Abstract
Kaempferia rotunda Linn. belongs to family Zingiberaceae has been traditionally used to cure several diseases and has been used as folk medicines because of having tremendous therapeutic potential. In the current study, the chemical constituents of the essential oil obtained from the fresh rhizome of K. rotunda by hydrodistillation method were analyzed by Gas Chromatography-Mass Spectrometry (GC–MS). Nine constituents have been identified in the essential oil of K. rotunda accounting for 96.79% of the total peak area. The major compounds were benzoic acid (58.25 ± 0.14%) followed by bornyl acetate (14.4 ± 0.35%), zingiberene (5.75 ± 0.08%) and β-myrcene (3.87 ± 0.08%), etc. Further, antioxidant, antimicrobial and cytotoxic activities of the essential oil were assessed. Antioxidant activity of the oil was determined by scavenging DPPH radical in terms of their 50% inhibition (IC50) values. Oil exhibited moderate to strong antioxidant activity. Antimicrobial activity was evaluated using Inhibition Zone Diameter (IZD) and Minimum Inhibitory Concentration (MIC) values, whereas cytotoxic activity was studied using MCF7 and HeLa human cancer cell lines. K. rotunda oil exhibited stronger antimicrobial activity against the gram-negative bacteria than the rest pathogens. Rhizome oil of K. rotunda exhibited 75.63% ± 5.66 percentage of cell inhibition for HeLa and 68.44% ± 2.51 for MCF7 cell line at 20 μg/ml concentration. Hence, it is concluded that K. rotunda rhizome essential oil can be considered as natural antioxidant and antimicrobial agent, and it can be used in food and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plants of Zingiberaceae family are widely distributed throughout the tropical regions, particularly in South and Southeast Asia comprising of about 50 genera and 1300 species [1]. Kaempferia, a genus of family Zingiberaceae, comprises of about 70 species of rhizomatous, aromatic perennial herbs. Kaempferia rotunda Linn. is popularly known as Bhumi-Champaka is a medicinal aromatic herb with subglobose tuberous rhizome and is found in various parts of India [2]. K. rotunda is well-known among the folk medicine practitioners for its wide therapeutic potential. Traditionally, the rhizomes of this plant are utilized in treating abdominal illness, cold, sputum laxative, obesity, dysentery and diarrhea. Furthermore, the rhizomes possess anti-inflammatory potential and provided in gastrointestinal complications [3]. Other plant parts such as leaves as well as rhizomes are also consumed as fresh or cooked vegetables. Further, these parts are used as food flavoring spice and cosmetic powders. The K. rotunda dried rhizome powder is well-known for preventing and treating malignancies [4]. This plant was also found to possess antimicrobial, antinociceptive, analgesic and antioxidant [5,6,7] activities. In Ayurveda, ‘Hallakam,’ a drug has been prepared from the rhizomes of this plant which is stomachic, heals burning sensitiveness, anti-inflammatory to wounds and bruises as well as useful in the treatment of mental disorders and insomnia [8].
Plants consist of some chemical substances which possess medicinal values. Secondary metabolism of aromatic plants results in the production of essential oils (EOs) which are widely known for their characteristic odor [9]. It plays an important role in the defense against several microorganisms and is also integrated with various functions required for the protection of plant [10]. The pharmaceutical properties attributed to aromatic plants are reasonably associated with the chemical constituents of EOs present within. Scientifically, phytochemical investigation reveals the screening of chemical constituents present in a plant is the primary step to validate its remedial values.
The production of potent free radicals is the major cause of oxidative stress [11]. Oxidative stress is a major reason behind several human diseases like malignancies, Parkinson, arthritis, immunodeficiency disorders, Alzheimer, aging, asthma and atherosclerosis [11]. Consumption of adequate dietary antioxidants can be helpful in protecting human health conditions caused by oxidative stress. The plant EOs having high antioxidant value are always in demand for their safety and efficacy toward proposing them as an alternative to synthetic products. These EOs are also broadly utilized in cosmetic, food and pharmaceutical industries [12].
