Abstract
The study aims to investigate and compare the proximate composition and the fatty acid profiles of Allium orientale Boiss., Eremurus spectabilis M. Bieb., Anchusa officinalis L. and Arum elongatum Steven collected in spring 2020 and 2021. C16:0, C18:0, C18:1ω9 and C18:2ω6 were found major fatty acids in four species. Essential fatty acids C18:2ω6 (34.4 and 31.2%) and C18:3ω3 (21.4 and 16.5%) were detected significantly high in A. elongatum in both years, respectively. The proportion of toxic C22:1ω9 in E. spectabilis (4.6 and 4.1%) was found close to the danger limit (5%). The C18:1ω9 level in A. orientale (27.3 and 29.9%) and A. officinalis (33.2 and 29.51%) was significantly higher than the C18:1ω9 level in E. spectabilis and A. elongatum in both years. C24:1ω9, involved in the biosynthesis and maintenance of nerve cell myelin, was found in relatively good levels in A. orientale (2.1 and 1.9%) and A. officinalis (2.8 and 2.2%). The ∑SFA level was found to be higher in A. orientale (53.9 and 52.1%), E. spectabilis (52.4 and 55.82%) and A. officinalis (48.9 and 50.80%) compared to their ∑MUFA and ∑PUFA levels in both years. Conversely, in A. elongatum, the ∑PUFA ratio was found to be quite high (55.8 and 47.7%). The total lipid level varied between 1% and 0.4% in 2020, and 0.6% and 0.9% in 2021. Crude protein content was found to be low, around 5% on average in both years. As a result, the study clarified that although the plant fatty acid profiles are quantitatively different, they are qualitatively similar, and that the species data will also contribute to the literature and conscious consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edible wild edible plants are used as food and traditional medicine for their beneficial ingredients such as vitamins, antioxidants, antimicrobial components, essential fatty acids, omega fatty acids, lipids, minerals, bioactive compounds, proteins, amino acids, carbohydrates, secondary metabolites, riboflavin, and folic acid. Allium orientale Boiss., Eremurus spectabilis M. Bieb., Anchusa officinalis L. and Arum elongatum are edible wild plants used for various purposes. A. orientale (Eastern onion) native to the eastern Mediterranean, Egypt, the Levant, Libya, Cyprus, Sinai and Turkey, is a species of the Alliaceae family, and its bulb and leaves are used as vegetable in many traditional dishes. Its flowers are also eaten as an accompaniment to salads. In most rural areas, people use it as an antimicrobial medicine due to its high antioxidant and antibacterial content [1]. E. spectabilis (Foxtail Lilly), native to Turkey, Iran, Lebanon, Iraq, Ukraine, Syria, northern Caucasus, Palestine, South European Russia, Transcaucasia and Turkmenistan, is a member of the Liliaceae family and its short stems and leaves are traditionally cooked as vegetables in a wide variety of dishes. Cell-based studies have shown it to be effective in prostate cancer, breast cancer, antifungal and antimicrobial activities and increase white blood cell count [2, 3]. A. officinalis (Alkanet) is a species belonging to the family Boraginaceae and its native range extends from Europe to the Caucasus. Its leaves and young shoots are cooked and eaten like spinach. In parts of Anatolia, it is believed to have a calming and relaxing effect by wrapping its powdered forms in wet cloth and placing it on the forehead of epileptic patients [4]. A. elongatum (Cuckoo pint) is a species in the Araceae family and is distributed in Turkey, Bulgaria, Ukraine, Greece, Palestine, Crimea, southern Russia, northern Caucasus and Transcaucasia. Species of the Araceae family are known as medicinal plants and have high antioxidant effect [5]. The sap of the plant is drunk to ease the pain of women giving birth in Anatolia and is considered beneficial to postpartum women for regeneration [4].
A lot of research has been done on fatty acids and the effects of fatty acids, especially essential and omega fatty acids on the human body have been proven by most of the studies. Many fatty acids have important contributions to the human body. For example, long-chained omega-3 fatty acids are essential for growth and development and play an important role in the prevention and treatment of coronary artery disease, hypertension, diabetes, arthritis, inflammatory diseases, autoimmune diseases and cancer [6, 7]. In addition, fatty acids participate in the structure of the lipid bilayer of the membrane, in the biosynthesis of eicosanoids, in the transfer of the cell signal, in the expression of genes, in the hormonal functions, etc. [7, 8]. The essential fatty acids, linoleic acid (LA, C18:2ω6) and α-linolenic acid (ALA, C18:3ω3), are mainly involved in the synthesis of the cell membrane, in the development of the brain and the nervous system, in the functions of the thyroid and adrenal glands, in the production of hormones, the regulation of blood pressure, liver functions, the transport of cholesterol in the blood, the coagulation of blood and the regulation of immune and inflammatory responses [7, 9]. The examination of these four edible plants has been considered important because of their popularity in traditional medicine, their high consumption, wide distribution, easy availability and many previously reported medicinal benefits.
