Abstract
Cellulolytic enzymes have received a lot of attention as prospective candidates for biomass conversion of agricultural byproducts. The current investigation is an attempt to investigate the production of the cellulolytic enzymes from local bacterial strains Bacillus cereus 3SME and Bacillus velezensis 3SME. Various agricultural residues such as rice straw, corn cob, corn stalk, bagasse, and wheat bran were utilized as enzyme production substrates in solid-state fermentation. The results indicated the highest percentage of fermentable sugar was produced from bagasse. B. cereus 3SME and B. velezensis 3SME were able to produce fermentable sugars 56.48 and 53.56 mg/g after 2 days of incubation at pH 6 and 6.5, respectively, at 37 degrees. Bagasse (0.25 g) was used as a carbon source in a 50-ml conical flask and corn steep liquor for both organisms. The functional groups in the presence and absence of both bacteria were studied using FTIR spectroscopy. SEM analysis was studied for the structural transformations of cellulolytic fibers after 2 days of incubation with both bacteria. The HPLC analysis of bagasse that was inoculated by B. cereus 3SME and B. velezensis 3SME indicated the two extracts were composed of glucuronic acid, fructose, glucose, and xylose with a molar ratio of 1:2.6:2.1:2.3 and 0.3:1.5:1:6.2, respectively. Bagasse can be utilized as a low-cost source of carbon for B. cereus 3SME and B. velezensis 3SME to produce hydrolytic enzymes effectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy crops, forest residue, agricultural residues, organic municipal solid waste, and components of wood are all forms of lignocellulosic biomass [1]. Approximately 80% of lignocelluloses contain cellulose (30–50%), hemicellulose (20–35%), and lignin (15–25%) as the main constituents, and this composition varies between plant species and plant tissues [2]. Biomass could be hydrolyzed to fermentable hexose and pentose, which could be used to make a variety of manufactured products food, fuel, paper, and pulp textile industries [3]. The effective hydrolysis of lignocelluloses to fermentable sugars has been a major challenge, various pretreatment techniques have been designed to overcome lignin's reluctance to yield fermentable sugars [1]. Among the well-known treatment, procedures are acid, alkali, ionic liquid, hot water, steam explosion, and ammonia treatments [4]. Low hydrolysis performance, inhibitor formation, lower fermentation efficiency, high waste management costs, insufficient cellulose, lignin separation, consumption of intensive energy, and a lot of chemicals are among the challenges that have yet to be overcome [5]. Biological pretreatments by bacteria, fungi, and enzymes that rely on their ability to break-down lignin and hydrolyze carbohydrates appear to be more eco-friendly because they use less energy and do not need any chemicals [1, 6]. The enzymatic techniques to hydrolyze plant biomass were chosen because of the non-inhibitory by-products and nontoxic effluents [7]. Bacteria, fungi, plants, and some protozoa, mollusks, and nematodes generated cellulase enzymes that hydrolyze the β-1, 4 links within cellulose chains [7, 8]. Bagasse is a lignocellulosic byproduct that was pretreated and enzymatically hydrolyzed to yield sugars, yielding around 40–51% glucose and 26–33% xylose upon completion of hydrolysis [9, 10]. Bacillus species had been found to generate cellulolytic enzymes for the agricultural feedstock hydrolysis, including sugarcane bagasse, into mono sugars [11]. In this work, cellulolytic enzyme-producing microbial isolates from Egyptian soils were screened for potential agricultural residue hydrolysis and could be used as a low-cost source of carbon in commercial production.
Material and Methods
Isolation of Microorganisms
Twelve decaying soil samples were obtained from Egypt across various districts. Clean plastic bags were used to preserve the samples at 4 °C until they were used. The basal medium was used for isolation and purification of cellulases producing fungi according to Gao et al. [12] (g/l): microcrystalline cellulose (27), KH2PO4 (6), (NH4)2SO4 (5), MgSO4.7H2O (1), CaCO3 (2.5), corncob steep liquor (3.3%), and Tween-80 (0.2%) and incubated at 28 °C for 7 days. The obtained growing fungal isolates were maintained on slants of Czapek-Dox medium and kept in a refrigerator at 4 °C for further investigation. The bacteria were isolated using the enrichment method in a minimal slate medium (MSM) according to Ahmed et al. [13] which contained (g/l): K2HPO4 (7), KH2PO4 (2), sodium citrate (0.5), (NH4)2SO4 (1), MgSO4.7H2O (0.1), and 1 cm of dry grass wood (10 g). In a 250 mL Erlenmeyer flask with a pH of 7.0, 5 g of each soil sample was added to 95 ml MSM then incubated at120 rpm and 37 °C for 5 days. One milliliter of the desired dilution was directly transferred into a Nutrient agar medium.