The increasing resistance potential of pathogens toward antimicrobial compounds has lethal effects on human health which is a global concern nowadays. Since ancient days, it is being observed that many plant derived products have been found in treating several infectious diseases [13]. EOs have been found to be an important source against various microorganisms. Thus, researchers have made plant derived products such as EOs their choice in order to reduce the utilization of synthetic chemicals.
Cancer is one of the most dangerous ailments in the present decade and a major cause of deaths happening worldwide due to various types of cancers. Uterine cervix, prostate and breast cancers are the most common types in the world. Since 1960s, investigation to find out natural anticancer substances from plants has been initiated and vinblastine, camptothecin, taxol, vincristine were discovered are currently also used in cancer therapies [14]. There are several literature reports of EOs from different plants examined for various types of cancers like oral, breast, liver, colon, brain, prostate and lung [15, 16]. The treatments of cancer include surgeries, radiotherapies, chemotherapies and anti-hormone therapies; however, antagonistic impacts, drug resistance and expenses are driving researchers to inspect different natural sources to find novel anticancer molecules. Among the well-known phytocomplexes, EOs, the natural products have gained interest because of their relevant chemical characteristics and bioactivities.
Nowadays, there is an increasing research interest has been observed in the EOs of aromatic plants and their bioactive components [17]. The pharmaceutical properties attributed to aromatic plants are reasonably associated with the chemical constituents of EOs present within. Various volatile bioactive constituents have been already identified in the EOs of K. rotunda, i.e., most abundantly α-pinene, camphene, β-pinene, camphor, cineole, linalool, bornyl acetate and benzyl benzoate [6, 18]. However, as far our knowledge, this is the first report regarding chemical constituent analysis and bioactivities study of K. rotunda from Eastern India. The present study was aimed to evaluate the phytoconstituents present in the rhizomes of K. rotunda Linn. and its different biological activities to validate its therapeutic potential.
Material and Methods
Plant Material
The tuberous rhizomes of K. rotunda were collected from Kalimpong, West Bengal (Latitude: 27.0594° N, Longitude: 88.4695° E) and were authenticated by a Taxonomist, Regional Plant Resource Center (RPRC), Bhubaneswar as well as deposited in the herbarium of RPRC bearing voucher specimen number 10125. The rhizomes were then planted in the green house of Center for Biotechnology, Siksha O Anusandhan Deemed to be University, Bhubaneswar.
Essential Oil Isolation and Quantification
Fresh rhizome samples (500 g) of K. rotunda were subjected to hydrodistillation for about 4 h using a Clevenger-type apparatus following the method of Guenther E (1972) with required modifications [19]. The yield percentage (v/w) was determined based on the fresh weight of the obtained essential oil (EO). Anhydrous sodium sulfate (Na2SO4) treatment was then followed for eradicating traces of moisture from the oil and stored in glass vials at 4 °C until further analysis. The experiment was carried out in triplicates.
Chemical Constituents Analysis by GC–MS
The phytochemical analysis of EO was carried out by gas chromatography equipped with mass spectrometry (GC–MS). The identification and analysis of constituents were obtained by using 6890 series instrument (Agilent Technologies, Palo Alto, CA, USA) fused with a Mass Selective Detector (model-MSD 5973) having a quadrupole analyzer. HP-5 silica capillary column incorporated with 30 m long column with 0.25 mm internal diameter and 0.25 µm film thickness was used. Helium gas was used as carrier gas which was supplied at a flow rate of 1.2 ml/min. 1 µl of neat EO sample was injected at the sample injection port. The oven temperature was programmed as follows: 50–240 °C at 4 °C/min; 240–270 °C at 15 °C/min; 50 °C/min for 1 min and lastly at 270 °C for 15 min. The electron ionization energy of 70 eV was maintained throughout the programming process with split ratio of 100:1. The temperature provided for auto-injector and detector was 280 °C. Data acquisition was performed with a mass scan ranges in between 20 and 600 amu at 230 °C. The retention indices (RI) of compounds were determined using homologous series of n-alkane (C8–C20) (Sigma-Aldrich, USA) under similar operating conditions. The compounds were identified by comparing the calculated Retention Index (RI) values with data reported in the literature and matching their mass spectra obtained from the chromatogram with National Institute of Standards and Technology Mass Spectral (NIST MS) search 2.0 library [20].