In this study, the proximate compositions and fatty acid profiles of edible leaves of medicinal and nutritional plants A. orientale, E. spectabilis, A. officinalis and A. elongatum consumed by most of the local population (mainly in the Middle East, Near East and Anatolia) are presented by comparing two consecutive years.
Material and Method
Sample collection and Preparation
Plant materials were harvested in fresh, edible form during the first two weeks of growth. A. orientale was collected from the village of Balveren (Şırnak) in April 2020 and 2021 (altitude 1342 m, coordinates: 37° 29′ 3.6960″ N and 42° 32′ 57.7464″ E); E. spectabilis was collected from the west of Mount Namaz in May 2020 and 2021 (altitude 1760 m, coordinates: 37° 32′ 19″ N and 42° 29′ 30″ E); A. officinalis and A. elongatum were harvested in March 2020 and 2021 in Kumçatı (altitude 522 m, coordinates: 37° 27′ 57.2832″ N and 42° 17′ 17.2896″ E). The plant species were brought to the laboratory and photographed with a high resolution camera. Then they were tagged and identified based on Turkish flora and plants databases. In addition, for the identification of the species, the assistance of expert biologists in diagnosis and taxonomy was requested. The preserved specimens are labeled according to the techniques of the herbarium. The edible parts (leaves) of the individual plants were separated and cleaned with water and the external moisture was wiped off with a dry cloth and then stored in the freezer at − 20 °C. The edible and consumed parts of plants have been studied as a whole.
Fatty Acid Methylation and Total Lipid Extraction
Approximately 2.5–3 g of samples was used for the analysis of total lipids. The samples were weighed into a 250 ml flask and 100 mL of a 4 M HCl solution was added. Acid hydrolysis was carried out by boiling on a hot plate for 1 h. The hydrolyzed sample was filtered through filter paper and washed with hot water until its acidity was eliminated. The filter papers were extracted in the apparatus for 3 h, after 20 min of washing and 20 min of evaporation, they were brought to constant weight in the oven at 105 °C for 1 h and the final weighing was carried out.
For fatty acid metal esters (FAME), the sample was placed in a Falcon tube. Ether was added thereto and shaken vigorously with a vortex. Then the Falcon tube was centrifuged. The upper phase was evaporated at low temperature with nitrogen. 8-9 drops of the oil obtained were placed in a 15 mL Falcon tube. 0.6 mL of 2 M KOH and 10 ml of nHeptane were added thereto. After mixing the sample with a vortex, some of the upper phases were added to the vial and prepared for analysis.
Gas Chromatography-Flame Ionization Detector (GC-FID) Conditions
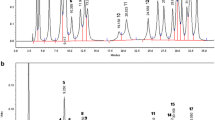
The determination of FAME was carried out in the flame ionization detector (FID) HP-88 of the gas chromatograph AGILENT 7890B, 100 m × 0.250 mm × 0.2 µm (maximum temperature 260 ºC). The detector temperature was 255 °C; 50:1 split ratio. Gas flows, He (29.061 mL/min), H2 (30 mL/min), Dry air (400 mL/min); inlet temperature 250 °C, Column temperature (oven): 130 °C, holding time 0 min; 170 °C at 4 °C/minute; 1.7 °C/minute to 215 °C, retention time 20 min; from 240 to 100 °C/minute, holding time 1 min; total analysis time: 57.7 min. The sample was injected into the instrument at 0.2 µl. A mixture of FAME (Sigma-Aldrich chemicals) was used as a standard for the identification of fatty acid metal esters. FAME chromatograms and total amounts of fatty acids were obtained from the computer program HP 3365 ChemStation (Chem32). Peaks on the chromatograms of the analyzed samples were identified by comparing the methyl ester retention times of all the fatty acids in the standard. The results are given in qualitative fatty acid value. Each measurement was performed in triplicate and the results were averaged.
