Abstract
Nostoc sp. NDUPC007 was isolated from agricultural fields of Varanasi, U.P., India. Cyanobacterium was characterized by morphological as well as molecular methods. 16S rRNA sequence was deposited to NCBI with accession no. KM281209.1. Chlorophyll-a content was 17.6 µg/ mg dry weight. The heterocyst frequency of both single and multiple contiguous heterocysts was 2.14%. The number of heterocysts in multiple contiguous heterocyst chains ranged from two to seven. Ammonia content in culture media increased up to the 9th day of growth and then remained approximately constant (2.34–2.43 µg/ ml) during the rest of the monitored period. Glutamine synthetase activity increased up to the 5th day of growth (maximum rate of 5.36 mM γ-glutamyl hydroxamate/ mg chl/ min). Approximately constant (3.21 mM γ-glutamyl hydroxamate/ mg chl/ min) rate of glutamine synthetase was maintained after the 9th day of growth. Algalisation with Nostoc sp. NDUPC007 increased the growth of rice plants. Length of radical and plumule was 2.6–3.1 cm and 14.3–17.1 cm, respectively in algalised plant, whereas it was 1.9–2.3 cm and 8.9–9.4 cm in non-algalised plants. 2.13 µg/ ml ammonia was noted in the algalised set and no ammonia was observed in the non-algalised set on 10 days after algalisation. The findings of the experiment proved the suitability of Nostoc sp. NDUPC007 as potential inocula for algalisation of rice fields of Varanasi, India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are photosynthetic prokaryotes with a range of morphological diversity, i.e., unicellular, colonial, filamentous unbranched, and with branching [1]. Three types of cells, i.e., vegetative, heterocyst, and spores are present in filamentous cyanobacteria. Heterocyst and spore perform the special function of nitrogen fixation and perennation, respectively [2,3,4]. Cyanobacteria have a worldwide distribution, including extreme habitats. Cyanobacteria play an important role in maintaining the structure and fertility of the soil by increasing nitrogen content, carbon content, phosphorus solubilization, and through various secretions [1, 5]. Cyanobacteria induce plant growth commonly by secretion of growth hormone, siderophore production, phosphate solubilization, and release of fixed nitrogen. These properties of cyanobacteria make them eco-friendly biofertilizers. The heterocyst is the main site for nitrogen fixation [3]. Most nitrogen fixed by cyanobacteria is not available for immediate use to plants. Nitrogen fixed by cyanobacteria is only available to plants on autolysis and mineralization of dead cyanobacteria [6]. A very small amount of fixed nitrogen is released by most nitrogen-fixing cyanobacteria during the growth period. The rate of release of fixed nitrogen was increased tremendously in cyanobacteria treated with glutamine synthetase inhibitor, i.e., L-methionine-DL-sulfoximine [7]. Aulosira fertilissima, Nostoc muscorum, Anabaena variabilis, and Tolypothrix Tenuis are being used in algal biofertilizer technology [8]. Indigenous cyanobacterial strains are suitable for algalisation [9]. A list of indigenous cyanobacteria with the potential to excrete ammonia is needed. Hence, an attempt was made in the present investigation to study on isolation, characterization, and ammonia excretion potential of Nostoc sp. NDUPC007.
Material and Methods
Cyanobacterium was isolated as described by Mishra et al. [10] and purified by repeated streaking method [11]. It was grown in BG-11 medium in a culture room temperature maintained at 28° ± 2 °C and illuminated by the fluorescent lamp (12 wm–2) with a 14:10 light and dark cycle.
Identification was done based on morphological as well as molecular parameters as followed by Mishra et al. [10]. The morphological parameters of strain, i.e., nature of filament, shape, and size of the vegetative cell, heterocyst, and spore were studied at 400 × and 1000 × using an Olympus 21Xi microscope. Dimensions of morphological parameters were measured with the help of Magnus PRO Micromeasurement & Image analysis software. The strain was assigned to cyanobacterial species following taxonomic descriptions provided in the literature [12, 13]. Molecular identification was done based on 16 s rRNA gene sequence analysis. Protocol devised by Singh et al. [14] was followed to isolate genomic DNA. For_5/-GAGTT(CT)GATCCTGGCTCAGGA-3/ and Rev_5/-TCCAGCCGCACCTTCCAGTA-3/ primers were used to amplify the 16S rRNA gene [14]. Sequencing of the purified PCR product was done at IIVR, Varanasi, India by the automated capillary sequencer (ABI 3130 Genetic Analyser, Applied Biosystems, Foster City, CA, USA). Partial 1371 base pair was obtained. NCBI-BLASTn program was used to search for similarity of sequence with other cyanobacterial strains.
