Abstract
Biofabrication of metal nanoparticles is a cost-effective, one-step and ecofriendly technique. Cell filtrate of an endophytic fungus isolated from Nyctanthes arbor-tristis was challenged with 1 mM solution of AgNO3 for the silver nanoparticles (AgNPs) synthesis. A reduction of silver ions into AgNPs was observed by surface plasmon resonance at absorption maxima 422 nm. The average size of AgNPs was 35.05 nm. The maximum inhibition zones by AgNPs were 14 mm each against E. coli and Pseudomonas aeruginosa compared to AgNO3 solution used as control (10 mm and 9 mm). The fungus was identified as Phomopsis helianthi by the sequencing of ITS region of rDNA. This experiment demonstrates a single-step and ecofriendly method for biosynthesis of AgNPs and their usage as an antimicrobial agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is a science of designing, characterization, production, and application of materials, devices, and systems by controlling the shape and size at the nanometer scale [1]. The exercise in nanotechnology is done for the transformation of materials in the form of nanoparticles, nanoclusters, nanodots, nanotubes, nanofibres, nanowires which reduce the cost and space for the application of those materials. The nanoparticles of gold are used in targeted drug delivery in cancer cells, whereas iron NPs are used for groundwater pollutants. Fabric treatment is done with AgNPs for the removal of microbial contaminations [2]. Interestingly, an excellent antiviral activity against HIV-1 was reported from silver nanoparticles. AgNPs bind to gp120 in a manner that prevents CD4-dependent virion binding, fusion, and infectivity, acting as an effective viricidal agent against the cell-free and cell-associated virus [3]. Despite other physical and chemical techniques, using a fungal endophyte Phomopsis helianthi, the authors report the one-step, ecofriendly, and cost-effective biosynthesis of AgNPs and their antibacterial potential. This endophytic fungus resides inside healthy plant tissues of Nyctanthes arbor-tristis without causing any symptoms to the host [4]. Interestingly, fungal endophytes are reported to produce a variety of bioactive and anticancer compounds [5].
Material and Methods
Isolation of Endophytic Fungus
The fungus was isolated from the healthy leaf of Nyctanthes arbor-tristis growing in the campus of Banaras Hindu University, Varanasi (25.5°N, 82.9°E; elevation 85 m) according to the standard protocol [4].
Biosynthesis of Silver Nanoparticles (AgNPs)
Biosynthesis of silver nanoparticles was done according to Singh et al. [6] with slight modifications. The fungus was grown in 1-l flasks containing 800 ml potato dextrose broth for three weeks on a rotary shaker at 200 rpm. Fungal biomass was filtered and resuspended in 100 ml deionized distilled water and agitated at 200 rpm for 24 h. The biomass was filtered with filter paper (Whatman No 1), and the filtrate was further centrifuged at 10,000 rpm to get the cell-free extract. The 50 ml cell-free extract was challenged with 50 ml of 01 mM ionic solution of AgNO3. A parallel control was kept using metal solution and distilled water in place of cell-free extract. The reaction mixture was left for 24 h on a shaker (200 rpm) at room temperature. The absorption spectra were recorded after 24 h from wavelength 250 nm to 700 nm on UV–visible spectrophotometer (Hitachi, UV-2910) at 1 nm resolution.
Characterization of AgNPs by Transmission Electron Microscopy (TEM)
A drop of the suspension of nanoparticles was placed onto a copper grid coated with 2% collodion in amyl acetate. After one minute, the extra solution was removed using blotting paper and stained with 2% phosphotungstic acid, air-dried, and examined under a transmission electron microscope (Technai-12 G2, FEI, The Netherlands) equipped with SIS Mega View III CCD camera at 120 kV. Measurements were done using the AnalySIS software (SIS, Germany).
Characterization of AgNPs by X-rays Diffraction (XRD) Analysis
The nanoparticles were further characterized by XRD spectrometry (Cu-Kα radiation source) using a 12 kW rotating Cu anode-based Rigaku (Tokyo, Japan) powder diffractometer (PRINT 2000/PC series) operating in Bragg–Brentano geometry and fitted with curved crystal graphite monochromator in diffraction beam and a high-temperature attachment.
Characterization of AgNPs by Fourier Transforms Infrared (FTIR) Spectroscopy
The nanoparticles solution was centrifuged at 14,000 rpm for 25 min. The pellet was washed three times with 20 ml of deionized water to get rid of the free proteins/ enzymes that were not capping the nanoparticles. The samples were dried and ground with KBr pellets and analyzed on a Varian 1000 FTIR Scimitar series.
