Abstract
Plant extracts are extensively used in folk medicine to treat the snake bites. In the present study, anti-venom potential of Canthium parviflorum root extracts against Naja naja venom was studied by in vitro and in vivo methods. Ethyl acetate and methanolic root extracts were analysed for in vitro neutralization of 5′-nucleotidase, phospholipase A2, acetylcholinesterase, phosphodiesterase, hyaluronidase, phosphomonoesterase and protease enzyme activities. Neutralization of pharmacological activities like fibrinogenolytic, and direct and indirect haemolytic activities with active methanol extract was performed. Lethal toxicity determination and its inhibition by root extract were carried out in vitro on chick embryo and in vivo on mice model. Further, venom induced edema, myotoxicity and its neutralization were carried out in the mice model. Both extracts inhibited all enzyme activities in a dose dependent manner with various IC50 values. However, only methanol extract effectively neutralized protease activity. Active methanol extract significantly neutralized all the pharmacological activities at various concentrations. Lethal dose (LD50) of venom was 2.5 µg/egg and effective dose of plant was 0.79 mg/egg for 2 LD50 of the venom. LD50 value of venom was 0.38 mg/kg body weight of mice and survival time was prolonged due to plant extract. At 1:10 and 1:20 of venom: plant extract concentration (w/w) significantly neutralized edema and myotoxic effects respectively in mice model. The present study revealed the potential of C. parviflorum root extract for its anti-venom properties and the plants could be considered as a potential source of anti-venom phytoconstituents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snake bite is a medical emergency for rural people in tropical and subtropical countries like India and it leads to high mortality and morbidity among the rural population. Snake bite has been included in the World health Organization (WHO) list of neglected tropical diseases. It is estimated that 2.5 million people envenomed each year on a global basis and approximately 35,000–50,000 deaths are reported in India annually [1]. An accurate measure of snake bite remains elusive as most victims approach traditional healers for treatment and statistics solely depends on medical record. Though there are 52 poisonous varieties of snakes in India, only four such as Naja naja (Indian cobra), Bangarus caeruleus (Krait), Daboia russelli (Russell’s viper) and Echis carinatus (Saw Scaled Viper) are responsible for maximum poisonous bites [1]. N. naja venom is highly neurotoxic in nature, it leads to respiratory paralysis and death even before any medical help can reach. The venom mainly consists of cardiotoxin and post-synaptic neurotoxin which act on the synaptic gap of neuron. The cardiotoxin from cobra acts on cardiac cells and depolarize them leading to systolic arrest [2, 3]. Presence of a higher quantity of basic phospholipase and other low molecular weight polypeptides in Indian cobra as compared to other species of Naja genus is responsible for their potent lethality [4]. Several pharmacological activities associated with the venom are due to multi molecular forms of phospholipase A2 which is the single most abundant toxic enzyme present in N. naja venom [5].
The widely accepted therapy for snake bite is immediate administration of anti-venom. In India, only polyvalent anti-venom is available which is often associated with hypersensitivity, anaphylaxis reaction and serum sickness [5, 6]. Due to geographical variations in venom composition of snakes, anti-venom raised against one geographical location may not be able to produce full neutralization of venom from other geographical location [4]. Antiserum was not efficient in giving protection against venom as it induced local effect such as necrosis, edema and degradation of extracellular matrix [7]. Neurotoxic envenomation like cobra and krait bite can be treated successfully with the mechanical ventilator and anti-AChE drug without the anti-venom administration [8].
In rural area, traditional healers use the plants to treat a snake bite without prior administration or after administration of antiserum as an accepted treatment by snake bite victims. According to the WHO, nearly 80% of the snake bite victims depend on herbal medicines to meet primary health care needs due to low cost and availability. During recent years, more attention has been paid to systematic evaluation of the plant for anti-venom properties. Many medicinal plants were used for snake bite and were systematically evaluated for the same. Methanol extract of Curcuma aromatica, Aristolochia indica, Andrographis paniculata and Curcuma zeodaria were reported for inhibition of Daboia russelli, Echis carainatus, Ophiophagus hannah, and N. kaouthia venom toxic effects by in vitro and in vivo methods [9].
The Canthium parviflorum plant is well known for its medicinal properties like anti-inflammatory, anti-cancer, anaemia, toothache, cough, snake bite, antioxidant, diuretic activity, constipation, antihelminthic, diarrhoea, fever, leucorrhoea and general disability. Though the ethno-medicinal report on this plant is well known for treating snake bites but it has not been scientifically evaluated [10]. The plant was selected based on extensive literature and individual survey conducted with tribal practioners (Western Ghats of Karnataka, India) using plant as source of anti-venom. The present study was aimed to evaluate the anti-venom potential of C. parviflorum root extract against N. naja (Indian cobra) venom by in vitro and in vivo methods.
