Abstract
The demand for cooling, especially in the developing economies, is rising at a fast rate. Fast-depleting sources of fossil fuel and environmental concerns necessitate looking for alternative cooling solutions. Solar heat driven adsorption based cooling cycles are environmentally friendly due to their use of natural refrigerants and the thermal compression process. In this paper, a performance simulation study of a basic two-bed solar adsorption chiller has been performed through a transient model for two different climatic locations in India. Effect of operating temperatures and cycle time on the chiller performance has been studied. It is observed that the solar hot water temperature obtained in the composite climate of Delhi (28.65°N, 77.25°E) can run the basic adsorption cooling cycle efficiently throughout the year. Whereas, the monsoon months of July and August in the warm and humid climate of Durgapur (23.48°N, 87.32°E) are unable to supply the required driving heat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for cooling is found to be proportional to the standard of living of people in a country. Comfort, preservative cooling load and the industrial requirement for cooling are increasing at a fast rate in the densely populous countries like India, China, etc. This is putting pressure on the peak energy demand of the countries; more and more fossil fuels are being burnt to produce electrical energy to run the vapour compression based cooling systems. The resources of fossil fuel are not limitless; burning of fossil fuels is generating greenhouse gases. The refrigerants in use are also adding to global warming, in addition to causing the depletion of stratospheric ozone layer. There is an urgent need to look for alternative refrigerants and refrigeration process to counter the ill effects of vapor compression cycles.
Heat driven sorption based cooling systems do not employ mechanical compressors, the major consumer of electrical energy in a vapour compression cooling system. The natural refrigerants used in the adsorption systems do not contribute to global warming or ozone layer depletion. Low driving source temperature requirement of adsorption chillers make them suitable to be run with simple flat plate or evacuated tube solar thermal collectors. Use of solar heat for cooling is all the more attractive as the demand for cooling is more or less in phase with the available solar irradiation.
Solar thermal energy can be used in adsorption chillers in two ways
-
(i)
Adsorbent bed can be directly heated by solar radiation. This process works in a 24 h long cycle and is purely intermittent. Cooling is obtained only during night when the bed gets cooled by releasing heat to the ambient and adsorption starts. This method is mostly used for ice production during night.
-
(ii)
Adsorbent bed can be indirectly heated by circulating a fluid through solar thermal collectors and the adsorbent beds. This method allows quasi-continuous operation through multiple beds and cooling can be availed during the day.
Use of solar energy in adsorption cooling system was first reported by Tchernev [1] in 1978. For a solar energy input of 6 kWh, Tchernev [2] could achieve a cooling of 0.9 kW/m2 of collector area with a zeolite–water basic adsorption cooling cycle.
In late eighties and during the last decade of twentieth century, a number of works on solar adsorption ice makers with flat plate or evacuated tube collectors have been published. Critoph [3,4,5,6] worked with activated carbon-ammonia systems, Pons and Guilleminot [7], Wang, et al [8], Sumathy, et al [9] and Li, et al [10] did experiments with activated carbon-methanol pair. The daily ice production, on an average, was 4–6 kg/m2 of collector area with a solar insolation of 18–20 MJ/m2/day.
On the solar air-conditioning field, Sakoda and Suzuki [11] provided a quantitative assessment of a solar powered silica gel-water adsorption cooling system. Freundlich adsorption isotherm was used to compute the amount adsorbed in equilibrium and the values of constants (k = 0.346 kg/kg and n = 1.6) in the Freundlich equation were evaluated experimentally for packed bed of silica gel (Fuji Davison Type A silica gel). Linear driving force (LDF) model was used to compute the adsorption rate with the overall mass transfer coefficient given by Gluckauf [12].
Working with Fuji Davison Type RD silica gel, Cho and Kim [13] found that the adsorption equilibrium was well represented by the Freundlich equation with k = 0.552 kg/kg and n = 1.6. However, Saha, et al [14], working with the experimental data from an adsorption chiller manufacturer (NACC, 1992), found good agreement with Freundlich equation plot (with k = 0.346 kg/kg and n = 1.6) only for a narrow range of pressure and temperature. To adapt the Freundlich equation to the manufacturer’s experimental data, the authors replaced the constants in Freundlich equation with two temperature dependent parameters, polynomial functions of silica gel temperature.
Xia, et al [15] compared the adsorption equilibrium data obtained from Freundlich equation, Dubinin-Astakhov (DA) equation and modified Freundlich equation with the experimental data from a weight method test rig of silica gel-water system. Results showed that the modified Freundlich equation agreed well with the experimental data, whereas the Freundlich equation and the DA equation showed large deviations, especially at low water uptake.
