Abstract
This is the first account of molecular and morphological characteristics of the stingless bee, Tetragonula iridipennis (Smith, 1854) from the Punjab (Northern India). The species was originally described from Sri Lanka. Using the standard barcoding protocols, cytochrome c oxidase subunit I marker (standard DNA barcode region) based DNA barcode sequence of the species has been established, as a first step towards DNA barcode library for stingless bees of Punjab. The barcode sequence generated for the species has been registered by GenBank, National Centre for Biotechnology Information under accession ‘KT960851’ and Barcode of Life Data Systems under Barcode Index Number ‘BOLD:ACT1038’. The host plant associations for T. iridipennis in Punjab are provided. Taxonomic comments on T. iridipennis and metric values of 40 morphological characters are also presented. The results can be used to further study the ecotypes in different parts of country, plant-pollinator interactions, habitat management and conservation programmes for stingless bees. Further, the precise identification of T. iridipennis and the inventory of its foraging plants would enhance its use as potential pollinator of crops, especially grown under protective cultivation wherein the Apis species are little useful and the hand pollination is highly laborious and costly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetragonula Moure, 1961 is a complex and the most widespread genus [1] of stingless bees in Indo-Malayan region, reported from India to Solomon and Caroline Islands and contains 32 described species [2]. The genus was proposed with Trigona iridipennis Smith, 1854 as the type species from Sri Lanka [3]. Worldwide, there are 505 named species of stingless bees [4]. Stingless bees are distinguished from other corbiculate Apinae by reduced forewing venation and the presence of the jugal lobe in hindwing.
Among a total of 50 genera of stingless bees, Trigona and Melipona are the major ones; most of the Asian and African stingless bee species belong to the former genus [5]. Until 2010, six named species of stingless bees belonging to three genera were reported from India [6–9] are Lepidotrigona arcifera (Cockerell), Lisotrigona cacciae (Nurse), Lisotrigona mohandasi Jobiraj and Narendran, Tetragonula bengalensis (Cameron), Tetragonula iridipennis (Smith) and Tetragonula ruficornis (Smith). In the year 2013, new record of Tetragonula gressitti (Sakagami, 1978) from Arunachal Pradesh, India [10] raised their number to seven. Taxonomic revisions of the species of Indian subcontinent lead to addition of Tetragonula praeterita (Walker) as distinct species following its removal from synonymy [2], which has further raised the total number of named species of stingless bees to eight in India.
Stingless bees are eusocial insects with organized system of division of labour and are commonly known as ‘dammer bees’ as they collect dammer (a clear to yellow resin derived from dipterocarp trees, used in varnishes and inks) [2] for construction of their nests along with the wax produced from their body [11]. The colony strength may vary from around 100 to 1,00,000 individuals depending upon species, and honey production varies from 200 to 5000 g per season [12, 13]. As the pollinators of crops of some families like compositae, cruciferae and leguminosae, the stingless bees outperform the honey bees [13]. The term ‘stingless’, though a misnomer, indicates weak or vestigial stinger in females, which is unable to inflict pain to humans unlike the other species of honey bees. Instead of stinging, inflicting a mild bite with mandibles, crawling into ears or nostrils of invaders, emitting a caustic liquid from mouth causing skin irritations, are some common defence mechanisms in them.
The information on diversity and distribution of stingless bees in different agroclimatic regions of Punjab had been lacking. The complete account of molecular and morphometric characterization of T. iridipennis will help to further investigate the species richness, diversity of stingless bees in Punjab.
Material and Methods
Specimen Collection
The bee specimens examined in the study were collected during the day time while sweeping the flowers of Brassica napus L. in central plain, Helianthus annuus in sub-mountain undulating and Trifolium alexandrinum in western agroclimatic zone of Punjab (India) during spring 2013.