Screening the Cellulases Enzymes Producing Microorganisms
Screening for cellulase-producing microorganisms (fungi and bacteria) was done using iodine on carboxymethylcellulose (CMC) plates according to Kasana et al. [14] which contained (g/l): K2HPO4 (1), NaNO3 (2), KCl (0.5), MgSO4 (0.5), peptone (0.2), CMC sodium salt (2), and agar (17) each isolate was cultured on the medium and incubated for 24 and 72 h at 37 °C and 30 °C in case of bacteria and fungi, respectively, then the plates were flooded with iodine described by Bartholomew et al. [15] which contained (g/l): iodine (3.33), and KI (6.67) which produced a bluish-black complex with CMC and a clear zone surrounding the colonies in 3 to 5 min as an indication of cellulases activity.
Identification of Selected Strains
Morphological (Gram stain, motility, and spore-former), culture characteristics (appearance of the colony surface, color, elevation, edge, pigmentation, and opacity of the bacterial colony), and biochemical characterization (starch hydrolysis, Voges-Proskauer, citrate utilization, nitrate reduction, oxidase, catalase, and acid production from different sugars) of the promising two bacterial strains Sz5 and HII5 that produce the maximum cellulase were determined using established procedures [16] combined with 16S rRNA gene sequencing using universal primers 27F 5′(AGAGTTTGA CCTGGCTCAG)3′ and 1492R 5'(GGTTACCTTGTTACGACTT)3′ PCR products were purified by using a PCR purification kit and sequenced bi-directionally by the SolGent Company (SolGent, Daejeon, Korea). The partial 16S rRNA gene sequences were compared with sequences in the nucleotide data base of GenBank. Multiple alignments of the gene sequences were performed using the CLUSTAL W program in the Mega 6 software package [17]. 16S rRNA gene sequences for type strains (the most closely related species) were obtained from the EZ Taxon-edatabase (http://eztaxon-e.ezbiocloud.net) and NCBI-BLAST programs. The neighbor-joining method with Kimura's two-parameter distance correction model was used to construct phylogenetic trees. Bootstrapping analysis of 1000 replications was performed to estimate the confidence of tree topologies.
Enzymatic Activity of the Bacterial Strains on CMC Media
For the preparation of enzymes, the isolates which showed positive results in enzyme screening were pre-cultured in a medium described by Kogo et al.[18] which contained (g/l): glucose (30), (NH4)2SO4 (6), and corn steep liquor (0.6%) in 50 ml of the medium at 37 °C for 2 days and 120 rpm. The pre-culture was transferred into 100 ml of quantitative screening medium described by Kogo et al. [18] Which contained (g/l): Avicel (9), (NH4)2SO4 (1.8), and corn steep liquor (0.18%) and incubated for 3 days at 37 °C and 120 rpm. The microbial cells were removed from the culture by cooling centrifugation for 20 min at 5000 rpm. The supernatant was used for the determination of CMC-ase, FP-ase, xylanase, β-glucosidase, and cellobiase [21] by the DNS assay [19]. One unit of the enzyme was defined as the amount of enzyme which releases 1 µg of reducing sugars, expressed as glucose, per minute under a given condition.