Antioxidant Activity
The antioxidative potential of EOs obtained from rhizomes of K. rotunda was carried out using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. The experiment was conducted using ascorbic acid as positive control by following the protocol of Sahoo et al. [21]. 1 ml of EO in different concentrations was prepared and mixed with 1 ml of 0.1 M methanolic solution of DPPH. Then, the mixture was mixed properly and kept at room temperature for 30 min in dark conditions. The absorbance of the samples was measured at 517 nm using a UV–visible spectrophotometer against DPPH solution as control. Each experiment was performed in triplicates. The radical scavenging activity was expressed as percentage of inhibition of DPPH radical values and determined by the following equation:
where Acontrol is the absorbance of the control, and Atest is the absorbance of samples.
The concentration of sample that gives 50% inhibition (IC50) was determined by plotting a graph exhibiting percentages of inhibition against concentrations of samples.
Antimicrobial Activity
The efficacy of antimicrobial potential of K. rotunda EOs was evaluated by disk diffusion method, and the minimum inhibitory concentration (MIC) was determined by serial dilution method in accordance with Clinical and Laboratory Standards Institute (CLSI) guideline with slight modifications [22]. To perform the experiment, six microorganisms, two gram-positive bacteria: Enterococcus faecalis and Staphylococcus aureus, two gram-negative bacteria: Escherichia coli and Acinetobacter baumannii and two fungi: Candida albicans and Aspergillus niger were used. The microbial stock cultures of bacteria were maintained in Mueller Hinton Broth (MHB), and fungus was maintained in Sabouraud Dextrose Broth (SDB) at 4 °C. By following the filter paper disk diffusion method, the zones of inhibition around the disks were obtained in all cases. The experiment was performed in triplicates by taking Gentamicin as standard and DMSO (Dimethyl sulfoxide) as control. Similarly, MIC of the samples was also evaluated by following two-fold serial dilution method with MHB for bacteria and SDB for fungi in 96-microtiter plates. The experiment was conducted in a triplicate manner, and Gentamicin was taken as standard. The MIC was evaluated as the lowest concentration of the samples that inhibited the growth of microorganisms.
Cytotoxic Activity
The K. rotunda rhizome EOs were also screened for cytotoxic activity which was estimated by taking two cancer cell lines, i.e., human breast cancer lines (MCF7) and cervical cancer cells (HeLa). A media supplemented with 10% inactivated Fetal Bovine Serum (FBS), 5 mM L-glutamine and 5% CO2 known as Dulbecco’s Modified Eagle Medium (DMEM) was prepared for culturing both cancer cell lines at 37 °C in an incubator. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay of the EOs were performed in accordance with the protocol by Sahoo et al., [23]. Metabolically active cells are responsible for the conversion of yellow tetrazolium salt–MTT to purple-formazan crystals. The viable cells can be quantified by analyzing the above mentioned conversion. In the experiment, untreated cells were considered as a control of viability (100%) and the percentage of inhibition of treated cells relative to the untreated controls was quantified.
Statistical Analysis
The statistical analysis of all the obtained data was performed by determining Mean ± SD, one way ANOVA along with Tukey’s HSD test at p < 0.05. The analysis was carried out by Minitab version 17 statistical software (Minitab Inc, PA, USA).
