Abstract
Species of Pleurotus have gained tremendous interest due to its nutritional and various medicinal applications. The specific goals of this research work are to evaluate the effects of different carbon and nitrogen sources for mycelial biomass, average growth rate and exopolysaccharide (EPSs) production, estimation of total carbohydrate content and antioxidant activities of EPSs and determination of antibacterial properties of various extracts of Pleurotus flabellatus. The starch (3.84 ± 0.43 g/l and 0.55 ± 0.21 g/l/day) and peptone (1.57 ± 0.87 g/l and 0.22 ± 0.12 g/l/day) were the best (p < 0.05) carbon and nitrogen sources, respectively, for better mycelial growth and average growth rate of P. flabellatus, whereas EPSs production was highest in sucrose (3.467 ± 0.96 g/l) and beef extract (6.00 ± 0.59 g/l). The highest amount of carbohydrate from EPSs was observed in starch (3.39 mg/100 g) and peptone (4.05 ± 0.08 mg/100 g) medium (p < 0.05). The highest free radical scavenging activities of EPSs were observed in glucose (83.80 ± 0.87%) and calcium nitrate (89.01 ± 1.61%) at 16.0 mg/ml concentration (p < 0.05). The broth extract of P. flabellatus showed highest (p < 0.05) antibacterial activity against B. subtilis, whereas EPSs extract showed lowest activity against B. subtilis; meanwhile, all three types of extract did not show activity against S. aureus. Therefore, more research work is necessary to find out the active chemicals constituents of EPSs and their functional relationships.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exopolysaccharides (EPS) are produced by various microorganisms particularly mushrooms due to their various structural complexities [1] and have ability to fulfill different tasks during the growth on natural substrates, such as adhesion to surfaces, immobilization of secreted enzymes and prevention of hyphae from dehydration and increased residence time of nutrients inside the mucilage [2]. Most of investigations on fungal polysaccharides were mainly focused on higher basidiomycetes mushrooms as these have great structural and functional properties which in turn have diverse applications in industries, pharmaceuticals, food, etc [2]. Polysaccharides from mushrooms are used as a tonic, promoting longevity and improving the quality of life by possessing different medicinal properties like free radical scavenging activity, superoxide radical scavenging, reducing properties, lipid peroxidation inhibition, suppression of proliferation and oxidative stress, anti-inflammatory activity, antiproliferative effects, immunostimulating, anti-tumor, hypoglycemic activities, biosysnthesis and increasing cell proliferation, etc [3]. On the basis of the above discussion, the present work is carried out to evaluate the effects of different carbon and nitrogen sources for mycelial biomass, average growth rate of mycelia and EPSs production from P. flabellatus. The following works have been intended to work out during this programme: (1) to estimate the total carbohydrate content from EPSs produced by three carbon and nitrogen sources, (2) to evaluate the antioxidant activity of EPSs produced by three carbon and nitrogen sources, and (3) to evaluate the antibacterial property of three different extracts of mushroom against Gram-positive and Gram-negative bacteria.
Material and Methods
Sample Collection and Identification
The specimen (MCCT- P01) was collected from Lake Chowmuhani market, West Tripura, Northeast India. The mushroom sample was identified by comparing the descriptions with the work of Pegler [4] and some e-resources. The mycelia of the studied fungus was obtained by tissue culture method and maintained on Potato Dextrose Agar (PDA) medium.
Submerged Cultivation
Media Preparation
Mushroom mycelia were grown in Basal Synthetic Liquid (BSL) medium for optimum biomass and EPS production. The BSL Medium contained glucose (30 g/l), yeast extract (2 g/l), peptone (2.5 g/l), MgSO4. 7H2O (0.5 g/l), Ca(NO3)2 (0.5 g/l), (NH4)2SO4 (0.25 g/l), KH2PO4 (0.25 g/l), FeCl3 (10 mg/l), ZnSO4 (0.1 mg/l), Inositol (50 mg/l), Thiamine (100 µg/l), Biotin (50 µg/l), Folic acid (100 µg/l), CaCl2 (0.1 M in 5 ml/l) and distilled water (1 l) [2].