Antimicrobial Assay of AgNPs
The antimicrobial activity of AgNPs was observed by disk diffusion assay against Escherichia coli, Staphylococcus sp., Morganella morgenii, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella enteritidis, and Shigella boydii. The sterile filter paper disks (5.0 mm dia.) were impregnated with 5 µl suspension of AgNPs (0.5 mM) and placed onto agar plates pre-seeded with test bacterium. The filter paper disks impregnated with 5 µl solution of silver nitrate with the same concentration used for nanoparticles were used as control. The diameter of the inhibition zone of bacteria around the disks was measured and compared with control.
Identification of Endophytic Fungus
The genomic DNA of endophytic fungus was extracted and amplified following the protocol of Sim et al. [7]. The universal primers ITS1 and ITS4 (Metabion International, Martinsried, Germany) were used to amplify 5.8S rDNA and ITS2 regions flanked between the 18S and 28S r RNA genes. Amplified PCR products were resolved by electrophoresis in 1.5% (w/v) agarose gels stained with ethidium bromide (0.5 μg /ml) for visual examination. PCR product was sent to First BASE Laboratories (Malaysia) for sequencing. Obtained ITS rDNA sequences were compared by BLAST search among mentioned sequences at the NCBI GenBank for the identification.
Results and Discussion
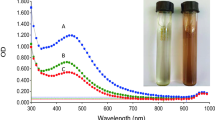
The conversion of colorless silver nitrate solution to yellowish-brown after treatment with cell filtrate of P. helianthi was considered a positive signal of AgNPs biosynthesis and was confirmed by UV–visible absorption peak at 422 nm of surface plasmon resonance (SPR) (Fig. 1). SPR excitation is the absorption and scattering of a wavelength-selective photon which leads to a remarkable enhancement of the local electromagnetic field near the surface of the AgNPs [8]. Variable shapes from spherical to hexagonal were found in AgNPs suggesting the polydispersed conditions (Fig. 2a). Through TEM observation, the maximum size of AgNPs was recorded up to 40 nm. Only Saifuddin and colleagues reported a similar size range (5–60 nm) of AgNPs which corroborates this study [9]; however, the considerable numbers of AgNPs in this study were below 05 nm which is quite impressive. The histogram of nanoparticles showed that more than 50% nanoparticles were below 10 nm (Fig. 2b). The smaller AgNPs were spherical, while larger were of pentagonal or hexagonal. Two fungal species Aspergillus tubingensis and Bionectria ochroleuca have been reported to biosynthesized silver nanoparticles in a spherical shape with a size of 35 ± 10 nm [10]. The XRD indexing of AgNPs was in accordance with Bragg’s reflection (JCPDS No: 03–0921) (Fig. 3) [11]. The lattice parameter was 4.074. The nanoparticles were crystallized in the face-centered cubic (FCC) structure with a space group of Fm3m (No. 225). In a similar study, Gajbhiye et al. [12] have reported the average size of spherical AgNPs as 32.5 nm synthesized by Alternaria alternata.
The FTIR analysis showed broad peaks around 3400 cm−1 and 1000 cm−1 (Fig. 4) due to the presence of a large number of hydroxyl, carboxyl and carbonyl groups, amides or amino-methyl stretching, and hydrogen bonding between them [13]. The peak around 3400 and 2900 cm−1 may refer to N–H stretch vibration of primary and secondary amides of protein and O–H stretch of alcohols and phenols. The peaks around 1300 cm−1 may refer to amine and amino-methyl stretching groups of protein [14]. These metabolites act as capping or stabilizing agents for AgNPs to protect them from agglomeration.
The bacterial inhibition was comparatively greater by AgNPs than AgNO3 (Table 1). AgNPs have displayed maximum inhibition zones (14 mm) against Escherichia coli and Pseudomonas aeruginosa. The combination of penicillin and AgNPs synthesized using Citrus reticulata juice showed an inhibition zone of 18 mm against E. coli [15]. AgNPs were active against both gram − ve and gram + ve bacteria. It differs from the antimicrobial activity of AgNPs synthesized by Aspergillus clavatus against Pseudomonas fluorescens and E. coli [16] where activity was only against gram-negative bacteria. The interaction between AgNPs and bacterial membrane causes structural changes and damage leading to death [17]. The biological synthesis of antimicrobial AgNPs was also characterized by an endophytic fungus Alternaria sp. isolated from the healthy leaves of Raphanus sativus [18].
Based on molecular sequence similarity (96%), the fungus was identified as Phomopsis helianthi with NCBI GenBank accession No. JN163858. Phomopsis is a very common filamentous endophytic species found in tropical and temperate medicinal plants as well [19]. Endophytic Phomopsis species is reported to produce antimicrobial compounds such as phomol and antimalarial phomoxanthones [20]. It is hypothesized that there may be NADH-dependent reductase which plays a role in the reduction of silver ions into AgNPs in cell filtrate of Fusarium oxysporum [21].
