Abstract
Pyrenacantha volubilis, an important medicinal plant, is reported to contain camptothecin (CPT), a high value anticancer compound with a global demand of USD 2.2 billion in 2008. Isolated root cultures of P. volubilis could be established using radicle-developed roots from the seeds cultured in 0.3 mg L−1 kinetin. The tap roots isolated from the germinated seeds were transferred to half-strength MS liquid media supplemented with 0.3 mg L−1 indole-3-acetic acid and 0.2 mg L−1 indole-3-butyric acid to establish isolated root cultures and were further subcultured into the same media to study the growth and CPT production over a period of 60 days at an interval of 10 days. Maximum biomass (3.34 ± 0.19 g) was obtained from roots cultured in MS liquid media augmented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA with alternating salt strength over a period of 45 (17 + 28) days. The concentration of CPT in the in vitro root-derived culture sample was estimated through HPLC and recorded as 0.135% (on g DW basis, 30th day) which is comparable to that of field grown roots (0.13%). The overall results obtained with the present experiment demonstrated the use of in vitro seedlings as a dynamic explant source for root culture establishment and the feasibility of the system for further use in the production of CPT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant secondary metabolites are a diverse group of organic compounds which facilitate the interaction of plants with the environment and also in the establishment of a defence mechanism. Among the important plant-derived compounds prescribed for cancer therapy, camptothecin (CPT), a water insoluble cytotoxic monoterpene-derived indole alkaloid, has been recognized as a very potent anticancer compound for its amazing inhibitory action against tumour cells by blocking the eukaryotic topoisomerase-I. Over a dozen derivatives of CPT are currently under clinical trials. Derivatives such as Hycamtin (topotecan) and Camptosar (irinotecan or CPT 11) are currently used widely for the treatment of ovarian, small lung and refractory ovarian cancers.
Due to the escalating demand (USD 2.2 billion in 2008) [1] for CPT coupled with limited availability of plant source, alternative methods need to be developed to circumvent the issue. In spite of the rapid growth of market demand, CPT raw material is still harvested by extraction from bark and seeds of Camptotheca acuminata and Nothapodytes nimmoniana, since its chemical synthesis is not cost-effective. Though many attempts have already been made to produce CPT from in vitro cultures of several plants, the yield of CPT was found to be comparatively low from natural sources. The optimization of culture parameters, the supply of biosynthetic precursors, and genetic engineering are among the strategies that can be adopted to increase its yield.
Recently, Pyrenacantha volubilis Hook., a woody climber (Icacinaceae), was reported to contain CPT and its derivatives and is one of the richest sources of CPT [2]. Maximum concentration of CPT was found in the cotyledons (1.35%) followed by ripened whole fruit and roots (0.13%, g DW) [2]. P. volubilis is a monoecious plant with low seed set. Its distribution in Kerala has been reported in certain evergreen patches and sacred grooves along the western coast of India [3]. Biotechnological methods including plant tissue culture and establishment of different in vitro culture system(s) have immense scope for mass production of plants along with enhanced compound yield. In this regard, the authors are reporting for the first time the in vitro production of CPT from normal root cultures of P. volubilis using in vitro-derived seedling explants.
Material and Methods
Pyrenacantha volubilis seeds were collected from a sacred groove of Thiruvananthapuram district, Kerala, India, and the voucher specimen (TBGT 79916) has been deposited at JNTBGRI herbarium, Palode, Thiruvananthapuram.