Inoculums and Culture Medium Preparation
A small portion of the actively growing mycelium from an agar slant of the test fungus was aseptically transferred to a sterile 250-ml conical flask containing 50 ml of BSL medium and was incubated on a shaking incubator (120 rpm) at 25 °C (± 5 °C) for 7 days in complete darkness. After 7 days, the mycelial mat was aseptically fragmented into small pieces with the help of a waring blender. The fragmented mycelium was washed several times with distilled water to remove any trace of adhering medium and suspended in a phosphate buffer medium (pH-5.5) for 24 h to overcome the shock encountered during blending. An aliquot of 1 ml of the mycelia cell suspension was used as the inoculum. Fungal biomass requirements were determined by the mycelia dry weight method. Each experiment was done in triplicates [2].
Utilization of Different Carbon Sources
The BSL medium with different carbon source was used for this experiment. Seven carbon sources, namely maltose, glucose, sucrose, starch, fructose, xylose and carboxymethyl cellulose (CMC), were evaluated separately to the fermentation medium as a sole carbon source at 30 g/l level. The basal medium that lacks any carbon compound (0%) served as the control. The most suitable carbon compound for yielding the highest biomass was selected as optimum carbon sources. The culture flasks were inoculated and incubated for 7 days at 25 °C on a shaker incubator (120 rpm), and the initial pH value was 6.0 [2].
Utilization of Different Nitrogen Sources
This experiment was performed using the BSL medium, but the control was without any nitrogen source. Different sources of nitrogen (peptone, yeast extract, beef extract, malt extract calcium nitrate, urea and ammonium sulfate) were used separately as sole nitrogen source at 3 g/l level. The most favorable nitrogen source for yielding the highest biomass was selected as best nitrogen source. The culture flasks were inoculated and incubated for 7 days at 25 °C in dark condition, and the initial pH value was 6.0 [2].
Analytical methods
Determination of Dry Cell Weight (DW) and Average Growth Rate
For measurement of cells dry weight, the cells were obtained by centrifuging a sample at 10,000 × g for 10 min, washing the precipitated cells for three times to a constant weight and then drying the cells at 105 °C until (maximum 12 h) a constant DW was obtained [2].
Measurement of Exopolysaccharide (EPS)
EPS of mushroom was determined by the slightly modified method of Fang and Zhong [5]. For the determination of EPS, after removal of mycelia by filtration, the fermentation filtrate was dialyzed and the crude EPS was precipitated with addition of 95% (v/v) ethanol by four times of volume and then separated by centrifugation (10,000 × g, 10 min). After washing three times with distilled water, the insoluble components were suspended in 1 M NaOH at 60 °C (1 h) and then the content of EPS in supernatant was measured by phenol–sulphuric acid method [2].
Carbohydrate Content of EPS
Total carbohydrate content was estimated by the method of Hedge and Hofreiter [6].
Medicinal Properties
Mushroom Mycelia Extraction
Preparation of water extracts of EPSs of mushrooms was done by slightly modified method of Mau et al. [7]. The soluble EPSs (5 g) were extracted by grinding with 50 ml of water with the help of pestle and mortar. After filtering through Whatman No.4 filter paper, the mycelium was then extracted twice with addition of 50 ml of water in each. The water extract of EPSs was then directly used for determination of antioxidant activity.
Free Radical Scavenging (FRS) Activity (DPPH)
Free radical scavenging (FRS) activity was measured by a little bit modified method of Shimada et al. [8]. 4 ml dried mushroom methanolic extract (0.25–16 mg/ml) was mixed with 1 ml of (0.0002 M) methanolic solution containing DPPH radical (Sigma). The mixture after shaking vigorously was allowed to stand for half an hrs, and the absorbance was measured at 517 nm against a blank in a spectrophotometer (Eppendorf AG 22331Hamburg). EC50 (mg/ml) is the valuable concentration at which DPPH radical was scavenged by 50% (w/v) and was obtained by interpolation from linear regression analysis. BHT and ascorbic acid were used as a control. Inhibition of free radical by DPPH in percent was calculated as follows: Percentage of inhibition: [(A Blank − A Sample)/A Blank] × 100, where A Blank and A Sample denote the absorbance of control and test compound, respectively.