Material and Methods
Chemicals
5′-Adenosine mono phosphate (5′AMP), acetyl thiocholine iodide, lecithin, danisidine hydrochloride, peroxidase, disodium nitro phenol phosphate and bovine fibrinogen were procured from Himedia Laboratories, India. Casein was procured from Sigma Aldrich, USA. All other chemicals and reagents were of analytical grade.
Venom, Experimental Mice and Eggs
The lyophilized venom of N. naja (Indian cobra) was procured from Irula Snake Catcher’s Co-operative Society, Kancheepuram, Chennai and was stored at 4 °C. In vivo studies were carried out in Nargund College of Pharmacy, Karnataka, India (Ethical committee approval number IAEC/NCP/92/2015). Swiss albino male mice weighing 25–30 g were procured from Sri Raghavendra Enterprises, Bangalore (Registration Number of the breeder 841/b/04/CPCSEA). Animal care and handling was conducted according to the regulations given by Institutional Animal Ethics Committee. Blood samples were obtained from healthy volunteers and consent was obtained from donors. The in vitro chick embryo experiment was performed at the Institute of Animal Health and Veterinary Biological (IAHVB) and eggs were procured from Department of Poultry, Bangalore Veterinary College, Bangalore, Karnataka, India.
Preparation of Extract
The roots of the C. parviflorum were collected in the month of October 2015 from Kalasa based on tribal information from Kudremukh, Karnataka, India with permission of the Forest department. The plant was identified and authenticated (RRCBI-MUS/03) at National Ayurveda Dietetics Research Institute, Bangalore, Karnataka, India. The collected plant material was shade dried and powdered. Dried plant material of 40 g was subjected to serial extraction by soxhlet apparatus using 400 mL of ethyl acetate and methanol as a solvent system. The extracts were concentrated by rotary vacuum evaporator and residue obtained was dried. Before use, it was dissolved in saline and centrifuged at 2000 rpm for 10 min and supernatant was used for further studies.
Qualitative Phytochemical Analysis
Preliminary phytochemical screening was done for alkaloids, phenols, tannins, saponins, terpenoids, flavonoids, steroids, quinones, glycosides and carbohydrates using standard procedure [11].
In Vitro Enzyme Inhibition Studies
For inhibition studies, N. naja venom was pre-incubated with plant extracts (ethyl acetate and methanol) of different concentration for 30 min at 37 °C. Inhibitory concentration value (IC50) values were determined for all enzyme activity.
5′-Nucleotidase Assay
5′-Nucleotidase was assayed using 5′-AMP as substrate [12]. The reaction mixture contained 1.0 mL of Tris–HCl buffer (pH 8.0), 0.1 mL of 0.1 M magnesium chloride and 0.8 mL of 0.15% 5′-AMP followed by 0.25 mL of 0.1% (w/v) crude venom. The reaction mixture was incubated at 37 °C for 15 min. At the end of incubation time, the reaction was terminated by adding TCA and filtered. The filtrate was assayed for inorganic phosphate at 625 nm using potassium dihydrogen phosphate as standard. One unit of enzyme activity was defined as the amount that yielded 0.01 μM of inorganic phosphate/min under the experimental conditions. For the inhibition studies, venom was pre-incubated with the plant extracts of different concentrations for 30 min at 37 °C (100, 200, 300, 400 µg/mL).
Phospholipase A2 assay
Phospholipase A2 activity was determined according to the acidimetric method given by Tan and Tan with little modification [13]. The reaction mixture consists of three equal parts of 1% lecithin, 18 mM calcium chloride and 8.1 mM sodium deoxycholate. The pH of the suspension was adjusted to 8.0 with 0.1 M of sodium hydroxide and stirred for 10 min to ensure homogenous mixing. Venom (0.1 mL of 0.1% w/v) solution was added to 15 mL of egg yolk suspension to initiate the hydrolysis. The initial decrease in pH was measured by a pH meter. Inhibition study was carried out by pre-incubating the venom with plant extract of different concentrations for 30 min (100, 200,300, 400 µg/mL). A decrease of 1 pH unit corresponds to 133 μM of fatty acid release.