Results and Discussion
Chemical Composition of Essential Oil
The hydrodistillation of fresh rhizomes of K. rotunda gave a yield of 0.15% essential oil (EO). GC–MS analysis was performed to determine detailed chemical composition of the rhizome EO which revealed nine major components, accounting for 96.79% of the total peak area. The result demonstrated the presence of < n- > hexyl benzoate with the maximum peak area (58.25 ± 0.14%) followed by bornyl ester (14.4 ± 0.35%), zingiberene (5.75 ± 0.08%), β-myrcene (3.87 ± 0.08%), respectively (Table 1). The current result corroborated with a report in which the aromatic (benzoates, salicylates) and aliphatic compounds were predominant [18]. In the article, the K. rotunda rhizomes were collected from Surabaya, East Java and the hydrodistilled oil was characterized by GC–MS (EI) analysis. The GC–MS characterization showed benzyl benzoate and n-pentadecane as the major constituents with 69.7% and 53.8% area percentages, respectively. Another report identified 13 compounds from the EOs of rhizome of K. rotunda collected from Herb Garden, Arya Vaidya Sala, Kottakkal in which bornyl acetate predominated with an area percentage of 30.121% followed by benzyl benzoate with 16.595% area percentage [6]. However, a report represented 57 volatile constituents identified from the K. rotunda EO and the GC–MS characterization showed Endo–borneol with an area percentage of 9.30% as the major one followed by dehydroisoandrosterone acetate with 9.12% area percentage [24]. The observed variation in the chemical composition of the EO of the species might be due to the differences in responding to the geographic, edaphic or climatic entities as well as variation in the maturity of plant materials [21].
Bioactivity Study
Antioxidant Activity
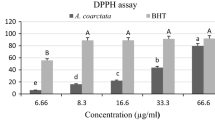
DPPH radical scavenging assay of K. rotunda rhizome EOs showed significant activity at a concentration of 25 μg/ml in terms of their 50% inhibition (IC50) values. DPPH radical scavenging potential of K. rotunda samples compared with the standard, i.e., ascorbic acid is depicted in the Fig. 1. It was observed that the rhizome oil possessed good radical scavenging activity. Assessed EO was able to reduce the stable violet DPPH radical to the yellow DPPH-H, reaching 50% of reduction with IC50 values. It was seen that, with increasing sample concentration, the activity was also increased.
Lotulung et al. [4] 2008 reported antioxidant activity of K. rotunda rhizome extracts with different solvents and resulted good radical scavenging activity. Overall, antioxidant properties of EOs depend on their phytoconstituents, their structural features, temperature, concentration, light and physical state of the system, along with on micro components acting as a pro-oxidant or synergists [25]. Hence, it can be concluded that the antioxidant potential of K. rotunda EO may be attributed to the presence of several phytoconstituents with different bioactivities. However, more advanced study is also required to identify antioxidant compounds present in this plant.
Antimicrobial Activity
The rhizome EO of K. rotunda was found to have good to moderate antimicrobial activities against two gram-positive, two gram-negative pathogenic bacteria and two fungi as determined from disk diffusion method and MIC assay. The MIC values of the rhizome oil were ranged between 8.34 and 10.91 µg/ml (Table 2). The zone of inhibition was highest in rhizome oil (15.72 ± 0.23 mm) against E. coli (gram-negative bacteria), whereas inhibition zones of the positive control, Gentamicin (5 µg/ml) were ranged from 17 ± 1.0 to 25.67 ± 0.59 mm. Both the assays resulted pathogens like E. coli and A. baumannii (gram-negative bacteria) were more sensitive toward the EO than the remaining microbes as shown in Table 2. This might be due to the structural differences in the cell wall which changes their susceptibility to different compounds [26]. The inhibition zone diameter increased with the increase in concentration of samples in almost all the strains tested. Kumar et al. [6] 2015 reported the antimicrobial activity of K. rotunda rhizome extract against respiratory tract pathogens and observed significant activity against them. The ethyl acetate extract showed maximum zone of inhibition against the Lactobacillus acidophilus, whereas in a report by Malahayati et al. [27] 2018 resulted that the ethyl acetate extract exhibited maximum zone of inhibition against Staphylococcus aureus and E. coli. Secondary metabolites like phenolics, flavonoids, alkaloids, terpenoids and essential oils are many beneficial plant derived antimicrobial compounds. [28].