Submerged Cultivation for Determination of Antibacterial Activities
Mushroom Mycelia Production
Pure culture of P. flabellatus was carried out in Potato Dextrose Agar (PDA) medium. To study antibacterial activity of mushroom mycelium was grown in sterile conical flask (250 ml) containing 50 ml of BSL medium. The medium was inoculated with disk of 6 mm diameter of mushroom mycelia obtained from 6- to 8-day-old grown culture on PDA plate. The growth was carried out under stationary condition at 28 °C (Shaking Incubator: LSI 4018R). After 30-day incubation in the dark, the liquid medium was filtered and the mycelium was separated from the liquid [9].
Extraction of Mycelial
Preparation of methanolic extracts of mushroom was done by slightly modified method of Mau et al. [7]. The dried powdered mycelium (5 g) was extracted by grinding with 50 ml of methanol with the help of pestle and mortar. After filtering through Whatman No.4 filter paper, the mycelium was then extracted twice with addition of 50 ml of methanol in each. The methanolic extract was then evaporated at 40 °C to dryness in rotary evaporator (Rotavap: PBV- 7D). The dried extract was used directly for determination of antibacterial activities.
Extraction of Cultured Medium
Seven to eight day-old cultures on PDA of the fungus were inoculated into BSL medium in Erlenmeyer flasks 250 ml, followed by static condition and incubated for 30 days at 25 °C. The fermentation broth of fungi was filtered and the filtrate was extracted three times with ethyl alcohol at room temperature. Evaporation of the extracted solution was done in a rotary evaporator [10], and it was used for determination of antibacterial activity.
Extraction of Exopolysaccharide
Equal volume of supernatant (mixture of sucrose and beef extract) was mixed with three times volumes of absolute ethanol and left for 24 h at 4 °C. The resulting precipitate was then separated by centrifugation at 5000 rpm for 1/6 h [1]. The dry weight of EPS was measured after drying at 70 °C for overnight to a constant weight [11]. The rest amount of crude supernatant EPS was used in antimicrobial assay. Yield percentage was calculated in relation to 20 g/l initial sugar concentration.
Preparation of Aqueous
Solution of the EPSs: 20 mg of the EPSs was dissolved in 1 ml of deionised water and prepared according to Akanni et al. [12].
Antibacterial Activity
Test Microorganisms
Antimicrobial activity was tested against Staphylococcus aureus (MTCC 96), Bacillus subtilis (MTCC 619) and Escherichia coli (MTCC 40), procured from IMTECH Chandigarh, India.
Evaluation of Antibacterial Activity
The antibacterial activities were evaluated by the disc diffusion method [13]. To evaluate the antibacterial activity, bacteria were grown in liquid LB (Luria Bertani) medium for 24 h and after this 100 µl bacterial cultures were spread on Petri dishes containing solid LB medium. The discs (4 mm diameter) were then impregnated with 10, 25 and 50 µl of various mushroom extracts (mycelial, broth and EPSs extracts) and then placed on solid LB medium.
Statistical Analysis
All the calculations were done with the help of Past software. All values were expressed as mean ± standard deviation (SD), and experiments were done in triplicate. The experimental results were analyzed using one-way ANOVA (analysis of variance) followed by Tukey’s Least Significant Differences (LSD) to determine the least significant difference at p < 0.05. Principal Components Analysis (PCA) was done by using PAST software.
Results and Discussion
Sample Collection and Identification
Mushroom sample was collected from Lake Chowmuhani market (West Tripura District) of Tripura. On the basis of morphological characters, mushroom was identified as P. flabellatus (Berk. Br.) Sacc. Dry mushroom samples were preserved in the Mycology and Plant Pathology laboratory, Department of Botany, Tripura University, having the accession tag as MCCT- P01.