DNA Extraction and PCR Reaction
For DNA barcoding, the specimens were preserved in a DNA-friendly fashion by immersing in 100 % ethanol and kept at −20 °C in vertical deep freezer till DNA was isolated. DNA extraction was done using previously standardized CTAB method [14]. CTAB was 2 % solution of cetyl trimethyl ammonium bromide (CTAB) in 100 mM Tris.Cl (pH 8.0), which additionally contained 20 mM of Na2EDTA (pH 8.0) and 1.4 M NaCl. DNA isolation was carried out using hind leg tissue of the bee. Genomic DNA isolation was carried out individually from three bee specimens, however, a single sample was finally processed for cloning and sequencing. The primers pair LepF1 (5′ATT CAACCAATCATAAAGATATTGG3′) and LepR1 (5′TAAACTTCTG GATGTCCA AAAAATCA3′) were used to amplify 654 bp fragment of COI gene. All PCR amplifications were accomplished in a programmable DNA thermalcycler (Mastercycler Gradient—Eppendorf™) using the following PCR programme: Step 1: Initial denaturation at 94 °C for 5 min (one cycle); Step 2: Initial denaturation at 94 °C for 1 min; Step 3: Primer annealing at 55 °C for 1 min; Step 4: Primer extension at 72 °C for 2 min; Step 5: Repeated step 2–4 (35 cycles); Step 6: Final extension at 72 °C for 5 min and storing the PCR product at 4 °C. Each PCR product was subsequently gel purified. The purified DNA fragments were ligated into a ‘PCR product cloning plasmid vector pTZ57R/T (Fermentas Life Sciences, USA)’. The ligation reaction product was transformed into Escherichia coli DH5-alpha host cells using ‘InsTAClone™ PCR cloning kit (M/s Fermentas Life Sciences)’ using manufacturer’s protocol followed by custom sequencing from Xcelris Labs, Ahmedabad. The natural orientation of sequence was determined by aligning of sequence with the reported sequences (in GenBank database, www.ncbi.nlm.nih.gov/pubmed/) for the species using ‘Gene align function’ of the DNA software program ‘CLC Free Workbench ver 7.5. of CLC Bio A/S’.
Morphometry
The measurements were made with image acquisition programmed zoom-stereo microscope (OLYMPUS CellA Imaging Solutions for Life Science Microscopy). Terminology and measurements follow those of Michener [15] and Ruttner [16]. The indices and their abbreviations used are as per given.
(1) body length (BL), (2) head/face length (HdL), (3) head width (HdW), (4) thorax length (ThL), (5) thorax width (ThW), (6) abdomen length (AbL), (7) abdomen width (AbW), (8) clypeus length (CL), (9) clypeus width (CW), (10) lower inter-orbital distance (LIOrD), (11) upper inter-orbital distance (UIOrD), (12) inter-orbital distance through antennal sockets (IOrDas), (13) clypeoantennal distance (CAD), (14) compound eye length (CEL), (15) compound eye width (CEW), (16) distance between antennal sockets (DbAS), (17) interocellar distance (IOD), (18) ocellocular distance (OOcuD), (19) antennocellar distance (AOD), (20) antennocular distance (AOcuD), (21) clypeocular distance (COcuD), (22) median ocellus diameter (MOD), (23) labrum length (LL), (24) labrum width (LW), (25) antennal socket maximum diameter (ASD), 26) scape length (SL), (27) scape diameter (SD), (28) pedicel length (PdL), (29) flagellum length (FgL), (30) 3rd flagellomere diameter (3FgmD), (31) forewing length (FwL), (32) forewing width (FwW), 33) hindwing length (HwL), 34) hindwing width (HwW), (35) jugovannal index (JVI), (36) hamuli number (HN), (37) hind tibia length (HTL), (38) hind basitarsus length (HbtL), (39) hind basitarsus width (HbtW) and (40) number of flagellomeres (FgmN). The observations pertaining to the bilateral body-parts such as eyes, antennae, legs and wings were taken on right side body part.
Results and Discussion
The identity of Tetragonula iridipennis Smith, largely depends on the works of Sakagami [6], Rosmussen [2, 7], and Sakagami and Inoue [17]. These authors considered the size differences as key morphological characteristics for species identification in addition to male genitilia and sternal characters. The metric values for the various morphological characters recorded in the present specimens are given in Table 1.