Acetylcholinesterase Assay
Acetylcholinesterase was determined using acetylthiocholine iodide (AI) as the substrate [14]. Crude venom of 50 µL (0.1% w/v) and 3 mL phosphate buffer (pH 8.0) was incubated at room temperature for 5 min and 10 µL 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) (10 mM/L) and 20 µL substrate acetylthiocholine iodide (158.5 mM/L) were added in order to reach a final concentration of 1 mM/L. The increase in absorbance at 412 nm was measured on a double beam spectrophotometer against control mixture prepared at the same time. For the inhibition studies, venom was pre-incubated with plant extracts of different concentrations for 30 min at 37 °C (100, 200, 300, 400 µg/mL).
Phosphodiesterase Assay
Phosphodiesterase was measured using disodium-p-nitrophenol phosphate (DNPP) as the substrate [15]. Reaction mixture consisted of 0.1 mL 0.25% (w/v) of venom solution, 0.5 mL 0.0025 M DNPP, 0.3 mL 0.01 M MgSO4 and 0.5 mL 0.17 M Tris–HCl buffer (pH 8.0). The reaction was measured at 400 nm. Phosphodiesterase activity was expressed in nM of product released/min. Inhibition study was carried out by pre-incubating venom with plant extract of different concentrations for 30 min (100, 200, 300, 400 µg/mL).
Hyaluronidase Assay
Hyaluronidase activity of crude venom was determined turbidometrically using sodium hyaluronate as substrate [16]. The assay mixture contained Tris–HCl buffer (pH 8.0), 50 mg hyaluronic acid (0.5 mg/mL in buffer) and the enzyme in the same buffer in a final volume of 1.0 mL. The mixture was incubated for 15 min at 37 °C and the reaction was stopped by the addition of 2 mL 2.5% (w/v) cetyltrimethyl ammonium bromide (cTAB) in 2% (w/v) NaOH. The absorbance was read at 400 nm (within 10 min) against a blank containing 1 mL of the same buffer and 2 mL 2.5% (w/v) cTAB in 2% (w/v) NaOH. Inhibition study was carried out by pre-incubating venom with the plant extract for 30 min (100, 200, 300, 400 µg/mL). Turbidity reducing activity was expressed as a percentage of the remaining hyaluronic acid, taking the absorbance of a tube in which no enzyme was added as 100%. One unit was defined as the amount of enzyme that provoked 50% turbidity reduction.
Phosphomonoesterase Assay
Method of Bessey et al. [17] was employed to determine phosphomonoesterase activity with slight modifications. To a mixture of 1.0 mL Tris–HCl buffer (pH 8.0) and 1.0 mL disodium-p-nitrophenol phosphate, 0.5 mL 0.25% (w/v) crude venom was added and incubated at 37 °C for 3 h. The absorbance was measured at 425 nm. p-Nitrophenol was used as the standard. One unit of enzyme activity was defined as the amount that yielded 0.1 μM of p-nitrophenol/h under the experimental conditions. For inhibition study venom was pre incubated with plant extract of different concentrations (50, 100, 15 0, 200 µg/mL).
Protease Assay
The reaction mixture was composed of 0.5% casein and 1.0 mL Tris–HCl buffer (pH 8.0), 0.5 mL 0.25% (w/v) of crude venom was added and the reaction mixture was incubated for 4 h, at 37 °C. At the end of 4 h the reaction was terminated by adding TCA and then filtered. Filtrate (1.0 mL) was used for protein estimation using l-tyrosine as a standard. In the above investigation, one unit of enzyme activity was defined as the amount that yielded 0.02 μM of tyrosine/h under the experimental conditions described by Greenberg [18]. For the inhibition studies, venom was pre-incubated with the plant extracts of different concentrations for 30 min at 37 °C (100, 200, 300, 400 µg/mL).
Inhibition of Pharmacological Activities
Neutralization of Fibrinogenolytic Activity
Venom (5 µg) was incubated with bovine fibrinogen (50 µg) for 1 h at 37 °C. Reaction was terminated by adding 20 µL of denaturing buffer containing 1 M urea, 4% SDS (w/v) and 4% β-mercaptoethanol (w/v). The hydrolysed product was analysed with 12% SDS and the protein pattern was visualized by staining with Coomassie brilliant blue. For inhibition study, venom (5 µg) was pre-incubated with methanol extract of different concentrations for 30 min at 37 °C (venom to methanol extract (w/w) 1:1, 1:2, 1:4, 1:8). Fibrinogenolytic pattern was analysed by incubating fibrinogen with different concentrations of venom [19].