Now, in this work, the performance of a single-stage, two-bed silica gel-water adsorption cycle has been investigated with evacuated tube collectors (ETC) under two different climatic conditions of India.
Methodology
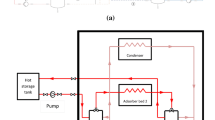
Adsorption chillers utilize the temperature dependent sorption property of adsorbents to provide cooling. Micro-pored silica gel, which has larger water adsorption capacity at low humidity, is suitable to be utilized in a closed cycle sub-atmospheric pressure refrigeration system. In solar heat driven adsorption chillers, solar thermal energy is used to heat up the adsorbent bed, causing desorption of refrigerant water vapour. The water vapour, thus released, gets cooled in the condenser, and then passed to the evaporator, wherein it again gets evaporated at low pressure, thereby providing cooling. In a two-bed chiller, the second adsorber, at the same time, adsorbs water vapour from the evaporator. Thus, the operation of the system follows a periodic succession of cycles. That is, at any time of operation, when one adsorber is in the desorption process (heating period); another adsorber will be in the adsorption process (cooling period). These periods are separated by isosteric heating and cooling of the adsorbers. In this way, a semi-continuous cooling effect may be obtained from the system. Figure 1 shows the flow diagram of a two-bed adsorption chiller.
Like any other solar energy application, weather plays an important role in the performance of a solar adsorption chiller. The round the year performance study of a silica gel-water paired single-stage, two-bed adsorption chiller under two different climates has been performed in this work. Two Indian climates, namely, warm and humid climate of eastern India (Durgapur, 23.48°N, 87.32°E) and composite climate of north-west India (Delhi, 28.65°N, 77.25°E) were selected for the performance study.
Round the year solar water heater temperature data were collected with the evacuated tube solar water heater installed at Durgapur. The hot water temperature data obtained from the collector were compared with the simulated hot water temperature data from TRNSYS [16] and a good matching was observed. Figure 2 shows the comparison between hot water temperatures obtained from the evacuated tube collector and TRNSYS simulated data.
The solar irradiation data of Delhi have been taken from ‘NREL Solar Resource Maps and Toolkit for Northwest India’ [17]. The data used are the average daily total radiation based on hourly estimates of radiation over a period of 6 years (2002–2007). The solar insolation data of Delhi, thus obtained, were fed into TRNSYS to simulate the hot water temperature data from an evacuated tube solar thermal collector.
The global horizontal solar radiation and hot water temperature data of Durgapur and Delhi are given in Figs. 3 and 4, respectively. A noteworthy difference between the hot water temperature data of Durgapur and Delhi is that in the warm and humid climate of Durgapur, the hot water temperatures achieved during the monsoon months of July and August are appreciably lower compared to other months.
The TRNSYS simulated hot water temperature data for Durgapur and Delhi were used in the performance simulation study of a basic cycle of silica gel–water adsorption chiller.
Modelling and Simulation
Adsorption Kinetics and Adsorption Equilibrium
The rate of adsorption or desorption for the silica gel-water pair is estimated from linear driving force kinetic equation;
where, \({\text{x}}^{\text{ * }}\) is the equilibrium adsorption capacity which is expressed by adsorption equilibrium equations. For silica gel-water system, the modified Freundlich model, which is expressed by the equation below, is used to estimate the equilibrium uptake.
where
The numerical values of A0 to A3 and B0 to B3 are given in Table 1 [18].
Mass Balance Equation
The mass balance for the refrigerant water is expressed as
Energy Balance Equations
Poor heat and mass transfer properties of the sorbent beds prescribe application of distributed parameter approach for heat and mass transfer modelling of adsorbent beds. However, the temperature profile of the adsorbent bed under heating (desorption) developed in an earlier work [19] by the present author and a number of published works [20, 21] suggest that a lumped parameter model can predict chiller performance with sufficient accuracy (error less than 10%), which saves computation complexity, cost and time.
Using a lumped parameter approach and neglecting heat losses, energy balance for the sorption bed, evaporator and condenser leads to following set of equations
Adsorber/desorber energy balance
where γ = 0 for desorbing bed, and γ = 1 for adsorbing bed.