Cytotoxic Activity
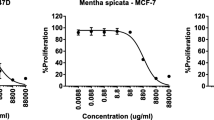
The rhizome EO of K. rotunda was evaluated for its cytotoxic activity against two cell lines, i.e., HeLa and MCF7 by MTT assay. The inhibition percentage increases with increase in the concentration of samples (Fig. 2). The cell inhibition percentage at 10 and 20 μg/ml concentrations is presented in Table 3. At 20 µg/ml concentration, the oil exhibited 75.63% ± 5.66 inhibition against HeLa cell line whereas that of MCF7 cell line was found to be 68.44 ± 2.51 (Table 3). In a report, the chloroform extract of K. rotunda rhizomes was subjected to cytotoxic activity screening against human breast cancer T47D cell line by MTT assay and exhibited significant cytotoxicity against T47D cells with IC50 value more than 10 µg/ml. Chloroform extract of K. rotunda showing their IC50 was 41.72 μg/ml [29]. It appears that the anticancer activities of EOs of K. rotunda against human cancer cell lines are innovative, and further work in this line may give the evidence for the use of this plant against a wide variety of disorders.
Conclusions
The present study deals with the chemical composition and bioactivity studies of K. rotunda rhizome essential oil (EO). The results revealed that this species has certain phytochemical constituents which may be responsible for several bioactivities like antioxidant, antimicrobial and cytotoxic activities. Thus, the identified important constituents of different species may be used as biomarkers for development of chemical fingerprint of respective plant species for authentic identification and quality control of herbal drug. The oil showed good antioxidant and antimicrobial activity which explains its potential against various oxidative diseases and its further utilization as natural antioxidants and antimicrobial agents in food and pharmaceutical industries. Advance work on separation and characterization of compounds of these classes will offer additional data on the dynamic principle accountable for their pharmacological properties. The EOs and the chemical constituents of the Kaempferia species with high bioactive potential can be used for formulation of novel drugs.
References
Wu TL, Larsen K (2000) Zingiberaceae. Flora China 24:322–377
Khare CP (2008) Indian medicinal plants-an illustrated dictionary. Springer, New York, p 352
Kirtikar KR, Basu BD (2006) Indian Medicinal Plants. Lalit Mohan Basu M.B, Allahabad
Lotulung PD, Kardono LB, Kawanishi K (2008) Antioxidant compound from the rhizomes of Kaempferia rotunda L. Pak J Biol Sci 11(20):2447–2450
Mohanty JP, Nath LK, Bhuyan N, Mariappan G (2008) Evaluation of antioxidant potential of Kaempferia rotunda Linn. Indian J Pharm Sci 70(3):362–364
Kumar A, Navneet KS (2015) Antimicrobial activity and phytochemical analysis of Kaempferia rotunda L. rhizomes. Der Pharm Lett 7:389–395
Sini S, Raj G, Shyamal S, Chitra S (2014) Safety assessment of tuberous rhizome of Kaempferia rotunda L. by acute and 28-days repeated dose toxicity studies. Glob J Pharmacol 8(2):128–139
Timai P (2017) Anti-inflammatory and analgesic activity of ethanolic extract of Kaempferia rotunda rhizome in rats. Doctoral dissertation. RVS College of Pharmaceutical Sciences, Coimbatore
Singh CB, Chanu SB, Bidyababy T, Devi WR, Singh SB, Nongalleima K, Lokendrajit N, Swapana N, Singh LW (2013) Biological and chemical properties of Kaempferia galanga L.–a Zingiberaceae plant. NeBio 4(4):35–41
Martinelli L, Rosa JR, Ferreira CSB, Nascimento GML, Freitas MS, Pizato LC, Santos WO, Pires RF, Okura MH, Malpass GRP, Granato AC (2017) Antimicrobial activity and chemical constituents of essential oils and oleoresins extracted from eight pepper species. Ciência Rural 47:5
Sen S, Chakraborty R, Sridhar C, Reddy YSR, De B (2010) Free radicals, antioxidants, diseases and phytomedicine: current status and future prospect. Int J Pharm Sci Rev Res 3:91–100