Culture Initiation
The fruits of the plants were collected during September–December. Fresh fruits were de-pulped and washed thoroughly in tap water with 5% teepol (commercial detergent) for 10 min. The seeds were again washed thoroughly in running tap water for 15 min and then surface sterilized with 0.1% HgCl2 (w/v) for 3 min. Later on, these seeds were washed with sterile distilled water for 2–4 times and the hard seed coat was removed before inoculating the embryos into half-strength Murashige and Skoog (MS) [4] solid medium containing 3% sucrose and supplemented with varied concentrations of plant growth regulators (PGRs) such as 6-benzylaminopurine (BAP) (0.5–4.0 mg L−1), naphthalene acetic acid (NAA) (0.2–0.8 mg L−1), indole-3-acetic acid (IAA) (0.1–0.4 mg L−1) and kinetin (Kn) (0.3–0.5 mg L−1). MS solid media devoid of PGRs were used as control. The pH of the media was adjusted to 5.8 before adding 0.15% CleriGel (w/v) (Himedia). Ten mL of the prepared media was poured into borosilicate culture tubes (150 mm × 25 mm) and autoclaved at 121 °C under 15 lbs pressure for 18 min. The cultures were kept under proper culture conditions (18/6 h photoperiod, 24 ± 2 °C).
Establishment of Isolated Root Culture
In order to establish isolated root culture, radicle-derived roots from the cultured seeds were dissected, either as intact 3-cm-long pieces (Fresh weight, 0.358 ± 0.008 g) or as chopped roots (0.356 ± 0.009 g FW) and transferred separately to both MS solid and liquid media supplemented with IAA (0.2–0.5 mg L−1) and IBA (0.2–0.3 mg L−1) either alone or in combination. The cultures were kept on a gyratory shaker at 80 rpm and also kept without shaking under complete darkness. Growth of roots and CPT production were studied up to 60 days at an interval of 10 days, and the growth index was determined by the following formula.
Optimization of Root Culture Protocol
In order to optimize the culture parameters, influence of media salt strength, varying concentrations of ammonium nitrate and sucrose were studied individually. Roots were inoculated on full strength, half-strength and quarter-strength MS liquid media containing same combinations of PGRs to find out suitable media for good growth of root samples. Root segments with an initial inoculum of 0.357 ± 0.006 g FW were inoculated onto 50 mL of nutrient media and studied the growth up to 30 days, since maximum biomass production was recorded for the 30th day and also estimated the concentration of CPT.
In another set of experiments, different concentrations of ammonium nitrate (NH4NO3) (1.650 g L−1, 1.2375 g L−1, 0.4125 g L−1 and 0 g L−1) as per the concentrations in full, ¾, and quarter-strength MS media, respectively, were also tested to optimize the root growth in half-strength media supplemented with same PGR combinations (0.3 mg L−1 IAA and 0.2 mg L−1IBA). Similarly, influence of different sucrose concentrations (1–6% w/v) on the root growth was also studied. In all experiments, root biomass level was estimated. Half-strength MS media (3% sucrose) supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA were used as the control. Data were recorded at 10 days interval over a period of 30 days.
Further, the cultures were first subcultured in quarter-strength MS supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA for 17 days and followed by subculture in half-strength MS media supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA for 28 days to study the influence of alternating salt strength on increased biomass production.
Extraction and Quantification of Camptothecin
Old root cultures of 28 days were harvested, dried up to constant final dry weight, ground to fine powder and extracted with methanol in Soxhlet apparatus for 4 h [5]. The extract was concentrated under vacuum using a rotavapour (Heidolph, Germany) at 50 °C and used for separation of the compound through TLC. TLC was performed on a pre-coated silica gel 60 F254 TLC plate (Merck, Germany). The samples were loaded onto the plate using a capillary tube. The mobile phase was chloroform and methanol (24:1 v/v). The authentic sample of CPT procured from Sigma Co., USA, was also loaded along with the extracted sample. After the compounds got separated, the plates were observed under UV light, and the Rf value of the separated compound was determined.
The methanolic extract of root culture was analysed using high-performance liquid chromatography (HPLC) attached with UV/VIS detector (Prominance SPD M20 A diode array detector) using a modified method as described by Roja and Heble [6]. Shimadzu Lab solution version 5.73 is used for data acquisition and instrumental control. Separation of the compounds was performed on a general purpose Shimadzu C-18 column (250 × 4.6 mm, 5 µm particle size, 5 µm), and the isocratic mobile phase consisted of 100% methanol (HPLC grade, Merck, Germany). The flow rate was 1.0 mL min−1, and the injection volume was 10 µL. The analysis was performed at room temperature (25 °C), and the compound was detected at 254 nm. Standard camptothecin (Sigma Co., USA) was used for the estimation of CPT in the root extract of P. volubilis. Standard CPT in the concentration range of 1–500 ppm was run in HPLC column for developing a calibration curve. The crude methanolic extract of plant sample was injected separately. The peak area of the crude extract was measured and compared with that of the standard and quantified equation from the calibration curve, f(x) = 22.294x + 7.4857. The results are expressed in mean ± standard deviation.