The bed outlet temperature of the cooling/heating fluid is calculated by log mean temperature difference (LMTD) method and is given by
Evaporator energy balance
Chilled water outlet temperature is given by
Condenser energy balance
and the cooling water temperature at condenser outlet is given by
Coefficient of performance (COP) is given by
Simulation Procedure
The set of coupled ordinary differential equations are solved using the numerical algorithm DIVPAG (IMSL library subroutine). The fifth order Gear’s Differentiation Formulae (GDF) was employed for the numerical integration. The calculation procedure starts with the initialization of variables, the system geometries and the adsorption characteristics of silica gel-water systems. Table 1 lists the parameters used for simulation. To calculate the adsorption isotherms and kinetics, a series of subroutines were used. Considering the initial conditions specified in the problem, the total system is allowed to operate from transient to cyclic-steady-state conditions. The computed values of the key variables are constantly updated with time. Double precision has been used, and the tolerance is set to 1 × 10−6. As the cycle is operated in a batched manner, that is, streams of hot and cold coolant flows are being alternated between beds at the end of each cycle period, the computer code adopts a special tracking ‘pointer’ or passing-variable routine to sequence the computed variables or data from one cycle to another.
Results and Discussion
Solar Hot Water Temperature Data
Figure 4 depicts the TRNSYS generated hot water temperature data from an evacuated tube solar collector for Delhi and Durgapur. The minimum temperature achieved in Delhi was 60°C in the month of January and the maximum temperatures of around 86°C were obtained in the months of April and May. The monsoon months of July and August, in the warm and humid climate of Durgapur, recorded the minimum temperatures of less than 50°C and April saw the maximum temperature of around 80°C.
Performance Analysis of the Chiller
The performance of the hot water driven silica gel-water adsorption chiller has been studied with variations in driving source temperature, cooling water and chilled water inlet temperatures. The effect of cycle time on the system performance has also been investigated. Cooling capacity and COP of the system have been chosen as the performance yardsticks. A comparative analysis of the performance of the chiller subjected to the climatic condition of Delhi and Durgapur has been undertaken.
Effect of Regeneration Temperature
Figure 5 shows the change in the cooling capacity of the chiller with changes in driving source temperature. It is observed that there is not much difference in chiller capacities for the two climates under study. Significant improvements in cooling capacity for both the chillers are noticed when the regenerating hot water temperature is increased from 60 to 95°C and the change is almost linear for both the climatic conditions. Higher regeneration temperature causes enhanced desorption adding to the amount of refrigerant circulating in the system. Evaporation of more refrigerant leads to increase in cooling capacity; comparatively drier adsorbent also has more potential to adsorb the refrigerant, triggering the evaporation.
Figure 6 shows the variation in COP of the chiller with changes in driving source temperature. As the cooling capacity increases with rising hot water temperature, the supply of input heat also increases. The COP of the chiller system is found to reach an optimum value as the driving source temperature is increased. For the Durgapur chiller, maximum value of COP is obtained at a regeneration temperature of around 80°C, exceeding which, the COP begins to fall. This may be attributed to the increased irreversible losses at higher regenerating temperatures. For the Delhi chiller, COP becomes a maximum at a regeneration temperature of around 88°C. The difference in optimum cycle times of the chillers (discussed in next section) contributes to this different behaviour of Delhi chiller. Larger cycle time for the Delhi chiller enables it to perform better at elevated regeneration temperature level. On the lower (regenerating temperature) side, the COP of the Delhi chiller suffers a sharper dip below 70°C. This might be caused by higher cooling water temperature of the Delhi climate, which reduces the regenerating temperature lift, that is, the temperature difference between the driving heat source and the heat sink of the chiller, the effect being more pronounced at low regeneration temperatures.
Effect of Cycle Time
Figure 7 shows the variation in COP and cooling capacity with changes in the cycle time. It has been observed that COP increases asymptotically with cycle time. Heat losses from intermittent cycle operation are largely due to the sensible heat load resulting from alternate heating and cooling, at short intervals, of the adsorbent bed with the heat exchanger metal. For a longer cycle time, the number of times a bed undergoes sensible heating and cooling, within a particular time period, is less. Thus the driving heat consumption tends to decrease with longer cycle time, with a favourable effect on COP. However, the cooling capacity, which is measured in kW, that is, per unit time, initially increases with cycle time and then it falls after reaching a peak value. Initially, more cycle time helps in more refrigerant being adsorbed or desorbed, which becomes less pronounced as the time progresses. For cycle times less than the optimum value, the sorption processes, particularly the comparatively slower adsorption process does not get sufficient time to occur satisfactorily and thus reduces the cooling output. The optimum cycle time (for cooling capacity) has been found to be around 450 and 700 s for the Durgapur chiller and the Delhi chiller respectively. Comparatively higher regeneration and cooling water temperatures for the Delhi chiller require longer cycle time for efficient utilization of driving heat and more importantly proper cooling of the adsorber.