Enzymatic Activity of the Bacterial Strains on Different Agricultural Byproducts
Five agricultural byproducts samples were collected from districts in Egypt such as corn stalk, wheat straw, corn cobs, bagasse, and rice straw. All agricultural byproducts samples were dried and crushed through a grinder to smaller particle size, with a size range of 0.5 to 2 mm. Then the chemical analysis of different agricultural byproducts moisture, ash, lipid content, lignin, hemicellulose, and cellulose was determined according to Nagah, [20]. Different agricultural residues were used as substrates for the production of cellulases under SSF without any pretreatment. Five grams of each substrate were weighed into 250 ml Erlenmeyer flasks and moistened with mineral salt medium [21] to achieve a moisture content (86%). The mineral salt medium contained (g/l): KH2PO4 (2), (NH4)2SO4 (1.86), urea (0.3) CaCl2 (0.03), MgSO4.7H2O (0.3), peptone (8), yeast extract (4.08) and (mg/l): MnSO4.H2O (1.6), FeSO4.7H2O (5), CoCl2 (2), ZnSO4.7H2O (1.4), and Tween 80 (0.1%) at pH 6 at 37 °C for 3 days. The enzyme from each microbial culture medium was extracted twice with 0.05 M sodium acetate buffer (pH 4.8) and thorough squeezing using a double-layered wet cloth. The extract was centrifuged for 10 min at 5000 rpm and 4 °C and the clear supernatant was used for assay of total reducing sugars and for the cellulase activities of CMC-ase, FP-ase, xylanase, β-glucosidase, and cellobiase [22] by the DNS assay [19]. One unit of enzyme is defined as the amount of enzyme which releases 1 µg of reducing sugar, expressed as glucose, per minute under a given condition.
Optimization for Hydrolysis of Bagasse
The promising bacterial strains (B. cereus 3SME and B. velezensis 3SME) were subjected to different physicochemical and nutritional conditions for hydrolysis of sugarcane bagasse. Subsequently, the medium component studied included the effect of different incubation periods (1, 2, 3, 4, and 5 days), different pH (5.5, 6, 6.5, 7, and 7.5), different temperatures (25, 30, 35, 37, 40, and 45 °C), and different inoculum size 100, 150, 200, 250, and 300 µl/g. Bagasse weight 0.25, 0.50, 1.00, 1.25, and 1.50 g using 50 ml Erlenmeyer flasks. Corn steep liquor as cheap nitrogen instead of peptone and yeast extract (25, 50, 75, 100%). All the experiments were carried out in a 50-ml Erlenmeyer flask containing one gram of untreated sugarcane bagasse and mineral salt medium [21] to achieve 86% initial moisture content in triplicate.
Fourier Transforms Infrared (FTIR) Spectroscopy
According to El-Gamal et al. [23], the chemical structures and functional groups of bagasse were determined. Adsorption and reactions on surfaces have been investigated extensively using Fourier transform infrared attenuated total reflection spectroscopy (FTIR-ATR). A JASCO-ATRFT/IR-6100 Fourier transform infrared spectroscope was used to capture the infrared spectra. The ATR crystal (2 mm/sec) was utilized with resolutions of 4 cm−1, the spectral range is 4000 to 400 cm−1.
Scanning Electron Microscopy (SEM) Analysis
A scanning electron microscope (SEM FEG Quanta 250 Czech) was used to investigate the surface morphology of bagasse that operated at 20 kV without any need for gold sputtering coating at a ratio of 56×-3000×. Firstly, to gain information on the shape distribution of particles in the observation, low magnification photographs were obtained. After that, greater magnification was used to focus on common surfaces. The SEM photographs illustrate how the raw structure can be expanded throughout the treatment.
Determination of Surges Content by Using HPLC
Sugars caused by the enzymatic actions of the B. cereus 3SME and B. velezensis 3SME strains for bagasse were determined using high-performance liquid chromatography (HPLC) after one millimeter of bagasse extraction was filtered through a double-layered wet cloth by squeezing (HPLC). Each sample was filtered via 0.22 µm Millipore membranes in a separate aliquot. An Agilent model 1100 Series (Agilent, USA) high-performance liquid chromatography equipped with a quaternary pump, refractive index detector, and Sim-pack SCR-101 N (300 mm L. × 7.9 mm I.D., 10 µm). The mobile phase was deionized water, degassed under vacuum in an ultrasonic bath. The flow rate was 0.7 ml/min at a temperature of 40 °C. The quantification was achieved by comparison with analytical curves using sugars standards from Supelco.