Growth was monitored by measuring chlorophyll-a content at 24 h intervals up to 20 days. Total chlorophyll was measured by the method of Myers and Kratz [15]. Extracellular ammonia release was measured by the Phenol hypochlorite method [16]. Added 2 ml of phenol solution, 2 ml of sodium nitroprusside solution, and 5 ml of the oxidizing reagent to 50 ml of the cyanobacterial extract with thorough mixing after each addition. The blue color developed after one hour at room temperature. Optical density was noted at 640 nm and the amount of ammonia was calculated through the standard graph of ammonia. Glutamine synthetase activity was measured by the method of Shapiro and Stadtman [17] and expressed as mM γ- glutamyl hydroxamate formed min−1 mg chl−1.
Heterocyst frequency was calculated by counting the number of heterocysts per 100 vegetative cells of strains with 100 replicates.
Two sets of autoclaved Petri plates were partially filled with autoclaved sand, and both were moistened with 25 ml of autoclaved soil extract. A total of 10 seeds (sterilized) of rice (Kaveri variety) were placed in each set and placed in the incubator for 36 h at 30 °C. One set was algalised with 1 ml of cyanobacterial culture and both sets were placed in a growth chamber maintained at 30 °C, illuminated with 14:10 light and dark duration. The growth of rice seedlings was measured after 10 days of growth.
Results and Discussion
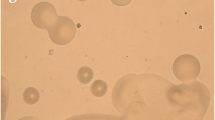
Cyanobacterium is filamentous, unbranched and heterocystous (Fig. 1). Trichome is blue-green to violet. Cells are cylindrical and barrel-shaped, constricted at the cross-wall with 4.16–6.58 µm length and 3.45–4.44 µm breadth (Fig. 1). Apical cells are rounded. Heterocysts are apical as well as intercalary, spherical, subspherical, and barrel-shaped with 3.42–10.39 µm length and 4.31–6.53 µm breadth (Fig. 1). There is a frequent occurrence of multiple contiguous heterocysts (Fig. 1). Spore formation always starts away from the heterocyst. Spores are formed in the chain and arranged in a zig-zag manner. Spores are elliptical, smooth hyaline with 7.91–12.15 µm length and 4.11–5.93 µm breadth (Fig. 1). Morphological features closely matched with cyanobacterial genera Nostoc [12]. The presence of knot-like structures and cell dimensions do not match with known species of Nostoc [12], hence this strain may be a new one but further characterization is needed. Hence, this cyanobacterium is being identified as Nostoc sp. with strain as NDUPC007. A genus of strain was further confirmed by molecular method. 16S rRNA of strain was sequenced. Partial 1371 base pair was obtained and deposited to NCBI with accession no. KM281209.1. BLASTin search of the sequence showed a maximum 96% similarity with different strains of Nostoc. The deposition of strain at NBAIM, Mau, India is under process.
Photomicrograph of Nostoc sp. NDUPC007 (Scale bar = 5.1 µm). A, filament with apical cell. B, Filament with apical heterocyst. C, Filament with intercalary heterocyst. D, Filament with apical heterocyst and knot-like structure. E, Filament with apical multiple contiguous heterocysts. F, Filament with intercalary multiple contiguous heterocysts. G, Spores in the chain
The growth of cyanobacterium was measured by monitoring total chlorophyll-a content at 24 h intervals for 20 days. Cyanobacterium reached a stationary phase after 13 days of growth (Fig. 2). Total chlorophyll-a was 17.6 µg/ mg dry weight in the stationary phase of growth. The heterocyst frequency of both single and contiguous multiple heterocysts was 2.14%. The number of heterocysts in multiple contiguous heterocyst chains ranged from two to seven (Fig. 1). Ammonia secretion in culture media was monitored from 6 h of culture to 20 days. Ammonia content in culture media increased up to the 9th day of growth and then remained approximately constant (2.34–2.43 µg/ ml) up to the monitored period (Fig. 3). Glutamine synthetase activity increased up to the 5th day of growth (maximum rate of 5.36 mM γ-glutamyl hydroxamate/ mg chl/ min). The rate of glutamine synthetase became approximately constant (3.21 mM γ-glutamyl hydroxamate/ mg chl/ min) after the 9th day of growth (Fig. 4).