Statistical Analysis
The experiment was conducted twice in four replicates in a completely randomized design. Data were recorded in 10 days interval, if not specified otherwise. Statistical analysis was done using SPSS software (IBM SPSS Statistics 21) using Duncan’s multiple range test (DMRT) at a probability of 5%.
Results and Discussion
Culture Initiation
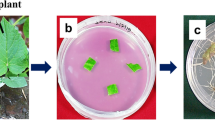
An average of 80% seeds was contamination free. The seeds inoculated on to different media have shown varied response to germination depending on the type and concentration of PGRs used in the media, and the best results (80%) were obtained in media supplemented with 0.3 mg L−1 Kn alone and a combination of BAP (1.0 mg L−1) and NAA (0.2 mg L−1). Emergence of roots (Fig. 1a, b) was observed within 5–15 days of culturing, and emergence of shoot was delayed by up to 35 days irrespective of the media combinations tried. Seeds cultured in all media combinations tested exhibited callus formation except the combinations of Kn (0.3 mg L−1) alone and a combination of BAP (1.0 mg L−1) and IAA (0.2 mg L−1).
a Seed germinated in ½ MS solid with 0.3 mg L−1 Kn, b germinated seed after 30 days of incubation, c lateral root formation in 25 days of subculture in ¼ strength MS media supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA and d established normal root culture in half-strength MS media supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA after 45 days of incubation
The seed development, dormancy and germination are controlled by specific endogenous growth promoting and inhibiting compounds [7]. The activation of metabolic machinery of embryo for seed germination is achieved through the application of various PGRs. The combined effect of hormones supplied along with the appropriate environmental conditions helps to achieve fast germination rate. In the experiments conducted, seed germination in media with a combination of BAP and IAA exhibited the lowest percentage of germination (0–40%) while media supplemented with a combination of BAP and NAA exhibited a germination percentage of 50–70%. The presence of IAA may have negative effect on seed germination possibly due to lack of hormonal receptors during the initial stages of seed germination or due to an intoxication process caused by IAA as evident from the studies on in vitro seed germination of Comparettia falcata [8]. The use of kinetin has shown a positive response in germination rate (60–80%) within 20 days of culture initiation.
Establishment of Isolated Root Culture
Roots transferred to liquid media showed very slow growth in early days of subculture. Profuse lateral branching was observed after 12 days. Roots kept without agitation did not exhibit any growth but had slight callusing. Root growth was observed only in intact roots and initiated with elongation of root tips. No growth was noticed in cultures initiated from chopped root pieces. Root growth was prominent in liquid media than solid culture. Roots of approximately 3 cm length having root tips(meristematic zones) only were found to grow up to a length of 15.4 ± 2.4 cm during the culture period of 30 days with 12.8 ± 3.4 lateral roots generated per root segment inoculated (Fig. 1c). Growth performance of roots was also recorded with the growth index of 4.95 in 30 days (Fig. 2). Maximum biomass production as well as CPT concentration (0.135%, g DW) was also recorded for the 30th day (Fig. 3).
In the experiment conducted, roots having meristematic cells were only found to achieve further growth after subsequent subcultures. The observation was in accordance with a study in Datura stramonium, suggesting that the growth rate in culture system varied with that of hairy root cultures mainly because of the presence of active intercalary meristematic zones as well on development of lateral roots rather than differences in cell division rate and the presence of larger meristem [9]. In the experiments conducted, it was observed that despite of a marked difference in the number of lateral root production, the final biomass was found to be higher in media with half the salt strength of MS compared to quarter strength. This suggests that the divided cells undergo cell proliferation by absorbing more water and nutrients in the second stage of development in half-strength media leading to cell elongation rather than fast meristematic division during the first stage as evident from previous reports [9].