Effect of Cooling Water Inlet Temperature
Cooling water inlet temperature was varied from 20 to 40°C to see its effect on cooling capacity and COP of the chillers. Figure 8 shows that the cooling capacity increases significantly as the cooling water inlet temperature is lowered from 40 to 20°C. This is due to the increased amount of refrigerant getting adsorbed at low temperature of the adsorbent. At low cooling water inlet temperatures up to 25°C, COP values are more or less steady as the effect of increasing capacity at low temperature is nullified by increasing amount of driving heat required for sensible heating of the adsorber. Above 25°C, COP gradually declines with increasing cooling water inlet temperature, the effect being more pronounced when the temperature exceeds 32/33°C. This may be attributed to less refrigerant being adsorbed at higher adsorbent temperature. The negative effect of increasing cooling water temperature is more pronounced for lower regeneration temperatures, as the regenerating temperature lift becomes proportionately smaller.
Effect of Chilled Water Inlet Temperature
Chilled water inlet temperature was varied from 10 to 20°C, keeping the hot and cooling water inlet temperatures constant. Figure 9 shows that the COP curves are almost flat with slight rise above 14°C. Cooling capacity improves as the chilled water inlet temperature is increased; however, it is impractical to utilize this benefit as because, in an adsorption chiller, the chilled water from evaporator outlet is used to provide the cooling load. Generally, requirement of higher cooling load demands lower chilled water temperature.
Conclusion
A parametric performance analysis of a silica gel-water paired adsorption chiller, driven by hot water from a solar thermal collector system, has been carried out for two different climatic conditions of India and the following conclusions may be arrived at from this study.
-
(i)
A basic cycle of single-stage, two-bed adsorption chiller is able to provide cooling, though at reduced capacity, with a low driving source temperature up to 60°C.
-
(ii)
For a particular chiller configuration, an optimum value of regeneration temperature can be found out to get maximum COP. Similarly, to achieve maximum cooling capacity, an optimum cycle time can be calculated. The operating strategy of the chiller may be planned likewise depending upon the requirement.
-
(iii)
The hot water temperatures obtained through evacuated tube solar thermal collectors in the composite climate of Delhi, India can run the adsorption chiller under study throughout the year. Lean months of October to January may experience somewhat reduced capacity and COP of the chiller, but the requirement of cooling is also at its minimum during that period.
-
(iv)
For the warm and humid climate of Durgapur, India, the hot water temperatures achieved during the monsoon months of July and August are not sufficient to effectively run the chiller; it may require the employment of multi-stage cycles to exploit such low regeneration temperature.
Abbreviations
- A0 :
-
coefficient in equilibrium equation, kg (kg of dry adsorbent)−1
- A1 :
-
coefficient in equilibrium equation, kg (kg of dry adsorbent, K)−1
- A2 :
-
coefficient in equilibrium equation, kg (kg of dry adsorbent, K2)−1
- A3 :
-
coefficient in equilibrium equation, kg (kg of dry adsorbent, K3)−1
- B0 :
-
coefficient in equilibrium equation (–)
- B1 :
-
coefficient in equilibrium equation, K−1
- B2 :
-
coefficient in equilibrium equation, K−2
- B3 :
-
coefficient in equilibrium equation, K−3
- A:
-
area, m2
- COP:
-
coefficient of performance
- Cp :
-
specific heat capacity, J kg−1 K−1
- Ds :
-
surface diffusion coefficient, m2 s−1
- Dso :
-
pre–exponential constant, m2 s−1
- ETC:
-
evacuated tube collector
- Ea :
-
activation energy, J mol−1
- hfg :
-
latent heat of evaporation, J kg−1
- m:
-
mass, kg
- P:
-
pressure, Pa
- Ps :
-
saturation pressure, Pa
- Qchill :
-
cooling output, kW
- Qhot :
-
driving heat, kW
- Qst :
-
isosteric heat of adsorption/desorption, J kg−1
- R:
-
gas constant, J kg−1 K−1
- Rp :
-
average radius of adsorbent particle, m
- SCP:
-
specific cooling power
- SWH:
-
solar water heater
- T:
-
temperature, K