Submerged Cultivation
Effects of Different Carbon Sources
In order to identify a suitable carbon source for mycelial biomass and EPS production, seven different carbon sources (carbohydrates), i.e., maltose, glucose, sucrose, starch, fructose, xylose and CMC, were used and without carbon source were used as a control (Fig. 1a). The results of cell growth, average growth rate and EPS production under various carbon sources were compared in Fig. 1a, where cell growth and average growth rate vary significantly (p < 0.05). The maximum mycelial yield (3.84 ± 0.43 g/l) and average growth rate (0.55 ± 0.21 g/l/day) were obtained in starch medium after 7 days of cultivation, which showed significance difference (p < 0.05) from other carbon sources, but the highest production of EPS (3.467 ± 0.96 g/l) was observed in sucrose medium followed by maltose (2.69 ± 0.51 g/l) and glucose (2.06 ± 1.78 g/l). Comparatively, good amount of EPS (1.11 ± 0.59 g/l) was observed in control (without carbon and nitrogen source) with less mycelial growth rate (0.31 ± 0.05 g/l and 0.04 ± 0.01 g/l).
Submerged mycelial biomass, average mycelial growth rate and EPSs production applying different carbon (a) and nitrogen (b) sources. Total carbohydrate contents (c) of EPSs produced by applying different carbon and nitrogen sources of P. flabellatus. All the experimental data average of triplicate with standard error and differs significantly at p < 0.05 along the same components
Present findings observed that out of seven carbon sources starch was the best carbon source for mycelial yield and average growth rate of P. flabellatus, but the highest production of EPS was observed in sucrose followed by maltose and glucose. Previous findings revealed that sucrose was the most suited carbon source for both mycelial growth and EPS production in Cordyceps militaris [14] while glucose for Phellinus gilvus [15], fructose and maltose for Collybia maculate and Phellinus gilvus [16]. Our finding also showed similarity with the finding of Hamedi et al. [17] where starch was the suitable carbon source for EPS and biomass production by Agaricus blazei. According to our previous finding, the highest cell weight and growth rate of Auricularia auricula-judae were observed in glucose, but the highest production of EPS was observed in starch as carbon source [2].
Effects of Different Nitrogen Sources
The results of cell growth, average growth rate and EPS production under various nitrogen sources were compared in Fig. 1b. The maximum mycelial yield and average growth rate of P. flabellatus were achieved significantly (p < 0.05) by using peptone as nitrogen source, which showed 1.57 ± 0.87 g/l and 0.22 ± 0.12 g/l/day, respectively. But EPS production was highest in beef extract (6.00 ± 0.59 g/l). From the above findings, it was evident that peptone and beef extract were the most beneficial nitrogen sources for mycelial growth and EPS production of P. flabellatus and it showed significant difference from other nitrogen sources.
Different complex media were found as good control of the exo-biopolymer production and mycelial growth from different mushrooms [2, 13], but we used only simple nitrogen source for EPS production. In our finding, it was observed that out of seven nitrogen sources peptone was the best nitrogen source for better mycelial yield and average growth rate, but EPS production was highest in beef extract followed by malt extract and yeast extract. Among the different nitrogen sources, yeast extract was suitable for the highest yield of EPS production in Cordyceps jiangxiensis and Lyophyllum decastes [18]. The maximum mycelial biomass and EPS production were achieved in medium containing yeast and peptone extract of Pleurotus citrinopileatus [19]. Different research findings showed that in comparison with inorganic nitrogen sources, organic nitrogen sources gave rise to relatively higher mycelial growth and EPS production in G. lucidum [5], which also positively correlated with our finding. But in case of A. auricula-judae maximum biomass yield, average growth rate and EPS production were recorded in urea as nitrogen source [2].
Total Carbohydrate Content of EPSs
In P. flabellatus the highest amount (3.39 mg/100 g) of carbohydrate from EPS was observed in starch medium, which varies significantly (p < 0.05) from glucose and sucrose. Production of carbohydrate (mg/100 g) from EPS of P. flabellatus is depicted in Fig. 1c. In case of nitrogen sources the highest amount of carbohydrate content from EPS was observed in the peptone (4.05 ± 0.08 mg/100 g) medium, which showed significant (p < 0.05) difference from malt extract and calcium nitrate. The mycelial biomass and EPS production by using different carbon and nitrogen sources are compared in Fig. 1c.
In this study, highest EPSs production was observed in sucrose and beef extract as a carbon and nitrogen sources, whereas highest carbohydrate content was observed in starch and peptone. As per the previous documentation, the biosynthetic pathways of mushroom polysaccharides are currently uncertain due to insufficient knowledge of relative enzymes and their roles in the pathways, which should be demonstrated by gene cloning and genetic transformation and their different intermediate compounds and enzymes were also responsible for various biological activities [20]. Therefore, this may be the reason that the differences in EPSs production and their various bioactivities were observed.