Results and Discussion
Cellulase Enzymes Production Isolation and Screening
Based on the different morphological characteristics that appeared on a basal medium total of 89 and 68 fungal and bacterial isolates, respectively, were obtained from various decayed soil samples from Egypt. Screening of the cellulase-producing bacterial and fungal isolates was carried out on a CMC agar plate using an iodine solution appeared HII5 and Sz5 bacterial isolates were selected. Our finding similar to another study that reported that after a certain time of incubation, clearing zones around microbial growth colonies showed their ability to produce cellulases [24, 25].
Bacterial Isolates Identification
The morphological, culture, and biochemical in Table 1 isolate Sz5 negative for starch hydrolysis, nitrate reduction, and citrate utilization while positive for oxidase, catalase, and Voges-Proskauer test. For isolate HII5 for starch hydrolysis test, oxidase test, and catalase test, nitrate reduction, and Voges-Proskauer test were positive, whereas the citrate test was negative and they were proven to be Bacillus species. The partial 16SrDNA sequence was evaluated in comparison to databases in the GenBank. Bacillus cereus 3SME and Bacillus velezensis 3SME were identified as the isolates and Gene Bank accession numbers MW522550 and MW523035, respectively, were given to the 16S rRNA sequences. Using the neighbor-joining approach, a phylogenetic tree was created (Fig. 1). Several investigations with cellulases production from bacteria had been used the molecular technique for their identification. The phylogenetic tree based on different species of Bacillus was constructed using the neighbor-joining method similar to Kanmani et al. [26] that isolated 52 bacteria from sediment and water samples.
Enzymatic Activity of the Bacterial Strains
Samples of agricultural residues such as corn stalk, wheat straw, corn cobs, bagasse, and rice straw were collected from Egyptian farms. Table 2 presents their chemical contents determination. The promising 2 strong positive microbial isolates B. cereus 3SME and B. velezensis 3SME were selected for growing on different five agricultural residues and CMC media were utilized as substrate for the cellulases production under solid-state fermentation without using any chemical pretreatment and the microbial isolates were inoculated. Results in Tables 3 indicated that the two bacterial isolates are the best hydrolysis of the bagasse residues and the total fermentable sugars by using B. cereus 3SME and B. velezensis 3SME were 26.57 and 27.98 mg/g of bagasse after 3 days, respectively. Similar that Namnuch et al. [3] was reported that lignocellulosic residues bagasse, rice straw, sawdust, rice bran, and commercial xylan and CMC substrates used in submerged fermentation for Aspergillus flavus KUB2 cultivation. In addition, the B. subtilis CD001 was discovered to hydrolyze the bagasse [11].
Optimization for Hydrolysis of Sugarcane Bagasse
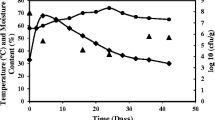
To improve enzyme production and make the process cost-effective, process parameters must be optimized. The media optimization technique for Bacillus species such as B. amyloliquefaciens SA5, B. megaterium BMS4, and B. subtilis BTN7 had shown that pH-7.0 is the best for cellulase production [27]. B. cereus 3SME and B. velezensis 3SME were exposed to a variety of culture conditions to determine the most effective parameters for cellulase production. The incubation time was gradually increased from 24 to 48 h, resulting in a significant increase in cellulase activity. Following that, a decrease in enzyme synthesis as the incubation time was extended above 2 days, as shown in Fig. 2a. The maximum total fermentable sugars after 2 days for B. cereus 3SME and B. velezensis 3SME were recorded at 33.42 and 30.08 mg/g, respectively. For the bacteria in this study, the maximum total fermentable sugars for both bacteria were 33.42 and 30.08 mg/g, respectively, at 37 °C in Fig. 2b and the production of enzymes was lowered as the temperature increased. The two strains in Fig. 2c showed the maximum total fermentable sugars were 42.19 and 36.94 mg/g at pH 6 and pH 6.5, respectively. Figure 2d shows the maximum total fermentable sugar productivity was 48.15 and 48.21 mg/g from B. cereus 3SME and B. velezensis 3SME, respectively, at inoculum sizes of 250 µl and 300 µl. The observed results are similar to B. subtilis SV1 cellulase production [28] and B. subtilis M-11 [29]. Figure 2e shows the best total fermentable sugars productivities were 56.48 and 51.25 mg/g for B. cereus 3SME and B. velezensis 3SME, respectively, at 0.25 g of bagasse in 50 ml Erlenmeyer flasks, and Fig. 2f for nitrogen source using different concentrations of corn steep liquor as cheap nitrogen instead of peptone and yeast extract (25, 50, 75, 100%) the results as the same so using the corn steep liquor as cheap nitrogen source.