- t:
-
time, s
- U:
-
heat transfer coefficient, W m−2 K−1
- \({\text{x}}^{\text{ * }}\) :
-
equilibrium uptake, kg kg−1
- x:
-
refrigerant amount adsorbed, kg kg−1
- ads:
-
adsorber
- bed:
-
adsorbent bed
- chill:
-
chilled water
- con:
-
condenser
- des:
-
desorber
- eva:
-
evaporator
- f:
-
heat transfer fluid
- hot:
-
hot water
- hex:
-
heat exchanger (adsorber/desorber)
- in:
-
inlet
- l:
-
liquid
- out:
-
outlet
- s:
-
saturation
- sg:
-
silica gel
- v:
-
vapour
- w:
-
refrigerant
- w:
-
refrigerant
References
D.I. Tchernev, Natural Zeolites: Occurrence Properties and Use (Pergamon Press, London, 1978)
D.I. Tchernev, Solar air conditioning and refrigeration systems utilizing zeolites, Proceedings of the Meetings of Commissions E1–E2, Jerusalem, International Institute of Refrigeration (1979), pp. 209–215
R.E. Critoph, Performance limitations of adsorption cycles for solar cooling. Sol. Energy 41, 21–31 (1988)
R.E. Critoph, Laboratory testing of an ammonia carbon solar refrigerator, ISES, Solar World Congress, Budapest, Hungary, 23–26 August 1993
R.E. Critoph, Rapid cycling solar/biomass powered adsorption refrigeration system. Renew. Energy 16, 673–678 (1999)
R.E. Critoph, Towards a one tonne per day solar ice maker. Renew. Energy 9, 626–631 (1996)
M. Pons, J.J. Guilleminot, Design of an experimental solar-powered, solid-adsorption ice maker. J. Sol. Energy Trans. ASME 108, 332–337 (1986)
R.Z. Wang, M. Li, Y.X. Xu, J.Y. Wu, An energy efficient hybrid system of solar powered water heater and adsorption ice maker. Sol. Energy 68, 189–195 (2000)
K. Sumathy, Z.F. Li, Experiments with solar-powered adsorption ice-maker. Renew. Energy 16, 704–707 (1999)
M. Li, R.Z. Wang, Y.X. Xu, J.Y. Wu, A.O. Dieng, Experimental study on dynamic performance analysis of a flat plate solar solid-adsorption refrigeration for ice maker. Renew. Energy 27, 211–221 (2001)
A. Sakoda, M. Suzuki, Fundamental study on solar powered adsorption cooling system. J. Chem. Eng. Jpn. 17, 52–57 (1984)
E. Gluckauf, Trans. Faraday Sci. 51, 1955, 1540. Cited by A. Sakoda, M. Suzuki, Fundamental study on solar powered adsorption cooling system, J. Chem. Eng. Jpn. 17, 52–57 (1984)
S.H. Cho, J.N. Kim, Modelling of a silica gel/water adsorption cooling system. Energy 17, 829–839 (1992)
B.B. Saha, E.C. Boelman, T. Kashiwagi, Computer simulation of a silica gel-water adsorption refrigeration cycle—the influence of operating conditions on cooling output and COP. ASHRAE Trans. 101, 348–357 (1995)
Z.Z. Xia, C.J. Chen, J.K. Kiplagat, R.Z. Wang, J.Q. Hu, Adsorption equilibrium of water on silica gel. J. Chem. Eng. Data 53, 2462–2465 (2008)
S.A. Klein et al., TRNSYS 17: A Transient System Simulation Program, (Solar Energy Laboratory, University of Wisconsin, Madison, USA, 2010), http://sel.me.wisc.edu/trnsys. Accessed June 2013
NREL Solar Resource Maps and Toolkit for Northwest India; Energy Analysis (CD–ROM), NREL/CD–6A2–45018, http://www.nrel.gov/docs/fy10osti/45018CD.zip. Accessed Nov 2009
K. Habib, B.B. Saha, S. Koyama, Study of various adsorbent—refrigerant pairs for the application of solar driven adsorption cooling in tropical climates. Appl. Therm. Eng. (2014). doi:10.1016/j.applthermaleng.2014.05.102
D. Sarkar, S.N. Tiwari, A. Yadav, B. Choudhury, Development of a solar-powered adsorption chiller. Int. J. Emerg.Technol. Adv. Eng. 3, 382–388 (2013)
X. Wang, H.T. Chua, Two-bed silica gel–water adsorption chillers; an effectual lumped parameter model. Int. J. Refrig. 30, 1417–1426 (2007)
G. Zhang, D.C. Wang, J.P. Zhang, Y.P. Han, W. Sun, Simulation of operating characteristics of the silica gel–water adsorption chiller powered by solar energy. Sol. Energy 85, 1469–1478 (2011)
Acknowledgement
Authors are thankful to the Ministry of New and Renewable Energy India (IN) for providing necessary grant to complete this study (Grant No. F. No. 2/7(12)/2007-SEC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choudhury, B., Chatterjee, P.K., Habib, K. et al. Performance Investigation of a Solar Heat Driven Adsorption Chiller under Two Different Climatic Conditions. J. Inst. Eng. India Ser. C 99, 347–354 (2018). https://doi.org/10.1007/s40032-017-0358-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40032-017-0358-x