Analysis of Proximate Composition
The protein content (nitrogen) was determined according to the Dumas method [10]. The analysis was carried out on a Velp brand, Dumas nitrogen analyzer NDA 701 device. Combustion tube operating temperature 900 °C, reduction tube operating temperature 650 °C, average gas pressure analysis 850 mbar, flow rates of gas (mL/min); MFC1 He 190 mL/min, MFC2 He 220 mL/min, MFC1 O2 400 mL/min. EDTA (ethylenediaminetetraacetic acid) was used for calibration and verification. About 100 mg of the sample was weighed into aluminum foil. Then the sample was placed in the autosampler of the device. The device was ignited in the combustion tube at approximately 900 °C, and then the released nitrogen was reduced in the reduction tube. A nitrogen peak has occurred in the detector. The results were read in % nitrogen and multiplied by the appropriate conversion factor to calculate the protein level. The moisture percentages (%) were calculated using the technique of drying the sample for 18 h in an oven (WISD/WON-105) at 103 °C. The ash content (%) was calculated by analysis for 12 h in the oven (Prothem/PLF 110/15) at 550 °C. Each measurement was performed in triplicate, and the results were averaged.
Statistical Analyses
The statistical program (SPSS 12.0) was used for the analysis data. All analytical values were performed in triplicate. Mean values were calculated with these three replicates. Statistical analyses of the proportions of fatty acids were carried out by analysis of variance (ANOVA), and comparisons between means were carried out with Tukey’s test. Differences between means were assessed as significant at p ≤ 0.05.
Result and Discussion
Fatty Acid Composition
In the fatty acid analysis of A. orientale, E. spectabilis, A. officinalis and A. elongatum, Caproic acid (C6:0), Caprylic acid (C8:0), Capric acid (C10:0), Lauric acid (C12:0), Myristic acid (C14:0), Palmitic acid (C16:0), Margaric acid (C17:0), Stearic acid (C18:0), Arachidic acid (C20:0), Lignoceric acid (C24:0) and Behenic acid (C22:0) were detected as saturated fatty acids (SFA). Palmitoleic acid (C16:1ω7), Heptadecenoic acid (C17:1ω8), Oleic acid (C18:1ω9), Eicosenoic (Gadoleic) acid (C20:1ω9), Nervonic acid (C24:1ω9) and Erucic acid (C22:1ω9) were detected as monounsaturated fatty acids (MUFA). Linoleic acid (LA, C18:2ω6), α-Linolenic acid (ALA, C18:3ω3) and Docosadienoic acid (C22:2ω6) were determined as polyunsaturated fatty acids (PUFA).
In several studies, the fatty acid composition of wild plants has been investigated and the components C16:0, C18:0, C18:1ω9, C18:2ω6 and C18:3ω3 have been determined as major. For example, in a study of the Lamiaceae family, the major fatty acids were determined as C16:0 (13.49-27.71%), C18:2ω6 (10.85-19.47%) and C18:3ω3 (40.68-56.53%), it is also pointed out that C16:0 was found as the main fatty acid in all taxa [11].
In another study, the seed oil samples from 57 mustard genotypes belonging to six species, (Brassica juncea, B. rapa, B. napus, B. nigra, B. arvensis and B. alba) C18:1ω9 (7.42-24.54%) and C18:2ω6 (9.61-25.11%) were determined as major components [12]. Besides, in the study of the fatty acid composition of the seeds of wild plants Carapa grandiflora sprague, C. procera, Maesopsis eminii, Pentaclethra macrophylla, Myrianthus holstii, M. arboreus, Tephrosia vogelii, Millettia dura, Podocarpus usambarensis, Cardiospermum halicacabum and Treculia africana, C16:0, C18:0, C18:1ω9, C18:2ω6 and C18:3ω3 have been reported to be dominant components [13]. The fatty acid composition of the seeds of four Anchusa species (A. azurea var. azurea, A. leptophylla, subsp. leptophylla, A. arvensis, subsp. orientalis and A. undulata subsp. hybrida collected from different parts of Turkey was investigated [14], and it was stated that C16:0 (4.4-16.3%), C18:1ω9 (29-42.7%), C18:2ω6 (12.5-32%), C18:3ω3 (0.3-10.8%) and C22:1ω9 (3.4-17.9%) were the major fatty acids [14]. In a study of Allium species, the fatty acid compositions of 10 different A. cepa’s seeds were investigated, and A. cepa’s seeds oil were rich in C18:2ω6 (49.42-60.66%), followed by C18:1ω9 and C16:0, respectively [15]. In another study on A. officinalis, the fatty acid composition of the seeds of the species was examined [16]. The species was examined in two groups and the samples were harvested from wild areas in Turkey's Marmara region, and dominant fatty acids were reported as C18:2ω6 (24.4–25.8%), C18:1ω9 (23.2-25.5%), C18:3ω3 (14.5–15.1%) and C18:3ω6 (12.5–12.6%) [16]. Additionally, significant differences were reported between the percentages of ∑PUFA (56.27–56.41%), ∑MUFA (26.47–31.38%) and ∑SFA (10.12–11.95%) [16]. The results of the present study indicated that major fatty acids were also C16:0, C18:0, C18:1ω9, C18:2ω6 and C18:3ω3 in A. orientale, E. spectabilis, A. officinalis and A. elongatum in both 2020 and 2021 (Tables 1 and 2). C16:0 was found at over 30% in A. orientale, E. spectabilis and A. officinalis in both 2020 and 2021 (Table 1), while in A. elongatum it was found at a level of 15.8% (p ≤ 0.05) in 2020 and 21.8% in 2021 (Table 2). The C18:0 level did not exceed 13% in four species in both years. C18:1ω9 was detected in A. orientale at 27.3% in 2020 and 29.9% in 2021, and in A. officinalis this component was detected in a proportion of 33.2% in 2020 and 29.51% in 2021. The major and minor fatty acid profiles of the species, which were determined at two consecutive years, were close to each other. This result indicates that the species probably exhibit the same physiological behavior each spring. Differences in species fatty acid profiles are known to be caused by most external factors such as season, soil structure and components, nutrients, minerals, sunlight, amount of water, temperature, etc. Considering the levels of ∑SFA ∑MUFA and ∑PUFA and major fatty acids, significant differences were observed compared to previous studies mentioned above. This should be considered normal as seeds contain a higher percentage of ∑PUFAs than leaves and other tissues. In addition, different fatty acid profiles can be observed among the tissues of wild species. The studies cited above are mostly related to the fatty acid composition of seeds or the inedible part of wild plants. However, local people never consume the seeds of A. orientale, E. spectabilis, A. officinalis and A. elongatum as food. Rural people harvest vegetative forms of these therapeutic and nutritional species from the wild in the spring and use them in many types of dishes and treatments. Therefore, studying the fatty acid content of the edible part of the species will contribute to the nutritional value of the species, human health and diet.
The level of ∑SFA was found to be higher in A. orientale (53.9 and 52.1%), E. spectabilis (52.4 and 55.82%) and A. officinalis (48.9 and 50.80%) in 2020 and 2021, respectively (Table 1, 2). However, in A. elongatum, the level of ∑PUFA was found to be quite high (55.8 and 47.7%) in 2020 and 2021, respectively (Table 1, 2). A high level of ∑PUFA is completely considered affirmative for the reduction of serum cholesterol and atherosclerosis, the preventing heart disease, the synthesizing of mediators for brain biochemistry [15]. In contrast, the level of ∑PUFA was found to be quite low in A. orientale (8.4 and 11.9%) and A. officinalis (9.5 and 13.1%) in 2020 and 2021, respectively (Table 1, 2). The levels were significantly different (p ≤ 0.05) from A. elongatum. In particular, it has been reported that growing at high temperatures like the Mediterranean area during vegetation stimulates the synthesis of PUFA particularly C18:1ω9 and inhibits the synthesis of C18:2ω6 [17].
The high percentage of C18:2ω6 (34.4 and 31.2%) and C18:3ω3 (21.4 and 16.5%) in A. elongatum would be associated with the harvesting the plant in March (still cold) month. As known, herbaceous plants in cold season generally tend to store PUFA rather than SFA [17]. On the other hand, the high content of C18:1ω9 MUFA (27.3 and 29.9%) in A. orientale would be related to the hot season because A. orientale was harvested at the end of April (hotter than March in the collected area). But above all, the fatty acid content of a plant varies according to its genetic heritage and many environmental conditions. In support of this, significant differences in the fatty acid profiles of some plants grown in ecological regions located in different latitude zones have been reported [18]. Increasing temperatures in southern latitudes have been reported to encourage plants to synthesize less than C18:2ω6 and more than C18:1ω9 [18].
C16:0 is one of the most abundant components in organisms. The high level of C16:0 in A. orientale, E. spectabilis, A. officinalis and A. elongatum is usual. As known C16:0 is used as the main source of energy needed by cells to fulfill their biological functions [19]. Additionally, C16:0 taken with food is used as a building block in the synthesis of other fatty acids and phospholipids, which are the structural building blocks of cell membranes [19]. In the current study, the high level of C16:0 was expected, because this fatty acid is commonly present in the seeds and tissues of many plants [11, 13, 16].