Hassan and Medany [21] reported that total carbohydrates content was found 85.17%, whereas our finding was slightly different. Hassan et al. [22] reported that Flammulina velutipes contained 74.85% of total carbohydrate extracted from EPSs. Our previous finding also documented that highest amount of total carbohydrate of A. auricula-judae from EPSs was obtained in medium containing starch as a carbon source and the calcium nitrate as a nitrogen source.
Principal Components Analysis (PCA) analysis
PCA was done by using PAST software. The components A (mycelial growth) and C (EPSs products) were mainly responsible for separating the M area and this variables are the highest positive loadings on PC1 and PC2 (Fig. 2a–f). This area was well known for components of A and C. The PC1 and PC2 contributed most significantly for the separation of R area, which was known for its growth-dependent carbon and nitrogen sources (Fig. 2a–f).
Showing PCA analysis of EPSs of carbon and nitrogen sources: a Loadings of PC1 (Principal component) and PC2 of carbon sources; b Screen plot, eigenvalues and % variance of the first three PCs; c Biplot of PC1 and PC2 for data of carbon sources in PCA1 model; d Loadings of PC1 and PC2 of nitrogen sources; e Screen plot, eigenvalues and % variance of the first three PCs; f Biplot of PC1 and PC2 for data of nitrogen sources in PCA1 model
Fig. 2a–f gives an overall view of the effect of different carbon and nitrogen sources on mycelial growth, average growth rate and EPSs with their significant differences by the results of the principal component analysis. The carbon and nitrogen sources which were closest to variables, i.e. A (mycelial growth), B (average growth rate) and C (EPSs products), are more important in producing effects while those with same direction or slightly distant show positive correlation. Glucose and starch are close to each other in the multivariate space and this was somehow expected as they play a similar role on three variables i.e. A (mycelial growth), B (average growth rate) and C (EPSs products), where fructose and xylose extracts were opposite to each other. But the component C (EPSs products) showed no significant difference from other carbon sources. On the other hand, peptone was significantly different from nitrogen sources the close to each other in case of variables A (mycelial growth) and B (average growth rate) and it was exact opposite of beef extract where highest EPS production was observed. This analysis also showed that urea and control were exact opposite to ammonium sulfate and malt extract.
Medicinal properties
Free Radical Scavenging (FRS) Activity by DPPH Method
EPSs produced from higher carbon and nitrogen sources of P. flabellatus showed varying scavenging ability. Among the three carbon sources, highest free radical scavenging effect was observed by glucose (83.80 ± 0.87%) at 16.0 mg/ml concentration, followed by sucrose (82.49 ± 3.49%) at 16.0 mg/ml concentration and starch (79.89 ± 2.43) at 8.0 mg/ml concentration, respectively (Fig. 3a). On the other hand P. flabellatus showed highest free radical scavenging effect on calcium nitrate (89.01 ± 1.61%) at 16.0 mg/ml concentration, followed by malt extract (85.56 ± 1.32%) and peptone (85.38 ± 0.76) at 16.0 mg/ml concentration, respectively (Fig. 3b). However, highest FRS activity of the synthetic antioxidant (BHT) was 95.98% at 2.0 mg/ml concentration. Variations in FRS activities by various EPSs of carbon and nitrogen sources from P. flabellatus are graphically represented in Fig. 3a and b. The highest free radical scavenging activity of P. flabellatus was observed in glucose and malt extract which were used as a carbon and nitrogen sources and they showed significant differences from other nutritional sources at the level of p < 0.05. The potent EC50 values of EPSs were observed in all the nitrogen sources peptone < malt extract < calcium nitrate at 1.16, 0.99 and 0.93 mg/ml concentrations, whereas carbon sources showed sucrose < starch < glucose at 2.25, 2.03 and 1.19 mg/ml concentrations.