Systematics
-
Genus Tetragonula Moure, 1961.
-
Tetragonula iridipennis (Smith, 1854) Sakagami, 1978.
-
Synonym Trigona iridipennis Smith, 1854.
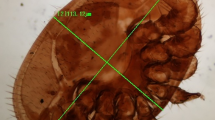
Material examined (Figs. 1–7).
Worker of Tetragonula iridipennis (Smith, 1854). 1 Mounted view. 2 Ocelli, compound eye and antennae. 3 Lower frontal view of head showing partially hidden labrum, mandibles and proboscis. 4 Thorax and Abdomen. 5 View towards propodeum, hindleg with corbicula. 6 Forewing view. 7 Hind wing with five hamuli
India (5 workers): 2 ♀♀, Ludhiana, in central plain zone of Punjab, from Brassica napus, 14.ii.2013, 30°53′5″ N and 75°48′21″ E, 256 m a.s.l., coll. G.S. Makkar; 2 ♀♀, Hoshiarpur, in sub-mountain undulating of Punjab, from Helianthus annuus, 4.v.2013, 31°22′51.9″ N and 75°53′2″ E, 296 m a.s.l., coll. G.S. Makkar; and 1 ♀, Muktsar, in western zone of Punjab, from Trifolium alexandrinum, 12.v.2013, 30°25′6.9″ N and 74°36′1.8″ E, 184 m a.s.l., coll. G.S. Makkar. Type specimens have been deposited in the collection of Insect Museum at Punjab Agricultural University and National Pusa Collection, Indian Agricultural Research Institute (IARI), New Delhi.
Molecular Characterization
DNA barcoding offers a highly precise means of species identification using mitochondrial gene, cytochrome c oxidase I (COI) [18]. A 654 bp DNA barcode sequence of T. iridipennis has been established by using protocols discussed earlier. The sequence composition was Adenine (172), Guanine (87), Cytocine (136) and Thymine (259). The edited sequence of T. iridipennis was put in a BLAST (Basic Local Alignment Search Tool) search to compare the present sequence with GenBank database of sequences to identify the database sequences that resemble the present sequence. Based on this alignment, T. laeviceps, T. sirindhornae, T. latigenalis, T. fuscobalteata and T. testaceitarsis showed 86.9, 85.21, 83.2, 80.43 and 80.43 % sequence similarity, respectively to T. iridipennis. The COI gene based sequence of this species has been made available in GenBank, NCBI (http://www.ncbi.nlm.nih.gov/) under accession ‘KT960851’ and Barcode of Life Data Systems (BOLD) (http://www.boldsystems.org) under BIN ‘BOLD:ACT1038’. The illustrative DNA barcode of the species is presented in Fig. 8.
Dimensions
The total body length ranged from 3.59 to 3.67 mm, head width from 1.59 to 1.61 mm, compound eye length from 1.05 to 1.06 mm, median ocellus diameter from 0.16 to 0.17 mm, diameter of third flagellomere from 0.11 to 0.12 mm, and forewing length including tegulae ranged from 3.73 to 3.75 mm. The other important measurements (means in mm) of the species were LIOrd = 0.82, IOrDas = 1.03, IOD = 0.35, OOcuD = 0.22, MOD = 0.17, and 3FgmD = 0.12. The hamuli number in the species was 5. These observations were compared with morphometrics and description (key identification characters of Tetragonula iridipennis based on worker caste from Indian subcontinent) given by Rasmussen [2] which supported the identity of the collected specimens. The description by the latter is as follows:
-
a.
Inner surface of hind basitarsus with differentiated basal sericeous area of short, dense hairs (Tetragonula Moure 1961).
-
b.
‘iridipennis’ species group includes smaller forms with forewing length including tegulae, between 3.5 and 4.2 mm; head width between 1.5 and 1.8 mm; having dark mesoscutum with four distinct bands of pubescence separated by broad glabrous interspaces.