It is a remarkable finding that C18:1ω9, which plays a crucial role in the synthesis of essential fatty acids in plants [20], is high in A. officinalis and A. orientale (Table 1, 2). Foods high in C18:1ω9 have been reported to have very important human health benefits [21]. In particular, C18:1ω9 is one of the most concentrated fatty acids in the human body [19, 20]. This component makes up about half of the fatty acids in organisms [19, 21]. Many studies have pointed out that it lowers blood pressure and has anti-inflammatory effects[19, 21], lowers the level of cholesterol and low-density lipoprotein (LDL) and increases levels of high-density lipoprotein (HDL) in the blood [21]. The concentrations of C18:1ω9 in A. orientale, E. spectabilis, A. officinalis and A. elongatum were 27.3 and 29.9%, 7.9 and 12.2%, 33.2 and 29.51%, 17.8 and 19.9% in 2020 and 2021 respectively. This component was found in close proportions in two consecutive years. The small fluctuations in C18:1ω9 for the species studied may be due to environmental factors.
The high content of essential fatty acids C18:2ω6 (34.4% in 2020 and 31.2% in 2021) C18:3ω3 (21.4% in 2020 and 16.5% in 2021) in A. elongatum were significant results. The level of C18:2ω6 in the other three species was detected in moderate amount between 7.2% and 10.02% in both years. In a study on the fatty acid composition of mustard genotypes of Brassica species, it was reported that the levels of C18:2ω6 and C18:3ω3 were about 20% and 10%, respectively [12]. In the study of the fatty acid of the seeds of 14 common wild plant species from 3 different families (Brassicaceae, Dipsacaceae and Asteraceae), C18:2ω6 and C18:3ω3 were reported to be between 7.04% and 66.22%, and 8.33% and 38.93%, respectively [22]. In the current study, the level of C18:3ω3 (18.7% in 2020 and 15.1% in 2021) in E. spectabilis differed significantly (p ≤ 0.05) from A. orientale and A. officinalis. This component increased to 3.1% in 2021 in A. officinalis; however, the last year, it was found that the C18:3ω3 content was 0.5% for the same species. There is a possibility that this increase is due to internal or external factors. The benefits of C18:2ω6 and C18:3ω3 (essential fatty acids) for human health have been described in many studies [6, 9, 19]. Today it is known that omega-3 fatty acids (especially C18:3ω3) are essential for growth and development and can play an important role in the prevention and treatment of coronary artery disease, hypertension, diabetes, arthritis, inflammatory diseases, autoimmune diseases and most cancers [6, 19]. In addition, C18:3ω3 is a pioneer in the synthesis of eicosapentaenoic acid (C20:5ω3), docosapentaenoic acid (DPA, C22:5ω3) and docosahexaenoic acid (DHA C22:6ω3), [20, 23] three types of long-chain omega-3 -fatty acids, which have vital roles in brain function, coronary artery disease, body development, cardiovascular and inflammatory responses, and most tissue function [9, 19, 23].
The trans fatty acid isomers (C18:1ω9-trans, C18:2ω6-trans and C18:3ω3-trans) were not detected in the tissues of A. orientale, E. spectabilis, A. officinalis and A. elongatum. Trans fatty acids have been shown to cause stress, preterm labor and miscarriage, and can cause serious problems during lactation, including milk production, immune system disorders, and even diabetes [24].
The harmful fatty acid C22:1ω9 occurs naturally in various plants along with other fatty acids and high levels of C22:1ω9 have been shown to be harmful to the body [25]. The fact that the level of C22:1ω9 (4.6% in 2020 and 4.1% in 2021) (Table 1, 2) in E. spectabilis is close to the danger limit indicates that when consumed Caution is advised. According to the regulations of the Turkish Food Codex Contaminants Regulation the maximum limit of C22:1ω9 is set at 5% in total fatty acids of vegetables and 10% in other foods (Turkish Ministry of Agriculture and Rural Affairs, Notice No.: 2008/26).