Different EPS productions with different biological activities by macrofungi through submerged cultures were reported by various researchers from different parts of the world [1, 2, 14, 15, 17]. Thai and Keawsompong [23] reported that the EPS of Tricholoma crassum increased DPPH scavenging activity rate from 32.80 to 57.54% at 100 to 500 mg/l concentration. Our finding also revealed that inhibition percentage of EPS was much lower than that of standard, which showed similarity with other findings [23, 24]. According to Xiao et al. [24] the polysaccharide fractions of cultured Cordyceps taii showed moderate activities against the DPPH radicals and their scavenging abilities were increased with increased doses ranging from 0.5 to 8.0 mg/ml concentration. The EC50 value of EPS from T. crassum for DPPH radical scavenging activity was 338 mg/l [23], whereas Xiao et al. [24] reported that the DPPH scavenging abilities of alkali-soluble crude polysaccharide and alkali-soluble refined polysaccharide with EC50 values of 9.8 mg/ml and 13.6 mg/ml concentrations, respectively, were significantly lower to the synthetic Thiourea (EC50 = 3.03 mg/ml). Both the findings showed slightly divergence with our findings.
Antibacterial Activities
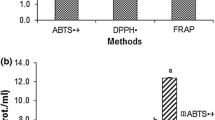
Three types of extract of P. flabellatus showed moderate inhibition against B. subtilis, E. coli and S. aureus in comparison with positive control. Highest zone of inhibition was observed in broth extract of P. flabellatus against B. subtilis, whereas EPSs extract showed lowest activity against B. subtilis. All three types of extract showed no activity against S. aureus. Different extracts of P. flabellatus inhibited the growth of three bacterial strains which are mentioned in Table 1 and presented in Fig. 4.
Present observation revealed that all three types of extract showed positive response against gram-positive bacteria in comparison with gram-negative one; however mycelial extract of P. flabellatus showed potent antibacterial activity against gram-negative bacteria. Previous findings also suggested that antimicrobial activities against gram-negative bacteria were much less common than against gram-positive [25].
The exopolysaccharides are contextually limited to all forms of polysaccharides synthesized and secreted into external environment in shaking condition which may remain loosely attached to the surface or completely detached. [1, 2] As more oxygen was added by agitation into the medium, there was more synthesis of EPS. Meade et al. [26] confirmed that aeration was necessary for EPS production, whereas Torino et al. [27] stated that agitation does not influence growth or EPS production markedly; it might, however, affect viscosity probably breaking some molecular associations among the EPS, the bacterial surface as well as certain medium components. Therefore, it might be the reason that broth extract of P. flabellatus was more effective against bacterial strains as compared to EPSs extract. Suay et al. [28] showed that extracts from mycelial cultures of Lepista nuda, Polyporus arcularius and several Ganoderma species have potent antibacterial activity. Younis et al. [29] documented that the extracts from cultural broth had the lowest antimicrobial activity and possible reason behind this might be that the active antimicrobial components may not be of secretive nature. They also reported that the water extracts from the fruiting bodies and broth of P. ostreatus had much higher antibacterial activities against gram-negative and gram-positive bacteria in comparison with the ethanol and methanol extracts. According to Özdal et al. [30], mycelia extracts from P. eryngii, P. sajor-caju, P. citrinopileatus, P. ostreatus and P. florida showed the antibacterial potentiality against both the gram-negative and gram-positive bacteria.
Conclusion
Present findings revealed that starch and peptone were the best carbon and nitrogen sources for better mycelial growth and average growth rate of P. flabellatus, whereas EPS production was higher in sucrose and beef extract. EPSs produced from best carbon and nitrogen sources were found to be a significant source of carbohydrate. EPSs of various carbon and nitrogen sources have the potentiality to scavenge the free radicals, which will be valuable source material for pharmaceutical industry. Present findings also documented that the various extracts of P. flabellatus (EPSs, broth and mycelia) have antibacterial activity against B. subtilis and E. coli. The further a lot more work is required to explore the active chemicals constituents of EPS and their various functional relationships.