Cyanobacteria are known to promote plant growth through the secretion of growth regulators, fixed nitrogen and phosphate solubilization, etc. Algalisation of rice fields with cyanobacteria increased the nitrogen content by up to 14% [18, 19]. Kaushik, [8] reported 15–53 kg Nitrogen (N)/hectare (h)/year(y) fixation by cyanobacteria in our country. N2-fixing cyanobacteria are the main contributors to the nitrogen economy of paddy fields [20]. Most of the fixed nitrogen of cyanobacteria is made available to plants by autolysis and mineralization after the death of cyanobacteria [6]. Nitrogen fixed by free-living cyanobacteria is released in insignificant quantities during the growth period. Treatment of cyanobacteria with mutagen L-methionine-DL-sulfoximine, a highly specific, irreversible inhibitor of glutamine synthetase, increased the rate of ammonia release several times [7]. Heterocyst is the main site of nitrogen fixation in cyanobacteria [3] though some non-heterocystous form, i.e., Trichodesmium sp. [21], Plectonema boryanum [22], Gleoecapsa [23] also fix nitrogen. Nitrogen fixed in heterocysts is assimilated to amino acid glutamine by GS-GOGAT pathway [24]. Glutamine is transported to surrounding vegetative cells [25]. Excess ammonia not assimilated by GS-GOGAT pathway is released by heterocyst by simple diffusion so the rate of ammonia assimilation is the key determinant of ammonia release. Low GS activity has been observed in some ammonia secreting cyanobacterial mutants [26, 27]. Nostoc sp. NDUPC007 is continuously secreting ammonia in culture medium as well as in Petri plates used for bioassay. The frequent presence of multiple contiguous heterocysts, appears to be responsible for surplus nitrogen fixation. GS activity of strain is not enough at all stages of growth to fully assimilate the nitrogen fixed by cyanobacteria and consequently the continuous release of ammonia. Heterocyst differentiation and pattern formation are regulated by the HetR gene [28]. PatS gene also plays role in heterocyst pattern formation [29]. Reported causes for multiple contiguous heterocysts are inactivity of PatS gene [29], multiple copies of HetR genes, inactivation of calcium sequestering proteins [30], overexpression of HetF gene [30], etc. The multiple contiguous heterocysts of this cyanobacterium might be due to these reasons. Ammonia release and pattern of heterocyst were monitored for five years.
An increase in plant growth was observed in a set algalised with cyanobacterium (Fig. 5). Length of radical and plumule was 2.6–3.1 cm and 14.3–17.1 cm, respectively in algalised plant, whereas it was 1.9–2.3 cm and 8.9–9.4 cm in non-algalised plants (Fig. 5). 2.13 µg/ ml ammonia was noted in the algalised set and no ammonia was observed in non-algalised even 10 days after algalisation. Ammonia is a well-known utilizable form of nitrogen and is necessary for the growth of plants. Ammonia in the Petri plate is inducing the growth of rice seedlings (Fig. 5). Ammonia secreting strains of cyanobacteria are being searched as suitable inocula for algalisation of rice fields. This cyanobacterium has shown the capacity to continuously release ammonia and is indigenous to rice fields of Varanasi, India. Hence, Nostoc sp. NDUPC007 may prove as suitable inocula for algalisation of rice fields of Varanasi, U.P., India.
Conclusion
Cyanobacterium Nostoc sp. NDUPC007 was isolated from the agricultural field of Varanasi, India. It was characterized based on morphological as well as molecular parameters. The frequent occurrence of contiguous multiple heterocysts was noted. The number of heterocysts in multiple contiguous heterocyst chains ranged from two to seven. It has shown the potential for continuous excretion of ammonia in the culture medium. Algalisation by this cyanobacterium induced the growth of rice seedlings. Indigenous cyanobacterium with biofertilizer potentials are sought for algalisation. Hence, Nostoc sp. NDUPC007 might prove as potential inocula for algalisation of rice fields of Varanasi, India.