Research has found that vegetable oil containing greater than 5% C22:1ω9 causes significant myocardial fat accumulation and cardiac tissue damage in experimental animals [25]. Fat accumulation in the myocardium has been reported to result from the negative effect of C22:1ω9 on enzymes that break down fat [25]. For this reason, foods and vegetables that contain little or no C22:1ω9 are now preferred in many countries. The level of toxic C22:1ω9 in E. spectabilis (4.6% in 2020 and 4.1% in 2021) was found close to the danger limit (5%). However, the rural population consumes E.spectabilis unknowingly, unaware that the C22:1ω9 content is near the dangerous level. Although the C22:1ω9 level was near the danger limit for both years, a detailed study of C22:1ω9 of E. spectabilis is recommended. On the other hand, the C22:1ω9 level was very low in A. orientale, A. elongatum and A. officinalis. The results were significantly different (p ≤ 0.05) from E. spectabilis.
Another significant fatty acid, C24:1ω9 was detected at 2.1% in 2020 and 1.9% in 2021 in A. orientale; 2.8% in 2020 and 2.2% in 2021 in A. officinalis (Table 1, 2). C24:1ω9 is involved in the biosynthesis and maintenance of nerve cell myelin and forms the basic building block of neurons in the brain and nerve tissue [26, 27]. C24:1ω9 is difficult to synthesize and is sometimes ingested from food or in pill form when deficient. Therefore, the detection of C24:1ω9 in plant species is considered an important outcome. Previous studies have reported that the plants Acer truncatum, Tropaeolum speciosum, Malania oleifera, Borago officinalis and Xanthoceras sorbifolium contain C24:1ω9 [26]. In a seed oil analysis study of 138 accessions from 14 populations of Acer truncatum (Aceraceae) native to China, the level of C24:1ω9 was reported to range from 3.9% to 7.85% [26]. Wild plant Malania oleifera was examined and C24:1ω9 (55.70%) was found as the main component [28]. The presence of significant amounts of C24:1ω9 in A. officinalis and A. orientale suggests that these plants can synthesize C24:1ω9 and this makes them nutritionally important. In addition to genetic factors, environmental factors such as temperature, pH, nutrients, precipitation and mineral concentration now also play a role in the fatty acid composition of plants. The fatty acid composition of plants can vary according to many ecological, morphological, physiological, and cultural factors, as well as the genotype of the plant.
Proximate Composition
The total lipid levels of the edible parts of A. orientale, E. spectabilis, A. officinalis and A. elongatum in 2020 were determined to be 0.6%, 0.5%, 0.4% and 1.0%, respectively (Table 3). In spring 2021, the total lipid concentration was found to be 0.7%, 0.6%, 0.6% and 0.9% for A. orientale, E. spectabilis, A. officinalis and A. elongatum, respectively (Table 3). The lipid levels of A. elongatum differed from those of the other three species (p ≤ 0.05). The total protein levels of A. orientale, E. spectabilis, A. officinalis and A. elongatum were observed as close levels as 5.1%, 5.6%, 5.0% and 5.2%, for 2020, and 5.4%, 5.3%, 5.2% and 5.5% for 2021, respectively (Table 3).
In a study conducted on the seeds, the crude protein content of Brassicaceae and Dipsacaceae species was given as between 9.4% and 32.6% [22]. Cinar et al. reported that the total lipid level was 0.46 g/100 g and total protein was 0.12 g/100 g for E. spectabilis on a wet basis [29]. In addition, in a study on A. elongatum, the total protein content was given as 8.47% [30]. In a study on the seeds of Allium species, the total lipid level of A. cepa (2.96%) was lower than that of A. sativum (5.98%). In the same study, total protein levels were reported to be between 12.53% (A. cepa) and 15.82% (A. sativum).
In another study of Allium species, 10 different seeds of A. cepa were examined. Analysis showed that A. cepa seeds contained high levels of total lipids (between 21.86% and 25.86%) [15]. The fatty acid composition of the seeds of four Anchusa species (A. azurea var. azurea, A. leptophylla, subsp. leptophylla, A. arvensis, subsp. orientalis and A. undulata subsp. hybrida) harvested from different parts of Turkey was investigated [14]. The total seed oil content was found to be 11.2% in A. azurea var. azurea, 22.8% in A. leptophylla, subsp. leptophylla, 19.80% in A. arvensis, subsp. orientalis and 21.5% in A. undulata subsp. hybrida. [14]. Various factors can directly or indirectly affect the quantity of protein and lipid accumulation of plants tissues. For example, soil factors such as inorganic and organic matter content, pH, nutrients and water content; weather and climatic factors such as temperature, precipitation and light intensity, and genetic and physiological factors can affect the plant physiology. Considering the above data, it can be concluded that the species A. orientale, E. spectabilis, A. officinalis and A. elongatum are low in protein and cannot be recommended as a diet in terms of protein and lipid. The ash content of the plants varied between 0.87% and 1.04% for 2020, and 0.94% and 1.01% for 2021 (Table 3). The moisture ranged from 87.7 to 90.02% in 2020 and from 85.7% to 91.01% in 2021. These results were quite normal for fresh plant leaves.