Optimization of Root Culture Protocol
Among the various media salt strengths tested, best result was obtained with half-strength media containing 0.3 mg L−1 IAA and 0.2 mg L−1 IBA. The results are presented in Table 1. The media supplemented with 0.3 mg L−1 IBA also yielded almost similar results. Media containing very low concentration of IAA did not support growth but favoured chlorophyll development in roots. The major aim of the experiment was to identify the feasibility of establishing a novel culture system for the in vitro production of CPT from seedling-derived root cultures of P. volubilis. Experimental strategies were adopted for the enhancement of biomass production in various combinations tried, since enhanced biomass production directly correlates with compound production. Basic knowledge about the biosynthesis of a particular secondary metabolite and the factors influencing the productivity of the cultured cells like manipulating media components, PGR augmentation, varying concentrations of carbon sources, nitrogen sources, phosphates, nitrate to ammonia ratio and micronutrients is imperative in standardizing culture conditions for optimized production [10]. Lateral root formation was observed to be more in MS quarter-strength media (Fig. 1c) supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA (data not furnished), but higher biomass production was obtained in half-strength media containing 0.3 mg L−1 IAA and 0.2 mg L−1 IBA. Hence, this influence of alternate media strength is utilized for enhanced root production as evidenced from previous reports on shoot cultures of Ophiorrhiza trichocarpos [11]. Similar reports by Kumar et al. [12] on micropropagation of Rosa damascena showed that the increase in the number of shoots on transfer to lower concentration may be because of the attainment of juvenility by cells through various biochemical changes occurring under the specific culture condition. The roots were first subcultured in quarter strength for a period of 17 days for increased lateral root formation followed by subsequent subculture in half-strength media (28 days) attaining an increased biomass production of 3.34 ± 0.19 g FW in 45 days (Fig. 1d). The study on the effect of varying concentrations of ammonium nitrate and sucrose was also studied. The results revealed that modified level of ammonium nitrate and sucrose in MS media did not favour biomass production. Though lower and higher concentrations of ammonium nitrate supported increase in biomass at the initial period of growth and later showed negative response. Nitrogen and sucrose are reported to play a vital role in the root system for effective growth of plants. Ammonium nitrate is reported to play an important role in the root induction [13] and growth as well as maintaining the pH of the culture media [14]. However, there are reports of varied responses also [15]. Studies conducted on the effect of ammonium nitrate concentration revealed that both increasing and decreasing concentrations of ammonium nitrate had a negative impact on root growth. This is in agreement with the findings of Woo et al. [16] and Roycewicz and Malamy [17] in experiments conducted with normal root cultures of Hyoscyamus niger and Arabidopsis thaliana seedlings, respectively.
Results on influence of varied concentrations of sucrose also indicated that 3% (w/v) sucrose was optimum for maximum biomass production (2.082 ± 0.333 g) for a period of 30 days. But, the roots grown in 6% sucrose also yielded considerable biomass and lateral root formation. Low concentrations of sucrose had a negative impact in biomass accumulation (Table 2). Sucrose is reported to act as a stimulant in lateral root formation. The sugar-induced lateral root formation is in concurrence with the amount of sugar in roots [18]. Hence, increased sucrose concentration can stimulate lateral root formation regardless of whether it is supplied exogenously or produced within the roots itself [18, 19]. Reports regarding varied sucrose concentration reveal that 2–3% (w/v) sucrose concentration is optimal for biomass production in suspension cultures and higher sucrose concentration resulted repressed growth [20]. But in the present experiments, it was observed that 3% (w/v) sucrose was optimum for maximum biomass production, whereas higher concentration of sucrose (5–6%) also favoured biomass production than lower concentration (1–2%), indicating the sucrose tolerant nature of the roots. The results showed that the nutrients in half-strength MS media are adequately balanced to provide optimal growth, and metabolism in in vitro roots of P. volubilis as has been observed in several other tissue culture systems [21].