Conclusion
The authors report the extracellular, single-step and safe biosynthesis of AgNPs by endophytic fungus P. helianthi which gives advantage over intracellular nanoparticles synthesis because that requires an additional effort to purify from the adhering biomass and exploiting them as an antibacterial agent. This method is ecofriendly using a biological agent for metal nanoparticle synthesis which does not affect our environment. Further research is needed for the investigation of the mechanism involved in nanosynthesis by biological molecules.
References
Mansoori GA (2005) Principles of nanotechnology—molecular-based study of condensed matter in small systems. World Scientific Publishing Co., Hackensack
Yeo SY, Jeong SH (2003) Preparation and characterization of polypropylene/silver nanocomposite fibers. Polym Int 52:1053–1057
Lara HH, Nuñez NVA, Turrent LI, Padilla CR (2010) Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol 8:1–10
Gond SK, Mishra A, Sharma VK, Verma SK, Kumar J, Kharwar RN, Kumar A (2012) Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis L., a well known medicinal plant of India. Mycoscience 53:113–121
Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D (2011) Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat Prod Rep 28:1208–1228
Singh DK, Sharma VK, Kumar J, Mishra A, Singh A, Kumari P, Verma SK, Kharwar RN (2018) Mycosynthesis of bactericidal silver and polymorphic gold nanoparticles: physicochemical variation effects and mechanism. Nanomedicine 13(2):191–207
Sim JH, Khoo CH, Lee LH, Cheah YK (2010) Molecular diversity of fungal endophytes isolated from Garcinia mangostana and Garcinia parvifolia. J Microbiol Biotechnol 20(4):651–658
Xu G, Chen Y, Tazawa M, Jin P (2006) Surface plasmon resonance of silver nanoparticles on vanadium dioxide. J Phys Chem B 110:2051–2056
Saifuddin N, Wong CW, NurYasumira AA (2009) Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E J Chem 6(1):61–70
Rodrigues AG, Ping LY, Marcato PD, Alves OL, Silva MC, Ruiz RC, Melo IS, Tasic L, De Souza AO (2013) Biogenic antimicrobial silver nanoparticles produced by fungi. Appl Microbiol Biotechnol 97:775–782
Shankar S, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 13:1822–1826
Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine 5:382–386
Schrand AM, Braydich-Stolle LK, Schlager JJ, Dai L, Hussain SM (2008) Can silver nanoparticles be useful as potential biological labels? Nanotechnol 19:1–13
Hamad MT (2019) Biosynthesis of silver nanoparticles by fungi and their antibacterial activity. Int J Environ Sci Technol 16:1015–1024
Sreekanth TVM, Nagajyothi PC, Prasad TNVKV, Lee KD (2013) Green synthesis of silver nanoparticles using Citrus reticulata juice and evaluation of their antibacterial activity and cytotoxicity against melanoma-B16/F10 cells. Curr Sci 9:457–462
Verma VC, Kharwar RN, Gange AC (2010) Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 5:33–40
Suresh AK, Pelletier DA, Wang W, Moon JW, Gu B, Mortensen NP, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2010) Silver nanocrystallite: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity in gram-negative and gram-positive bacteria. Environ Sci Technol 44:5210–5215
Singh T, Jyoti K, Patnaik A, Singh A, Chauhan R, Chandel SS (2017) Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol 15(1):31–39
Gond SK, Verma VC, Kumar A, Kumar V, Kharwar RN (2007) Study of endophytic fungal community from different parts of Aegle marmelos Correae (Rutaceae) from Varanasi (India). World J Microbiol Biotechnol 23:1371–1375
Weber D, Sterner O, Anke T, Gorzalczancy S, Martino V, Acevedo C (2004) Phomol, a new anti-inflammatory metabolite from an endophyte of the medicinal plant Erythrina crista-galli. J Antibiot 57:559–563
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28:313–318
Acknowledgements
SKG acknowledges the financial help of CSIR, New Delhi, in the form of JRF and SRF. The authors thank Head, Department of Botany, BHU, Prof. Gopal Nath and Dr. Madhu Yashpal (IMS, BHU) for antibacterial and TEM facility, respectively. The authors are also thankful to Prof. D. Pandey, IIT, BHU and Dr. Kedar Singh, Physics Department, BHU, for XRD analysis and Head, Ceramic Engineering, IIT, BHU, for FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement
Green synthesis of nanoparticles using fungal biomass is an eco-friendly and cost-effective process. This study signifies that endophytic fungus Phomopsis helianthi could be a good agent for the synthesis of antimicrobial silver nanoparticles.
Rights and permissions
About this article
Cite this article
Gond, S.K., Mishra, A., Verma, S.K. et al. Synthesis and Characterization of Antimicrobial Silver Nanoparticles by an Endophytic Fungus Isolated from Nyctanthes arbor-tristis. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 641–645 (2020). https://doi.org/10.1007/s40011-019-01137-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-019-01137-2