Conclusion
As a result, the consumption of the edible wild plants A. orientale, E. spectabilis, A. officinalis and A. elongatum is recommended due to valuable fatty acids such as C16:0, C18:0, C18:1ω9, C18:2ω6 and C18:3ω3. However, detailed studies are needed on E. spectabilis, whose C22:1ω9 level has been close to the danger limit (5%) for two consecutive years. Further research on these unconventional wild edible plants will show if they can be included in addition to local food sources and potentially be considered as new food sources. Although the current paper discusses the nutritional values of the wild plants, data of the fatty acid and proximal compositions of the species also contributes to taxonomic and physiological properties of the species studied. Since studies on the fatty acid content in A. orientale, E.spectabilis, A.officinalis and A.elongatum are limited in the literature, it is anticipated that the data will also contribute to the literature. Note again that the presentation of a plant nutritional value can serve as an important opportunity to identify some gaps in the literature and identify the need.
References
Ceylan O, Alıc H (2015) Antibiofilm, antioxidant, antimutagenic activities and phenolic compounds of Allium orientale BOISS. Braz Arch Biol Technol 58:935–943. https://doi.org/10.1590/S1516-89132015060309
Bircan B, Kırbağ S (2015) Determination of antioxidant and antimicrobial properties of Eremurus spectabilis Bieb. Artvin Coruh Univ J For Fac 16:176–186. https://doi.org/10.17474/acuofd.63418
Tosun M, Ercisli S, Ozer H, Turan M, Polat T, Ozturk E, Padem H, Kilicgun H (2012) Chemical composition and antioxidant activity of foxtail lily (Eremurus spectabilis). Acta Sci Polon Hortorum Cultus 11:145–153
Kardaş C (2019) Muş’ta yabani bitkilerin halk hekimliğinde kullanılması [Usage of savage plants in the folk medicine in Muş]. Lokman Hekim J 9:85–96. https://doi.org/10.31020/mutftd.468848
Jaradat N, Abualhasan M (2016) Comparison of phytoconstituents, total phenol contents and free radical scavenging capacities between four Arum species from Jerusalem and Bethlehem. Pharmaceutical Sci 22:120–125. https://doi.org/10.15171/PS.2016.19
Corrêa APA, Peixoto CA, Cabral GLAG, FA (2008) Fractionation of fish oil with supercritical carbon dioxide. J Food Eng 88:381–387. https://doi.org/10.1016/j.jfoodeng.2008.02.025
Kones R, Howell S, Rumana U (2018) n-3 polyunsaturated fatty acids and cardiovascular disease: principles, practices, pitfalls, and promises-a contemporary review. Med Princ Pract 26:497–508. https://doi.org/10.1159/000485837
Johnson M, Bradford C (2014) Omega-3, omega-6 and omega-9 fatty acids: implications for cardiovascular and other diseases. J Glycom Lipidom 4:1–8. https://doi.org/10.4172/2153-0637.1000123
Çelebi Ş, Kaya H, Kaya A (2017) Effects of omega-3 fatty acids on human health. Alınteri J Agric Sci 32:105–112. https://doi.org/10.28955/alinterizbd.319437
Helrich K (1990) Association of Official Analytical Chemists. Official Methods of Analysis. 15th ed., Association of Official Analytical Chemists Inc, Arlington
Cacan E, Kokten K, Kilic O (2018) Leaf fatty acid composition of some Lamiaceae Taxa from Turkey. Prog Nutr 20:231–236. https://doi.org/10.23751/pn.v20i1-S.5930
Kayaçetin F, Efeoğlu B, Sarıoğlu G (2018) Evaluation of fatty acid compositions of some important wild and domestic Turkish mustard genotypes (Brassica spp.). Int J Second Metab 5:270–278. https://doi.org/10.21448/ijsm.474894
Minzangi K, Kaaya AN, Kansiime F, Tabuti JRS, Samvura B, Grahl-Nielsen O (2011) Fatty acid composition of seed oils from selected wild plants of kahuzi-biega national park and surroundings, democratic republic of congo. Afr J Food Sci 5:219–226
Kucukboyaci N, Doğru-Koca A, Yıldırımlı Ş, Gören KE, A (2013) γ-Linolenic acid content and fatty acid profiles of the seed oils of some Anchusa species. Turk J Pharm Sci 10:87–94
Yalcin H, Kavuncuoglu H (2014) Physical, chemical and bioactive properties of onion (Allium cepa L.) seed and seed oil. J Appl Bot Food Qual 87:87–92. https://doi.org/10.5073/JABFQ.2014.087.013