Extraction and Quantification of Camptothecin
The methanolic extract of 28 days old in vitro root sample was subjected to TLC, and the presence of CPT in the sample was identified (Rf—0.6) (Fig. 4). Linear regression revealed to have good correlation between the concentration of the standard solutions and the samples. The peak response with a correlation coefficient (r2) of 0.999 ± 0.01 (y = 22.294x + 7.4857) for CPT was within the concentration range of 1.9–125 ppm which was used to generate the calibration curve. The HPLC chromatogram of the standard CPT and sample showed retention time in the range of 4.1 and 3.7 min (Fig. 5a, b). The concentration of CPT in the root sample (28th day) was found to be 0.135% (on g DW basis) which is comparable to that of field root (0.13%) [19], suggesting the feasibility of the culture system for large scale production of the compound. Similar results were observed in multiple shoot cultures of O. mungos and O. trichocarpos where the concentrations of CPT under in vitro samples were found to be more than that of the field samples [11, 22].
Conclusion
The root culture system established in half-strength MS liquid media supplemented with 0.3 mg L−1 IAA and 0.2 mg L−1 IBA using the seedling-derived explants was found to be a viable alternative for establishing root culture for the production of CPT. The optimization of culture parameters like media salt strength, plant growth regulators, varying concentrations of ammonium nitrate and sucrose resulted in significantly enhanced biomass production. The optimized media produced a biomass of 3.34 ± 0.19 g FW with a CPT concentration of 0.135% (on g DW basis, 28 days old roots) which is comparable to the concentration of CPT in field grown roots. Moreover, enhanced CPT production can also be achieved through elicitation and precursor feeding techniques. In conclusion, the authors are reporting for the first time the establishment of in vitro root cultures of P. volubilis for the production of CPT and suggest that the root culture system can be chosen as an alternative source for CPT. However, further efforts towards enhancing the biomass production would be crucial for such endeavours.
References
Cui L, Ni X, Ji Q, Teng X, Yang Y, Wu C, Zekria D, Zhang D, Kai G (2015) Co-overexpression of geraniol-10-hydroxylase and strictosidine synthase improves anti-cancer drug camptothecin accumulation in Ophiorrhiza pumila. Sci Rep 5:8227. https://doi.org/10.1038/srep08227
Suma HK, Kumar V, Senthilkumar U, Kumara PM, Ravikanth G, Santhoshkumar TR, Shaanker RU (2014) Pyrenacantha volubilis Wight, (Icacinaceae) a rich source of camptothecine and its derivatives, from the Coromandel Coast forests of India. Fitoterapia 97:105–110. https://doi.org/10.1016/j.fitote.2014.05.017
Santhosh Kumar ES, Yeragi SS, Babu KN, Shanavas Khn AE (2001) Pyrenacantha volubilis Hook. f.(Icacinaceae): a new record for Kerala. J Econ Taxon Bot 25(3):729–731
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Rajan R, Varghese SC, Kurup R, Gopalakrishnan R, Venkataraman R, Satheeshkumar K, Baby S (2013) Search for camptothecin-yielding Ophiorrhiza species from southern Western Ghats in India: a HPTLC-densitometry study. Ind Crops Prod 43:472–476. https://doi.org/10.1016/j.indcrop.2012.07.054
Roja G, Heble MR (1994) The quinoline alkaloids camptothecin and 9-methoxycamptothecin from tissue cultures and mature trees of Nothapodytes foetida. Phytochemistry 36:65–66. https://doi.org/10.1016/S0031-9422(00)97013-4
Hartman H, Kester D, Davies F, Geneve R (1997) Plant propagation: principles and practices, vol 6. Prentice-Hall, Upper Saddle River, pp 125–144
Pedroza-Manrique J, Fernandez-Lizarazo C, Suarez-Silva A (2005) Evaluation of the effect of three growth regulators in the germination of Comparettia falcata seeds under in vitro conditions. Vitro Cell Dev Biol Plant 41:838. https://doi.org/10.1079/IVP2005698
