raheman, A.G. Phadatare, Diesel engine emissions and performance from blends of Karanja methyl ester and diesel. Biomass Bioenerg. 27(4), 393–397 (2004)
A.S. Ramadhas, C. Muraleedharan, S. Jayaraj, Performance and emission evaluation of a diesel engine fueled with methyl esters of rubber seed oil. Renew. Energy 30(12), 1789–1800 (2005)
A. Datta, B.K. Mandal, Numerical prediction of the performance, combustion and emission characteristics of a CI engine using different biodiesels. Clean Technol. Environ. Policy 20(8), 1773–1790 (2018)
F. Moreno, M. Munoz, J. Morea-Roy, Sunflower methyl ester as a fuel for automobile diesel engines. Trans. ASAE 42(5), 1181 (1999)
M.T. Raj, M.K.K. Kandasamy, Tamanu oil-an alternative fuel for variable compression ratio engine. Int. J. Energy Environ. Eng. 3(1), 18 (2012)
M.A. Kalam, H.H. Masjuki, Biodiesel from palm oil: an analysis of its properties and potential. Biomass Bioenergy 23(6), 471–479 (2002)
L.G. Schumacher, S. Soylu, J.V. Gerpen, W. Wetherell, Fueling direct injection diesel engines with 2% biodiesel blends. Appl. Eng. Agric. Trans. ASAE 21(2), 149–152 (2005)
G. Labeckas, S. Slavinskas, The effect of rapeseed oil methyl ester on direct injection diesel engine performance and exhaust emissions. Energ. Convers. Manag. 47(13–14), 1954–1967 (2006)
M. Canakci, Combustion characteristics of a turbocharged DI compression ignition engine fueled with petroleum diesel fuels and biodiesel. Bioresour. Technol. 98(6), 1167–1175 (2007)
A.N. Ozsezen, M. Canakci, A. Turkcan, C. Sain, Performance and combustion characteristics of a DI diesel engine fueled with waste palm oil and canola oil methyl esters. Fuel 88(4), 629–636 (2009)
A. Monyem, J.H. Van Gerpen, M. Canakci, The effect of timing and oxidation on emissions from biodiesel-fueled engines. Trans. ASAE 44(1), 35 (2001)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasnain, S.M.M., Chatterjee, R. & Sharma, R.P. Spectroscopic Performance and Emission Analysis of Glycine max Biodiesel. J. Inst. Eng. India Ser. C 101, 587–594 (2020). https://doi.org/10.1007/s40032-020-00570-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40032-020-00570-x