Abbreviations
- EPS:
-
Exopolysaccharide
- MCCT:
-
Mushroom culture collection tube
- BSL:
-
Basal synthetic liquid
- CMC:
-
Carboxymethyl cellulose
- DW:
-
Dry weight
- FRS:
-
Free radical scavenging
- DPPH:
-
1,1diphenyl-2-picrylhydrazyl
- BHT:
-
Butylated hydroxytoluene
- PDA:
-
Potato dextrose agar
- SD:
-
Standard deviation
- PCA:
-
Principal components analysis
- EC:
-
Effective concentration
References
Bae JT, Sinha J, Park JP, Song CH, Yun JW (2000) Optimization of submerged culture conditions for exo-biopolymer production by Paecilomyces japonica. World J MicrobBiot 10:482–487
Debnath S, Halam V, Saha DP, AK, (2018) Proximate composition, Effects of carbon and nitrogen sources on biomass and exopolysaccharides (EPSs) production of Auricularia auricula- judae. Vegetos 31(3):30–36. https://doi.org/10.5958/2229-4473.2018.00070.8
Wani BA, Bodha RH, Wani AH (2010) Nutritional and medicinal importance of mushrooms. J Med Plant Res 4(24):25–26. https://doi.org/10.5897/JMPR09.565
Pegler DN (1977) A preliminary agaric flora of east Africa. Kew Bull AdditSer 6:1–615
Fang QH, Zhong JJ (2002) Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites–ganoderic acid and polysaccharide. BiochemEng J 10:61–65. https://doi.org/10.1016/S1369-703X(01)00158-9
Hedge JE, Hofreiter BT (1962) Carbohydrate chemistry (Whistler RL, Be Miller JN eds.). Academic Press, New York.
Mau LJ, Huang PN, Hung SJ, Chen CC (2004) Antioxidant property of methanolic extracts from two kinds of Antrodia camphorata mycelia. Food Chem 86:25–31. https://doi.org/10.1016/j.foodchem.2003.08.025
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40(6):945–948. https://doi.org/10.1021/jf00018a005
Kalyoncu F, Oskay M, Kayalar H (2010) Antioxidant activity of the mycelium of 21 wild mushroom species. Mycology 1(3):195–199. https://doi.org/10.1080/21501203.2010.511292
Sutjaritvorakul T, Whalley AJS, Sihanonth P, Roengsumran S (2011) Antimicrobial activity from endophytic fungi isolated from plant leaves in Dipterocarpous forest at Viengsa district Nan province. Thailand J AgricTechnol 7(1):115–121
Iwan S (2009) Exopolysaccharide production and its bioactiveties of the edible Pleurotus ostreatus in submerged culture. Biotropia 162:96–104
Akanni KT, Ikeobi OM, Adebambo F (2010) Genetic difference in hen-day production egg quality traits in pure and cross bred chickens in a humid environment. In: Ifut OJ (eds) Proceedings of the 15th Annual Conference of the Animal Science Association of Nigeria (ASAN); Nigeria, pp. 78–81
Collins CH, Lyne PM (1987) Microbiological methods. Butter Worths and Co (Publishers) Ltd, London
Kim SW, Hwang HJ, Xu CP, Sung JM, Choi JW, Yun JW (2003) Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J ApplMicrobiol 94:120–126. https://doi.org/10.1046/j.1365-2672.2003.01754.x
Xu CP, Yun JW (2003) Optimization of submerged-culture conditions for mycelial growth and exo-biopolymer production by Auriculariapolytricha (wood ears fungus) using the methods of uniform design and regression analysis. BiotechnolApplBiochem 38:193–199. https://doi.org/10.1042/BA20030020
Lim JM, Kim SW, Hwang HJ, Joo JH, Kim HO, Choi JW, Yun JW (2004) Optimization of medium by orthogonal matrix method for submerged mycelial culture and exopolysaccharide production in Collybia maculate. ApplBiochem Biotech 119:159–170. https://doi.org/10.1385/abab:119:2:159
Hamedi A, Ghanati F, Vahidi H (2012) Study on the effects of different culture conditions on the morphology of Agaricus blazei and the relationship between morphology and biomass or EPS production. Ann Microbiol 62:699–707. https://doi.org/10.1007/s13213-011-0309-3
Xiao DM, Song Y, Xiao JH (2006) Antioxidant activities of alkali-soluble polysaccharides from medicinal mushroom Cordyceps taii and its chemical characteristics. Biomed Res 27(1):199–206
Wu CY, Liang ZC, Lu CP, Wu SH (2008) Effect of carbon and nitrogen sources on the production and carbohydrate composition of exopolysaccharide by submerged culture of Pleurotus citrinopileatus. J Food Drug Anal 16:61–67