In Vitro Human Red Blood Corpuscles Stabilization Properties of C. parviflorum Methanol Extract Against N. naja Venom Induced Haemolysis (Direct Haemolysis Assay)
Hypo-saline induced haemolysis was modified in the present study by venom induced haemolysis of human red blood corpuscles (HRBC) [20]. Different tubes were filled with 1 mL of venom (100 μg/mL), 1 mL phosphate buffer pH 7.4, 1 mL of 1% HRBC and varying concentrations of methanol extracts (100, 200, 300 and 400 μg/mL). Control consisted of the same composition but was free of plant extract. The mixtures were incubated at 37 °C for 30 min and then centrifuged at 1000 rpm for 3 min. The absorbance of the supernatant was measured at 540 nm using a spectrophotometer. The inhibition percent of haemolysis was calculated by the following equation.
where, Ac is absorbance of control without extract and At is absorbance of test with extract in venom solution.
Indirect Haemolysis Assay (Phospholipase A2 Activity)
Phospholipase A2 activity was measured on agarose-erythrocyte-egg gel plate [7]. The venom was added to agarose-erythrocyte-egg gel plate and saline served as a control. The neutralization of haemolysis was carried out by incubating constant amount of venom with different concentrations of methanol extract (venom to methanol extract ratio w/w, 1:5, 1:10, 1:15, and 1:20). The plates were incubated at 37 °C overnight and haemolytic halos were measured. The minimum indirect haemolytic diameter (MIHD) corresponds to dosages of venom, which produced haemolytic halo of 10 mm diameter.
Assessment of Venom Toxicity and Its Neutralization by Plant Extract on Chick Embryo and Mice Experimental Model
Determination and Neutralization of Lethal Toxicity Using Chick Embryo Model
The lethal toxicity of N. naja venom was determined using the chick embryo model method developed by Sells et al. [21]. As a modification to this method, contents in the egg were not transferred out of egg instead test sample was transferred inside the eggs at the apex without disturbing the content inside [22]. Six days old eggs were surface sterilized with alcohol and a small opening was made on the apex of the eggs. Venom of different concentration in constant volume of saline was transferred to the egg using syringe (1–5 µg/0.1 mL), while control group of eggs (n = 6) were transferred with saline. The holes were sealed using wax to avoid the contamination and desiccation of the eggs contents. Eggs were incubated for 24 h in swinging incubator at 37 °C and 65% relative humidity for embryogenesis. After 24 h of incubation, eggs were observed for survivality by using candling technique.
For the venom neutralization, 2LD 50 was taken as challenging dose and challenging dose of the venom was incubated with methanol extract of C. parviflorum at different concentrations for 30 min and survivality was recorded at 24 h. Effective dose (ED50) is the concentration of anti-venom/plant extracts needed for survival of 50% of the embryos injected.
Determination and Neutralization of Lethal Toxicity in Mice Model
Different concentration of venom in 0.2 mL of saline was injected in mice (n = 5) by the intraperitoneal route [22]. Lethal toxicity calculation was done according to the method of Meier and Theakston [23]. For inhibition of N. naja venom induced lethal toxicity, 3LD50 of venom was selected as challenging dose. Neutralization of lethal toxicity was performed by pre-incubation of venom with plant extract of different concentrations (n = 5) and separate injection of venom followed by plant extract of different concentrations (n = 5). As a separate injection protocol, two different concentrations of plant extract were injected into same spot exactly after 5 min after venom injection (3LD50). Survival time was recorded at 24 h and signs of neurotoxicity were observed.
Neutralization of Edema Activity
Group of mice (n = 5) were injected with 5 µg of venom in 20 µL of saline into right foot pad and left footpads injected with 20 µL of saline served as control. After 1 h, mice were anaesthetized, foot pad was cut at the ankle joint and increase in weight due to edema was measured. Minimum edema dose (MED) was defined as amount of venom required to cause an edema ratio 120%. For the neutralization studies, MED of venom was incubated with different concentrations of methanol plant extract [5].
Neutralization of Myotoxic Activity
The myotoxic activity was determined by measuring the elevation of cytoplasmic marker enzyme lactate dehydrogenase (LDH) in the serum [24]. Group one mice (n = 5) and group two mice (n = 5) were injected with saline and half the LD50 of venom in 50 µL saline respectively by intramuscular route. Group three (n = 5) and four mice (n = 5) were injected with venom pre-incubated with methanol extract of C. parviflorum at two different concentrations. The fifth group (n = 5) of mice was injected with the plant extract alone at a higher concentration. After 3 h mice were anesthetized and blood was drawn by retro orbital method. Serum LDH enzyme activity was assayed.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 6. The significance between the groups was calculated using student unpaired t test. Statistical symbol ‘a’ represents P < 0.05, ‘b’ represents P < 0.01, ‘c’ represents P < 0.001 and ‘d’ represents P < 0.0001. Inhibitory concentration (IC50) is dosage of extract required to produce 50% inhibition of enzyme activity was calculated using regression analysis. Effective dose (ED50) was calculated for pharmacological activity similar to inhibitory concentration determination.