Tuttolomondo T, La Bella S, Licata M, Virga G, Leto C, Saija A, Trombetta D, Tomaino A, Speciale A, Napoli EM, Siracusa L (2013) Biomolecular characterization of wild Sicilian oregano: phytochemical screening of essential oils and extracts, and evaluation of their antioxidant activities. Chem Biodivers 10:411–433
Wadud APP, Rao MM, Narayana A (2007) Evolution of drug: a historical perspective. Bull Indian Inst Hist Med Hyderabad 37:69–80
Tamrat T, Yesudass DR (2018) A review on anticancer activity of some plantderived compounds and their mode of action. Nat Prod Chem Res 6:4
Aziz ZAA, Ahmad A, Setapar SHM, Karakucuk A, Azim MM, Lokhat D (2018) Essential oils: extraction techniques, pharmaceutical and therapeutic potential-a review. Curr Drug Metab 19(13):1100–1110
Blowman K, Magalhães M, Lemos MF, Cabral C, Pires IM (2018) Anticancer properties of essential oils and other natural products. Evid Based Complement Altern Med. https://doi.org/10.1155/2018/3149362
Xiang H, Zhang L, Yang Z, Chen F, Zheng X, Liu X (2017) Chemical compositions antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind Crops Prod 108:6–16
Woerdenbag HJ, Windono T, Bos R, Riswan S, Quax WJ (2004) Composition of the essential oils of Kaempferia rotunda L. and Kaempferia angustifolia Roscoe rhizomes from Indonesia. Flavour Frag J 19(2):145–148
Guenther E (1972) The production of essential oils. In: Robert E (ed) The essential oils, vol I. Krieger, New York, pp 361–391
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Publishing Corporation, Carol Stream
Sahoo S, Singh S, Nayak S (2014) Chemical composition, antioxidant and antimicrobial activity of essential oil and extract of Alpinia malaccensis Roscoe (Zingiberaceae). Int J Pharm Pharma Sci 6(7):183–188
Clinical and Laboratory Standards Institute (CLSI). (2013). Performance standards for antimicrobial susceptibility testing: twenty-fourth informational supplements M100, 28 ed. 950 West Valley Road, Suite 2500, Wayne, PA 19087 USA
Sahoo S, Kar B, Dash S, Ray M, Acharya KG, Singh S, Nayak S (2018) Anticancerous and immunomodulatory activities of Alpinia nigra (Gaertn.) Burtt. J Essent Oil Bear Plants 21(4):869–875
Kumar A (2014) Chemical characterization of essential oil from the rhizomes of Kaempferia rotunda L. by GC/MS technique. Int J Pharm Bio Sci 5(4):458–462
Koleva II, Beek TAV, Linssen JPH, Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Romaniuk JA, Cegelski L (2015) Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos Trans R Soc Lond B Biol Sci 370(1679):20150024
Malahayati N, Wardani Widowati T, Febrianti A (2018) Total phenolic, antioxidant and antibacterial activities of curcumin extract of Kunci Pepet (Kaempferia rotunda L.). Res J Pharm Biol Chem Sci 9(3):129-135
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Mustahil NA, Sukari MA, Abdul AB, Ali NA, Lian P (2013) Evaluation of biological activities of Alpinia mutica Roxb. and its chemical constituents. J Pharm Sci 26(2):391–395
Acknowledgements
Authors are thankful to Dr. Manoj Ranjan Nayak, President, Siksha O Anusandhan (Deemed to be University) for their support and encouragement throughout.
Author information
Authors and Affiliations
Contributions
SS was contributed to Writing—original draft, conceptualization, formal analysis and Investigation, MD was contributed to formal analysis, methodology and writing. DS was contributed to GC–MS analysis, methodology. SKK was contributed to sample collection, formal analysis and methodology. BK was contributed to visualization, supervision and writing—review and editing. SN was contributed to investigation, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement: The present study revealed the chemical composition of essential oils of K. rotunda rhizomes and its bioactivities toward validating the therapeutic potential.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahoo, S., Dash, M., Sahoo, D. et al. Chemical Composition and Biological Activities of Essential Oil from Kaempferia rotunda. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 93, 669–675 (2023). https://doi.org/10.1007/s40011-023-01458-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-023-01458-3