Baíza AM, Quiroz A, Ruíz JA, Maldonado-Mendoza I (1998) Growth patterns and alkaloid accumulation in hairy root and untransformed root cultures of Datura stramonium. Plant Cell Tissue Organ Cult 54:123–130. https://doi.org/10.1023/A:1006110518548
Gajula H, Vadlapudi K, Vijendra PD, Rajashekar J, Sannabommaji T, Basappa G, Santhosh TU (2018) An alternative approach for anticancer compounds production through plant tissue culture techniques. In: Akhtar M, Swamy M (eds) Anticancer plants: natural products and biotechnological implements. Springer, Singapore, pp 529–549. https://doi.org/10.1007/978-981-10-8064-7_22
Sibi CV, Renjith R, Gopalakrishnan Roja, Ravichandran P, Satheeshkumar K (2016) A novel and efficient method for the enhanced production of multiple shoots and camptothecin from Ophiorrhiza trichocarpos blume through subculture passages in media of alternating strength. Eur J Biotechnol Biosci 4:12–16
Kumar A, Sood A, Palni U, Gupta A, Palni LM (2001) Micropropagation of Rosa damascena Mill. from mature bushes using thidiazuron. J Hortic Sci Biotechnol 76:30–34. https://doi.org/10.1080/14620316.2001.11511322
Bennett IJ, McDavid DAJ, McComb JA (2003) The influence of ammonium nitrate, pH and indole butyric acid on root induction and survival in soil of micropropagated Eucalyptus globulus. Biol Plant 47:355–360. https://doi.org/10.1023/B:BIOP.0000023877.21262.a5
George EF, Sherrington PD (1993) Factors affecting growth and morphogenesis. Plant propagation by tissue culture. Exegetics Ltd, London, pp 231–271
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193. https://doi.org/10.1007/BF03030532
Woo SH, Park JM, Yang JW (1995) Production of scopolamine by normal root culture of Hyoscyamus niger. Biotechnol Lett 17:921–926. https://doi.org/10.1007/BF00127427
Roycewicz P, Malamy JE (2012) Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philos Trans R Soc B 367:1489–1500. https://doi.org/10.1098/rstb.2011.0230
Freixes S, Thibaud MC, Tardieu F, Muller B (2002) Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ 25:1357–1366. https://doi.org/10.1046/j.1365-3040.2002.00912.x
MacGregor DR, Deak KI, Ingram PA, Malamy JE (2008) Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell 20:2643–2660. https://doi.org/10.1105/tpc.107.055475
Tian HQ, Russell SD (1998) Culture-induced changes in osmolality of tobacco cell suspensions using four exogenous sugars. Plant Cell Tissue Organ Cult 55:9–13. https://doi.org/10.1023/A:1026472527872
Cui XH, Murthy HN, Wu CH, Paek KY (2010) Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult 103:7–14. https://doi.org/10.1007/s11240-010-9747-z
Namdeo AG, Priya T, Bhosale BB (2012) Micropropagation and production of camptothecin form in vitro plants of Ophiorrhiza mungos. Asian Pac J Trop Biomed 2:S662–S666. https://doi.org/10.1016/S2221-1691(12)60292-5
Acknowledgements
The authors are thankful to Kerala State Council for Science Technology and Environment (KSCSTE) (Grant No. 03/FSHP/2013/CSTE) for financial assistance as Ph.D. fellowship and the Director, JNTBGRI for providing facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement
In vitro production of camptothecin (CPT) from root cultures of Pyrenacantha volubilis using in vitro-derived seedling explants was established for the first time which yielded more camptothecin than the field grown roots. Root culture can be used as an alternative source for CPT production instead of the conventional sources.
Rights and permissions
About this article
Cite this article
Hima, S., Midhu, C.K., Krishnakumar, G. et al. In Vitro Seedlings as Dynamic Explants for Establishment of Root Cultures of Pyrenacantha volubilis Hook. for Camptothecin Production. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 405–413 (2020). https://doi.org/10.1007/s40011-019-01113-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-019-01113-w