Results and Discussion
Plant Extraction And Qualitative Phytochemical Analysis
The extracts were dried under rotary vacuum evaporator, the yield of ethyl acetate extract was 0.61 g and methanol extract was 1.26 g. The extracts were stable at room temperature for more than 6 months. Preliminary phytochemical analysis revealed the presence of alkaloids, phenols, saponins, terpenoids, flavonoids, steroids, glycosides, tannins and carbohydrates in both the extracts, whereas quinones were present only in methanol extract.
In Vitro Enzyme Inhibition Studies
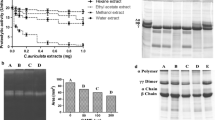
Ethyl acetate and methanol extract were analysed for neutralization of enzyme activities (in vitro enzyme neutralization). Both the extracts significantly neutralized 5′- nucleotidase, phospholipase A2, acetylcholinesterase, phosphodiesterase, hyaluronidase and phosphomonoesterase activities at various concentrations. Methanol extract neutralized protease activity whereas ethyl acetate extracts did not neutralize protease enzyme activity up to 1000 µg/mL concentration (Figs. 1 and 2). Inhibitory concentration value of plant extracts for enzyme inhibition studies are tabulated (Table 1).
Inhibition of 5′-nucleotidase activity (A), phospholipase A2 activity (B), acetylcholine estrase activity (C) and phosphodiesterase activity (D) of N.naja venom by methanol and ethyl acetate extracts of C. parviflorum root extracts. Results were expressed mean ± SEM. ‘a’ represents P < 0.05, ‘b’ represents P < 0.01, ‘c’ represents P < 0.001 and ‘d’ represents P < 0.0001
Inhibition of hyaluronidase activity (A), phosphomonoesterase activity (B) and protease activity (C) of N.naja venom by methanol and ethyl acetate extracts of C. parviflorum root extracts. Results were expressed mean ± SEM. ‘a’ represents P < 0.05, ‘b’ represents P < 0.01, ‘c’ represents P < 0.001 and ‘d’ represents P < 0.0001
Neutralization of Fibrinogenolytic Activity
Fibrinogenolytic activity of N. naja venom was analyzed electrophoretically using fibrinogen as substrate on 12% agarose gel (Fig. 3). N. naja venom at 5 µg concentration degraded only Aα band of the fibrinogen and further increase in venom concentration up to 30 µg did not degrade Bβ and γ bands of fibrinogen (not shown in the result). Venom to methanol extracts ratio 1:1(w/w) effectively neutralized venom induced fibrinogenolytic activity.
Inhibition of N. naja venom induced fibrinogenolytic activity by C. parviflorum methanol extract. Lane A- fibrinogen control, Lane B and C- fibrinogen + 5 µg of N. naja venom (F + V), Lanes D, E, F and G-F + V incubated with plant extract of different concentration (venom to extract (w/w), 1:1, 1:2, 1:4 and 1:8)
Direct Haemolysis Assay
Venom (100 µg/ml) was pre-incubated with different concentration of C. parviflorum methanol extract ranging from 100 to 400 µg/mL. Results are expressed as percentage inhibition of haemolysis compared to control. Methanol extract neutralized N. naja venom induced HRBC lysis in a dose dependent manner (Fig. 4). Dose dependent inhibition was observed and ED50 values of C. parviflorum for N. naja venom induced haemolysis was 360.91 µg/mL.
Indirect Haemolysis Assay (Phospholipase A2 activity)
Methanol extract effectively neutralized the indirect haemolytic activity on agarose-erythrocyte-egg yolk gel plate (Fig. 5). N. naja venom of 2 µg produced haemolytic halo of 12 mm, which was completely inhibited by venom to methanol extract ratio of 1:20 w/w.