References
Whitton BA, Potts M (2000) Introduction to the cyanobacteria. In: Whitton BA, Potts M (eds) Ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht, pp 235–255
Thomas JJC, Meeks CP, Wolk PW, Shaffer SM, Austin CWS (1977) Formation of glutamine from (13N) ammonia, (13N) dinitrogen, and (14C) glutamate by heterocysts isolated from Anabaena cylindrica. J Bacteriol 129:1545–1555
Wolk CP, Wojciuch E (1971) Photoreduction of acetylene by heterocysts. Planta 97:126–134
Yamamoto Y (1975) Effect of desiccation on the germination of akinetes of Anabaena cylindrica. Plant Cell Physiol 16:749–752
Tiwari DN, Kumar A, Mishra AK (1991) Use of cyanobacterial diazotrophic technology in rice agriculture. Appl Biochem Biotechnol 28(29):387–396
Martinez MR (1984) Algae: biofertilizer for rice. Philippines Council for Agricultural Research and Resources Development (PCARRD) Monitor 12 9–12
Ronzio R, Rowe W, Meister A (1969) Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochem 8:1066–1075
Kaushik BD (2014) Developments in cyanobacterial biofertilizer. Proc Indian Natn Sci Acad Spl Sec 80(2):379–388
Grant IF, Roger PA, Watanabe I (1985) Effect of grazer regulation and algal inoculation on photodependent nitrogen fixation in wet land rice field. Biol Fert Soils 1:61–72
Mishra SK, Singh J, Pandey AR, Dwivedi N (2019) Indole-3-acetic acid production by the cyanobacterium Fisherella muscicola NDUPC001. Curr Sci 116(7):1233–1237
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Desikachary TV (1959) Cyanophyta. Indian Council of Agriculture Research, New Delhi
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure culture of cyanobacteria. J Gen Microbiol 111:1–61
Singh SP, Rastogi RP, Donat-P H, Sinha RP (2011) An improved method for genomic DNA extraction from cyanobacteria. World J Microbiol Biotechnol 27:1225–1230
Myers J, Kratz WA (1955) Relation between pigment content and photosynthetic characteristics in blue-green algae. J Gen Physiol 39:11–92
Solorzano L (1969) Determination of ammonia in natural waters by the phenol-hypochlorite method. Limnol Oceanogr 14:799–801
Shapiro BM, Stadtman ER (1970) Glutamine synthetase (E. coil). Meth Enzymol 17A:910–922
Rao DLN, Burns RG (1990) Use of blue-green algae and bryophyte biomass as a source of nitrogen for oil-seed rope. Biol Fertil Soils 10:61–64
Singh PK, Biosyi RN (1989) Blue green algae in rice fields. Phycos 28:181–195
Irisarri P, Gonnet S, Monza J (2001) Cyanobacteria in uruguayan rice fields: diversity, nitrogen-fixing ability and tolerance to herbicides and combined nitrogen. J Biotechnol 91:95–103
Carpenter EJ, Price CC (1976) Marine Oscillatoria (Trichodesmium): Explanation for aerobic nitrogen fixation without heterocysts. Science 191:1278–1280
Stewart WDP, Lex M (1970) Nitrogenase activity in blue-green algae Plectonema boryanum strain 594. Arch Microbiol 73:250–260
Wyatt JJ, Silvey JKG (1969) Nitrogen fixation by Gloeocapsa. Science 165:908–909
Martin-Figueroa E, Navarro F, Florencio FJ (2000) The GS-GOGAT pathway is not operative in the heterocysts cloning and expression of glsF gene from the cyanobacterium Anabaena sp. PCC 7120. FEBS Lett 476:282–286
Meeks JC, Elhai J (2002) Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol Mol Biol Rev 66(1):94–121
Boussiba S, Liu X-Q, Gibson J (1984) Endogenous ammonia production by Anacystis nidulans R-2 induced by methionine sulfoximine. Arch Microbiol 138:217–219
Spiller H, Littore HC, Hassan ME, Shanmugan KT (1986) Isolation and characterisation of nitrogenise depressed mutants of cyanobacterium Anabaena variabilis. J Bacteriol 165:412–419
Buikema WJ, Haselkorn R (1991) Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev 5(2):321–330
Cheng-Cai Z, Laurent S, Sakr S, Peng L, Bédu S (2006) Heterocyst differentiation and pattern formation in cyanobacteria: a chorus of signals. Mol Microbiol 59(2):367–375
Kumar K, Mella-Herrera RA, Golden JW (2009) Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol 2(a000315):1–19
Acknowledgements
We are thankful to the Head, Department, of Botany and Principal, Udai Pratap College, Varanasi, Uttar Pradesh, India for providing necessary laboratory facilities. The authors also thank Prof. Padmanabh Dwivedi, Institute of Agricultural Sciences, BHU, Varanasi for suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement Cyanobacterium Nostoc sp. NDUPC007 is indigenous to the agricultural fields of Varanasi, India. It has shown the potential for continuous excretion of ammonia in the culture medium. Algalisation by this cyanobacterium induced the growth of rice seedlings. Indigenous cyanobacterium with biofertilizer potentials are sought for algalisation. The findings of the experiment prove the suitability of Nostoc sp. NDUPC007 as potential inocula for algalisation of rice fields of Varanasi, India.
Rights and permissions
About this article
Cite this article
Prakash, O., Singh, J., Mishra, S.K. et al. Isolation and Characterization of Ammonia Secreting Cyanobacterium Nostoc sp. NDUPC007 from Agriculture Fields of Varanasi. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 92, 89–93 (2022). https://doi.org/10.1007/s40011-021-01323-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-021-01323-1