Sugarcane Bagasse Structural Change
After two days of bacterial cultivation, the SEM and FTIR spectroscopy studies were carried out to analyze the major structural changes induced by the catalytic enzymatic actions of the B. cereus 3SME and B. velezensis 3SME strains in bagasse. To determine the qualitative of lignocellulose materials, FTIR is a well-known approach. Peaks that are correlated with lignin, hemicellulose, and cellulose showed the β-glycosidic linkage at peak 898 cm−1, crystalline cellulose at 1098 cm−1, in lignin and xylan, syringyl ring and C-O expanding at 1266 cm−1, lignin aromatic skeleton vibrations at peak 1515 cm−1, Stretching of CH2 deformation in lignin and xylan at peak 1457 cm−1, and the unconjugated C=O in xylan at peak 1730 cm−1 [3, 30]. Figure 3 shows the FTIR spectral results of the spectra analysis corresponding to lignin, cellulose, and hemicellulose substrates changes without bacterial inoculation compared with bacterial cultivation. Similar FTIR results studied by Namnuch et al.[3] for sugarcane bagasse hydrolysis by A. flavus KUB2. Figure 4 shows SEM scans of bagasse structure morphology after cultivation with B. cereus 3SME and B. velezensis 3SME strains, as well as bacterial inoculation compared with bacterial cultivation. The surface of the control bagasse was smooth and flat. Bagasse, on the other hand, developed fissures and a large number of visible holes after culture with the B. cereus 3SME and B. velezensis 3SME strains as well as bagasse without any inoculation as a control the results similar to pore formation in the bagasse after culture with A. flavus KUB2 after 21 days of solid fermentation was recorded by Namnuch et al.[3].
Determination of Surges Content by Using HPLC
HPLC analysis of bagasse inoculated by the B. cereus 3SME and B. velezensis 3SME strains and the two extracts composed of glucuronic acid, fructose, glucose, and xylose with a relative molar ratio of 1:2.6:2.1:2.3 for B. cereus 3SME and 0.3:1.5:1:6.2 for B. velezensis 3SME. similar to the previous sugarcane bagasse enzymatic hydrolysis indicated that composed of glucose 40–51% and xylose 26–33% [9, 10].
Conclusions
B. cereus 3SME and B. velezensis 3SME isolated from Egyptian soil were found to be potential producers of cellulolytic enzymes. After two days of fermentation, sugarcane bagasse proved to be a potential source of carbon for submerged culture resulting in high enzyme activity. Using bagasse as an inexpensive carbon source and corn steep liquor as a supplement source, variables in the process were improved, resulting in a considerable increase in fermentable sugars yield.
Data Availability
Not applicable.
References
Tsegaye B, Balomajumder C, Roy P (2018) Biodegradation of wheat straw by Ochrobactrum oryzae BMP03 and Bacillus sp. BMP01 bacteria to enhance biofuel production by increasing total reducing sugars yield. Environ Sci Pollut Res 25(30):30585–30596
Zhang Y et al (2020) Low-cost cellulase-hemicellulase mixture secreted. Carbohydr Act Enzym Struct Act React Prod 21:51
Namnuch N, Thammasittirong A, Thammasittirong SN-R (2021) Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology 12(2):119–127
Smuga-Kogut M et al (2017) The use of ionic liquid pretreatment of rye straw for bioethanol production. Fuel 191:266–274
Tomás-Pejó E et al (2017) Valorization of steam-exploded wheat straw through a biorefinery approach: bioethanol and bio-oil co-production. Fuel 199:403–412
Chen Y et al (2015) Study of the rice straw biodegradation in mixed culture of Trichoderma viride and Aspergillus niger by GC-MS and FTIR. Environ Sci Pollut Res 22(13):9807–9815
Madhavan A et al (2012) Bioconversion of lignocellulose-derived sugars to ethanol by engineered Saccharomyces cerevisiae. Crit Rev Biotechnol 32(1):22–48
Singh A et al (2021) An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour Technol Rep. https://doi.org/10.1016/j.biteb.2021.100652
Ngamsirisomsakul M, Reungsang A, Kongkeitkajorn MB (2021) Assessing oleaginous yeasts for their potentials on microbial lipid production from sugarcane bagasse and the effects of physical changes on lipid production. Bioresour Technol Rep 14:100650