Determination of LD50 Value and Neutralization of Lethal Toxicity in Chick Embryo and Mice Model
LD50 value for chick embryo model was 2.5 µg/egg. All the embryos were dead on exposure to 2LD50 of the venom. Effective dose (ED50) in chick embryo model was 0.79 mg/egg for 2LD50 of the venom. The embryos were considered dead as they were motionless with no distinguishable vasculature (Fig. 6). LD50 of venom in the mice model was 0.38 mg/kg body weight. Neurotoxic symptoms like convulsion, lacrimation, urination, difficulty in breathing and respiratory failure were observed when venom was injected and survival time was 1 h and 15 min. No neurotoxic symptoms were observed in inhibition studies. Both method of inhibition using plant extract delayed these symptoms. Survival time of the mice increased more than two times in comparison to control and was similar among the two inhibition studies (Table 2).
Neutralization of Edema Inducing Activity
The N. naja venom (5 µg) produced edema ratio of 173.45%, for the inhibition study 5 µg of venom was pre-incubated with two different concentrations (venom to extract w/w 1:10 and 1:20) of methanol extract. Significant decrease in edema ratio was observed when venom was pre-incubated with methanol extract of a different concentration (Fig. 7).
Neutralization of Myotoxic Activity
N. naja venom induced skeletal muscle damage was quantified by measuring the elevated LDH enzyme activities in the serum. Half of the LD50 value of N. naja venom was responsible for elevation of LDH activities to 8485 ± 21.21 U/L against the control values of 568.33 ± 7.6 U/L in the serum of control mice. For the inhibition studies, venom was pre-incubated with C. parviflorum methanol extract of two different concentrations 1:10 and 1:20, respectively (venom: extract, w/w). Myotoxic effect was found to be significantly neutralized by methanolic plant extract in mice model. Plant extract alone was found to be non-myotoxic in mice model (Fig. 8).
All the above observations indicate that C. parviflorum possesses active phytochemicals responsible for inhibition of N. naja venom induced in vitro and in vivo activity. N. naja venom is rich in post-synaptic neurotoxin, phospholipase A2, protease, alkaline phosphatases, ATPase, hyaluronidase, acetylcholinesterase and more reported for mortality and morbidity. All these are major enzymes which contribute to easy movement of other enzymes, membrane degradation, early reaction, myotoxicity, neurotoxicity, cytotoxicity and hypotension in snake bite [4]. Hyaluronidase is a spreading factor but it has been neglected because of lack of its toxicity. This hydrolytic enzyme is involved in deterioration of structural integrity of extra cellular matrix and thereby helps in spreading of other enzymes. Inhibition of this enzyme retards the spreading of other enzymes into the body. However, anti-venom fails to neutralize the local effect and it is continued even after anti-venom treatment.
Both non-polar and polar extracts neutralized all these enzyme activities with various IC50 values. Some of the enzymes were completely neutralized by the plant extracts at lower concentration. Interestingly in the present study, methanol extract was more efficient in neutralization of the degradation of casein substrate by the protease enzyme. Based on this observation, methanol extract was considered to be active extract for anti-venom studies and further pharmacological and toxicological neutralization studies were carried out with active extract. Previous anti-venom studies also support the current observations. Methanol extract of Aristolochia indica, Andrographis paniculata, Curcuma aromatica and Curcuma zeodaria were potent inhibitor of snake venom induced pharmacological activities [9].
Direct haemolysis attributed by the venom is due to the combined action of phospholipase A2 and cytotoxin, which is regarded to be one of the key events in snake bite. These enzymes act on membrane phospholipid and liberate lysolecithin which further acts on HRBC causing haemolysis. The inhibition of percentage of haemolysis is due to stabilization of protein on HRBC [25]. C. parviflorum extract neutralized the N. naja venom induced haemolysis which may be due to stabilizing the protein in the membrane. Indirect haemolytic activity corresponds to PLA2 activity of venom which relates to the hydrolysis of phospholipid. Various phytoconstituents from plants have been identified for the neutralization of the PLA2 enzyme mediated pharmacological effects. Phenol or tannins from the plant either precipitate or form the complex with Ca2+ and reduce enzymatic action of the phospholipase A2.
The degradation of Aα band of the fibrinogen was observed at 5 µg of venom concentration, however increase in concentration did not alter the fibrinogen degradation pattern (Unpublished observation). Mangifera indica extracts neutralized fibrinogenolytic activity induced by the Russell’s viper venom at various concentrations [26].