Wang Q, Wang F, Xu Z, Ding Z (2017) Bioactive mushroom polysaccharides: a review on monosaccharide composition, biosynthesis and regulation. Molecules 22(6):955. https://doi.org/10.3390/molecules22060955
Hassan FRH, Medany GM (2012) Studies on submerged culture conditions for mycelial biomass production of wood ears mushroom (Auricularia polytricha). Middle East J Agric Res 1(1):33–39
Hassan FRH, MedanyGhada M, El-Kady ATM (2012) Mycelial biomass production of enoke mushroom (Flammulina velutipes) by submerged culture. Aust J Basic ApplSci 6:603–610
Thai LQ, Keawsompong S (2019) Production of exopolysaccharide from Tricholoma crassum in submerged culture and its antioxidant activities. Int J of AgricTechnol 15(1):141–156
Xiao DM, Yu S, Xiao JH (2016) Antioxidant activities of alkali-soluble polysaccharides from medicinal mushroom Cordyceps taii and its chemical characteristics. Biomed Res 27:199–206
Yamaç M, Bilgili F (2006) Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushrooms isolates. Pharm Biol 44(9):660–667. https://doi.org/10.1080/13880200601006897
Meade M, Tanenbaum SW, Nakas JP (1994) Optimization of novel extracellular polysaccharide production by an Enterobacter sp. on wood hydrolysates. Appl Environ 60(4):1367–1369
Torino MI, Mozzi F, Sesma F, Font de Valdez G (2000) Effect of stirring on growth and phosphopolysaccharide production by Lactobacillus helveticus ATCC 15807 in milk. Milchwissenschaft 55:204–206
Suay I, Arenal F, Asensio FJ, Basilio A, Cabello MA, Diez MT, Garcia JB, Val AG, Gorrochategui J, Hernandez P, Pelaez F, Vicente MF (2000) Screening of basidiomycetes for antimicrobial activites. Antonie Van Leeuwenhoek 78:129–139
Younis AM, Wu FS, El Shikh HH (2015) Antimicrobial activity of extracts of the oyster culinary medicinal mushroom Pleurotus ostreatus (higher basidiomycetes) and identification of a new antimicrobial compound. Int J Med Mushrooms 17:579–590. https://doi.org/10.1615/intjmedmushrooms.v17.i6.80
Ozdal M, Gulmez O, GurOzdal O, Algur OF (2019) Antibacterial and antioxidant activity of mycelial extracts of different Pleurotus species. Food Health 5(1):12–18. https://doi.org/10.3153/FH19002
Acknowledgements
The authors are grateful to the Head, Department of Botany, Tripura University for providing all sorts of laboratory facilities. The authors are also thankful to Forest Department of Tripura, Government of Tripura, India, for giving the permission of fieldwork in forest areas. The first author is thankful to the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India for the financial assistance received through a Project (Sanctioned Order No. BT/463/NE/TBP/2013).
Funding
This research work was funded by Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India (Sanctioned Order No. BT/463/NE/TBP/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement Present findings revealed that starch and peptone were the best carbon and nitrogen sources for better mycelial growth and average growth rate of P. flabellatus, whereas EPS production was higher in sucrose and beef extract. EPSs produced from best carbon and nitrogen sources were found to be a significant source of carbohydrate. EPSs of various carbon and nitrogen sources have the potentiality to scavenge the free radicals, which will be valuable source material for pharmaceutical industry. Present findings also documented that the various extracts of P. flabellatus (EPSs, broth and mycelia) has potent antibacterial activity against B. subtilis and E. coli. The further work is still needed to explore the active chemicals constituents of EPS and their various functional relationships.
Rights and permissions
About this article
Cite this article
Debnath, S., Debnath, B., Saha, R. et al. Production of Exopolysaccharides (EPSs) and Evaluation of Biological Properties of Pleurotus flabellatus (Berk and Br.) Sacc. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 91, 581–591 (2021). https://doi.org/10.1007/s40011-021-01261-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-021-01261-y