Özcan T (2008) Fatty acid profiles of the seed oils in two groups of Anchusa officinalis L. IUFS J Biol 67:65–71
Bellaloui N, Mengistu A, Kassem MA (2013) Effects of genetics and environment on fatty acid stability in soybean seed. Food Nutr Sci 4:165–175. https://doi.org/10.4236/fns.2013.49A1024
Lajara JR, Diaz U, Diaz Quidiello R (1990) Definite influence of location and climatic conditions on the fatty acid composition of sunflower seed oil. J Am Oil Chem Soci 67:618–623
De Carvalho CCCR, Caramujo MJ (2018) The various roles of fatty acids. Molecules 23:1–36. https://doi.org/10.3390/molecules23102583
Shanab SMM, Hafez RM, Fouad AS (2018) A review on algae and plants as potential source of arachidonic acid. J Adv Res 11:3–13. https://doi.org/10.1016/j.jare.2018.03.004
Johnson M, Chastity B (2014) Omega-3, omega-6 and omega-9 fatty acids: Implications for cardiovascular and other diseases. J Glycom Lipidom. https://doi.org/10.4172/2153-0637.1000123
Tonguç M, Erbaş S (2012) Evaluation of fatty acid compositions and some seed characters of common wild plant species of Turkey. Turk J Agric For 36:673–679. https://doi.org/10.3906/tar-1201-22
Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ (2008) Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 197:12–24. https://doi.org/10.1016/j.atherosclerosis.2007.11.008
Semma M (2002) Trans fatty acids: properties, benefits and risks. J Health Sci 48:7–13. https://doi.org/10.1248/jhs.48.7
Vargas-Lopez JM, Wiesenborn D, Tostenson K, Cihacek L (1999) Processing of crambe for oil and isolation of erucic acid. J Am Oil Chem Soci 76:801–809. https://doi.org/10.1007/s11746-999-0069-4
Qiao Q, Wang X, Ren H, An K, Feng Z, Cheng T, Sun Z (2019) Oil content and nervonic acid content of Acer truncatum seeds from 14 regions in China. Hortic Plant J 5:24–30. https://doi.org/10.1016/j.hpj.2018.11.001
Sargent J, Coupland K, Wilson R (1994) Nervonic acid and demyelinating disease. Med Hypotheses 42:237–242. https://doi.org/10.1016/0306-9877(94)90122-8
Tang TF, Liu XM, Ling M, Lai F, Zhang L, Zhou YH, Sun RR (2013) Constituents of the essential oil and fatty acid from Malania oleifera. Ind Crops Prod 43:1–5. https://doi.org/10.1016/j.indcrop.2012.07.003
Cinar A, Ay ST, Karabak S, Guzelsoy N, Ucurum O (2017) Foxtail lilly (Eremurus spectabilis M. Bieb.) as priority species of biodiversity for food and nutrition project of Turkey. Anadolu J AARI 27:69–73
Tunçtürk R, Tunçtürk M, Eryigit T, (2019) Chemical contents of some species of Teucrium genus distributed in Van flora. KSU J Agric Nat 22:138–142. https://doi.org/10.18016/ksutarimdoga.vi.440882
Acknowledgements
Most of the research was supported by Şırnak University Scientific Research Projects Unit (Project number is 2020.FNAP.06.03.01 which is carried out by the Author).
Funding
This research was supported and mostly financed by Şırnak University Scientific Research Projects Unit (Project number is 2020.FNAP.06.03.01).
Author information
Authors and Affiliations
Contributions
Conceived and designed the analysis; Collected the data; Contributed data or analysis tools; Wrote the paper; Design, preparing tables.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author. The research and the data presented in the current study have neither been published in whole/in part nor under consideration for publication in any other journal/periodical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement
Therapeutic and nutritional plants A. orientale, E. spectabilis, A. officinalis and A. elongatum are widely consumed in rural areas, particularly in Middle East and Anatolia. Thereby, the study will contribute to consumption and also literature.
Rights and permissions
About this article
Cite this article
Ekin, İ. Proximate and Fatty Acid Compositions of Four Therapeutic and Nutritional Plants for two Consecutive Years. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 92, 929–937 (2022). https://doi.org/10.1007/s40011-022-01392-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-022-01392-w