Lethality of the venom is a combined action of toxic components and also depends on the concentration of toxic and nontoxic proteins present. N. naja is highly neurotoxic in nature, neurotoxins act at neuromuscular junction either post synoptically or pre synoptically. The probable mechanism of inhibition of neurotoxic effect by C. parviflorum may be by interfering with the acetylcholine receptor sites, thereby antagonizing the action of neurotoxic substances in the venom at the acetyl choline receptor sites [27]. For neutralization study, the survival time was increased in the mice model when 3 × LD50 of the venom was used as a challenging dose. Challenging dose of N. naja venom was neutralized almost similarly by the plant extracts in both protocols. Various previous studies supported co-injection of venom with plant extract as it provides higher protection than separate injection [26]. However, snake bite victims are treated post envenomation and the present study on neutralisation of N. naja venom by plant extract shows beneficial protection against the venom suggesting its potential anti-venom property and could be an effective antidote for this snake bite.
The main problem associated with using the animal is that, a large number of animals are required in order get statistically significant results and pain of the animal during the experimental time [21]. In case of eggs, extract was analysed for their efficacy at various concentrations to get ED50 for venom induced lethal toxicity. The use of chick embryo model minimizes the experimental animal use in toxicity study. Thus, chick embryo model can be used as an alternative model for venom research. Modified protocol to Sell’s et al. used in the present study is less labor intensive [28]. Lethal toxicity determination for neurotoxic scorpion venom using chick embryo model was reported in the previous study [28]. Local edema is a common occurrence in snake bite, combined action of metalloprotease and PLA2 is responsible for release of endogenous inflammatory mediators [5]. Previous studies suggested that involvement of phospholipase A2 in the edema formation and activity was decreased by EDTA. The reduction of edema was due to chelation of divalent cation, resulted in the neutralization of phospholipase A2 activity. The myotoxic phospholipase acts on skeletal muscles and severely damages the muscles resulting in the elevation of CK and LDH which are released into serum. Several anti-venom plants are ineffective in neutralizing the venom induced myotoxicity. Mimosa pudica root extracts neutralized Naja kaouthia induced myotoxicity, similarly C. parviflorum root extracts exhibited significant neutralization [29].
Many anti-venom plants have been reported for several types of snake bite due to different phytochemicals present rather than single phytocompound. Because toxicity attributed by the venom is due to large number of proteins and peptides and composition of venom also varies greatly. Euphorbia hirta plant consists of active phytochemicals like sistosterols, camposterols, ellagic acid, tannins and many more were reported as active phytoconstituents for anti-venom properties [30]. C. parviflorum is a cocktail of various phytochemicals, which antagonized N. naja venom induced toxicological effects.
Conclusion
The results of the present study reveal the anti-cobra venom potential of C. parviflorum root extract that was not reported previously. Based on in vitro, pharmacological and toxicological neutralization studies, the present research scientifically proves anti-venom properties of C. parviflorum, a traditional Indian plant used against cobra bite. This research provides scientific evidence to the traditional use of the plants for snake bites and thereby they can be regarded as an efficacious herbal drug treatment.
References
Bawaskar HS (2004) Snake venoms and antivenom issues: critical supply issues. J Assoc Physicians India 52:11–13
Lee CC, Chang CC (1966) Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem Inst Butantan 33:555–572
Watt MD, Theakston DRG, Hayes CG (1986) Positive response to endrophonium in patients with neurotoxic envenoming by cobras (Naja naja philippinensis). N Engl J Med 315:1444–1448. https://doi.org/10.1056/NEJM198612043152303
Mukherjee AK, Maity CR (2002) Biochemical composition, lethality and pathophysiology of venom from two cobras-Naja naja and Naja kaouthia. Comp Biochem Physiol B: Biochem Mol Biol 131:125–132
Vishwanath BS, Kini RM, Gowda TV (1987) Characterization of three edema inducing phospholipase A2 enzymes from Habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon 25:501–515. https://doi.org/10.1016/0041-0101(87)90286-8
Cannon R, Ruha AM, Kashani J (2008) Acute hypersensitivity reactions associated with administration of crotalidae polyvalent immune Fab antivenom. Ann Emerg Med 51:407–411. https://doi.org/10.1016/j.annemergmed.2007.09.036