Frassatto PAC et al (2021) β-Glucosidase production by Trichoderma reesei and Thermoascus aurantiacus by solid state cultivation and application of enzymatic cocktail for saccharification of sugarcane bagasse. Biomass Convers Biorefin 11(2):503–513
Malik WA, Khan HM, Javed S (2021) Bioprocess optimization for enhanced production of bacterial cellulase and hydrolysis of sugarcane bagasse. BioEnergy Res 2021:1–14
Gao L et al (2013) Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Biores Technol 147:658–661
Ahmed AAQ, Babalola OO, McKay T (2018) Cellulase-and xylanase-producing bacterial isolates with the ability to saccharify wheat straw and their potential use in the production of pharmaceuticals and chemicals from lignocellulosic materials. Waste Biomass Valoriz 9(5):765–775
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Current Microbiol 57(5):503–507
Bartholomew JW, Mittwer T (1952) The gram stain. Bacteriol Rev 16(1):1–29
Sneath PH (1986) Endospore-forming gram-positive rods and cocci. Bergey’s Manual Syst Bacteriol 2:1104–1207
Weisburg WG et al (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Kogo T et al (2017) Production of rice straw hydrolysis enzymes by the fungi Trichoderma reesei and Humicola insolens using rice straw as a carbon source. Biores Technol 233:67–73
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Nagah MM (2017) Conversion of some agricultural residues to fermentable sugars for bioethanol production. In: MC of science, Al Azhar university
Narra M et al (2012) Production of cellulases by solid state fermentation with Aspergillus terreus and enzymatic hydrolysis of mild alkali-treated rice straw. Biores Technol 121:355–361
Shawky B, Hickisch B (1984) Cellulolytic activity of Trichoderma sp. strain G, grown on various cellulose substrates. Zentralbl Mikrobiol 139(2):91–96
El-Gamal R et al (2018) Ftir analysis for the evaluation of some triazole fungicides for the treatment of wooden artifacts. Mediterr Archaeol Archaeom. https://doi.org/10.5281/zenodo.1297161
Sreedevi S, Sajith S, Benjamin S (2013) Cellulase producing bacteria from the wood-yards on Kallai river bank. Adv Microbiol 3(04):326
Nagah M et al (2016) Qualitative and quantitative screening of cellulases from different local Egyptian fungal strains. Middle East J Appl Sci 6(3):579–587
Kanmani R et al (2011) Studies on detergent additives of protease enzyme from an estuarine bacterium Bacillus cereus. Int Res J Biotechnol 2(7):157–163
Hussain AA et al (2017) Optimization and molecular identification of novel cellulose degrading bacteria isolated from Egyptian environment. J Genet Eng Biotechnol 15(1):77–85
Nargotra P, Vaid S, Bajaj BK (2016) Cellulase production from Bacillus subtilis SV1 and its application potential for saccharification of ionic liquid pretreated pine needle biomass under one pot consolidated bioprocess. Fermentation 2(4):19
Özmen I (2020) Optimization for coproduction of protease and cellulase from Bacillus subtilis M-11 by the Box-Behnken design and their detergent compatibility. Braz J Chem Eng 37(1):49–59
Corrêa RCG et al (2016) Spent mushroom substrate of Pleurotus pulmonarius: a source of easily hydrolyzable lignocellulose. Folia Microbiol 61(5):439–448
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
Not applicable.
Ethics Approval and Consent to Participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement Cellulolytic enzyme-producing from local bacterial isolates using to hydrolysis the agricultural residues and could be used as a low-cost carbon source used in economical production.
Rights and permissions
About this article
Cite this article
Abdelhamid, S.A., El-Shatoury, E.H., Asker, M.S. et al. Hydrolysis of Cellulose Rich Agricultural Waste Using Two Potent Local Bacterial Isolates. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 93, 225–234 (2023). https://doi.org/10.1007/s40011-022-01416-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-022-01416-5