Gutierrez JM, Avila C, Rojas E, Cerdas L (1988) An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 26:411–413. https://doi.org/10.1016/0041-0101(88)90010-4
Bawaskar HS, Bawaskar PH (2002) Profile of snakebite envenoming in western Maharashtra India. Trans R Soc Trop Med Hyg 96:79–84
Alam MI (2014) Inhibition of toxic effects of Viper and Cobra venom by Indian medicinal plants. Pharmacol Pharm 5:828–837. https://doi.org/10.4236/pp.2014.58093
Hiremath VT, Taranath TC (2010) Traditional phytotherapy for snake bites by tribes of Chitradurga District, Karnataka, India. Ethnobot Leafl 14:120–125
Harborne JB (1973) Methods of plant analysis. In: Phytochemical methods, 2nd edn. Chapman and Hall, London, pp 132
Rowe M, de Gast GC, Platts-Mills Asherson TA, Webster GL, Johnson SM (1980) Lymphocyte 5′-nucleotidase in primary hypogammaglobulinaemia and cord blood. Clin Exp Immunol 39:337–343
Tan NH, Tan CS (1988) Acidimetric assay of phospholipase A2 using egg yolk suspension as substrate. Anal Biochem 170:282–288
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Lo TB, Chen YH, Lee CY (1966) Chemical studies of Formosan cobra (N. naja atra) venom. Part 1. Chromatographic separation of crude venom on CM-Sephadex and preliminary protection by Mikania laevigata (guaco) extract against the toxicity of Philodryas olfersii snake venom’ characterization of its components. J Chin Chem Soc 13:165–177
Pukrittayakamee S Warrell, Desakorn DA, McMichael V, White AJ, Bunnag D (1988) The hyaluronidase activities of some Southeast Asian snake venoms. Toxicon 26:629–637
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem 164:321
Greenberg DM (1955) Plant proteolytic enzymes: methods in enzymology. In: Colowick SP, Kalpan NO (eds) Academic Press Inc., New York, pp 54–64
Ouyang C, Teng CM (1976) Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim Biophys Acta 420:298–308
Balu S, Alagesaboopathy C (1995) Anti-snake venom activities of some species of andrographis wall. Ancient Sci Life 14:187–190
Sells PG, Ioannou P, Theakston RDG (1998) A humane alternative to the measurement of the lethal effects (LD50) of non-neurotoxic venoms using hens’ eggs. Toxicon 36:985–991
Theakston RDG, Reid HA (1983) Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Organ 61:949–956
Meier J, Theakston RDG (1986) Approximate LD50 determination of snake venoms using eight to ten experimental animals. Toxicon 24:395–401
Gutierrez JM, Arce V, Brenes F, Chaves F (1990) Changes in myofibrillar components after skeletal muscle necrosis induced by a myotoxin isolated from the venom of the snake Bothrops asper. Exp Mol Pathol 52:25–37
Kumarapppan C, Jaswanth A, Kumarasunderi K (2011) Antihaemolytic and snake venom neutralizing effect of some Indian medicinal plants. Asian Pac J Trop Med 11:743–747. https://doi.org/10.1016/S1995-7645(11)60185-5
Dhananjaya BL, Zameer F, Girish KS, Cletus D’Souza JM (2011) Antivenom potential of aqueous extract of stem bark of Mangifera indica L. against Daboia russellii (Russell’s viper) venom. IJBB 48:175–183
Richard Lobo ISR, Punitha K Rajendran, Shirwaikar Arun, Shirwaikar Annie (2006) Preliminary study on the antisnake venom activity of alcoholic root extract of Clerodendrum viscosum (Vent.) in Naja naja venom. Nat Prod Sci 12:153–156
van der Valk T, van der Meijden A (2014) Toxicity of scorpion venom in chick embryo and meal worm assay depending on the use of the soluble fraction versus the whole venom. Toxicon 88:38–43. https://doi.org/10.1016/j.toxicon.2014.06.007 (Epub 2014 Jun 19)
Mahanta M, Mukherjee AK (2001) Neutralisation of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts. J Ethnopharmacol 75:55–60
Mors WB, Nascimento MC, Pereira BM, Pereira NA (2000) Plant natural products active against snake-bite the molecular approach. Phytochemistry 55:627–642
Acknowledgements
The authors thank Jain University for their financial support and infrastructure facilities to carry out the research work. They are thankful to Nargund College of Pharmacy, Karnataka, India to provide facilities to carry out the animal studies. They would like to acknowledge the Institute of Animal Health and Veterinary Biological (IVHVB), Bangalore, Karnataka, India to provide the facilities and valuable suggestions to carry out egg experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that they have no conflicts of interest to publish this manuscript.
Additional information
Significance of paper The present study demonstrated the anti-venom potential of C. parvifloum root extracts against the Naja naja venom. The outcome of the present study is encouraging and intended to bridge the gap between traditional knowledge.
Rights and permissions
About this article
Cite this article
Shrikanth, V.M., Janardhan, B. & More, S.S. Anti-Venom Potential of Canthium parviflorum Against Naja naja Venom by In Vitro and In Vivo Studies. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 483–492 (2019). https://doi.org/10.1007/s40011-017-0959-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0959-4