Geographical Distribution
In Indian subcontinent, studies on stingless bees’ diversity and distribution are a few. However, the species has been reported from Haryana [19], Rajasthan [20], Bangalore (Karnataka) [21], Kerala [22], Jammu & Kashmir, Maharashtra, south India (Andhra Pradesh, Tamil Nadu, Karnataka) and north east India (Assam, Arunachal Pradesh, Nagaland, Meghalaya) [13].
In Punjab, the species has been recorded in central plain agroclimatic zone and subsequently in sub-mountain undulating and western zone.
Floral Association
Stingless bees are ecologically important because of their role in pollination of various crops. The foraging plants visited by T. iridipennis in the region have been documented as a first step towards making a comprehensive inventory of its host plants. The species has been recorded foraging on 31 plant species including Brassica napus, Helianthus annuus, Brassica juncea, Brassica rapa, Trifolium alexandrinum, Pennisetum glaucum, Crotolaria juncea, Allium cepa, Coriandrum sativum, Anethum neveolens, Cajanus cajan, Psidium guajava, Syzgium cumini etc. in Punjab. Of the 31 foraging plants recorded, 17 provided both nectar and pollen, 7 provided only nectar and 6 provided only pollen. The detailed account on the species floral association in Punjab is presented in Table 2. In Kerala (India), the species has been reported to visit 77 plants comprising 21 for both nectar and pollen rewards, 37 for only nectar and 19 for only pollen [23].
Male
No males were recorded in the present collection of T. irridipennis.
Future Perspective
Stingless bees are highly evolved social insects and live in a colony with organized system of division of labour with a great potential to successfully pollinate large number of food plants in open and in protected systems of cultivation. The present state of knowledge of diversity, biology, colony organization, nesting characteristics, husbandry including colony multiplication and foraging plants of stingless bees of India and the Punjab in particular, is inadequate, and thus, systematic investigations are desired to completely understand all these attributes for realizing higher honey harvests as well as for their commercial utilization as pollen vectors particularly for the crops grown under various protected cultivation systems.
Conclusion
In conclusion, eight named species of stingless bees including four species from ‘iridipennis’ species group have been reported from India, but the species diversity of stingless bees in the Punjab needs to be investigated. The cytochrome c oxidase subunit I (COI) marker (standard DNA barcode region) based sequence of the species will serve as a permanent tool for species level identification. The morphometric data of the present bee specimens provided highly useful information for identification and comparison with metric values of T. iridipennis given by Rasmussen [2]. Since, the crop pollination under protected environments is a challenge due to existence of a physical barrier which prevents the access of natural pollinators to flowers, the research on potential pollinators under such conditions is of immense value. Their smaller colony size and short flight range put them at advantage than the Apis species as pollinator of crops under enclosures. Several stingless bee species have been reported to provide adequate pollination services to many crops grown under protected cultivation, such as strawberry, eggplant, sweet pepper, tomato and cucumber [24], although their use for pollination purposes at commercial level is still in developmental stage [25]. The present results serve as a potential basis for integration of this species for managed crop pollination. Further, systematic investigations on stingless bees in different agroclimatic zones of the Punjab needs additional collections to completely understand their species richness, diversity, distribution, floral associations, which in turn would assist in their potential commercial exploitation for pollination purposes.
References
Moure JS (1961) A preliminary supra-specific classification of the old world meliponine bees (Hymenoptera, Apoidea). Stud Entomol 4:181–242
Rasmussen C (2013) Stingless bees (Hymenoptera: Apidae: Meliponini) of the Indian subcontinent: diversity, taxonomy and current status of knowledge. Zootaxa 3647(3):401–428
Smith F (1854) Catalogue of the hymenopterous insects in the collection of the British Museum. Part II, Apidae. British Museum (Natural History), London, pp 199–465
Ascher JS, Pickering J (2016) Discover life: apoidea species guide. http://www.discoverlife.org
Michener CD (2013) The meliponini. In: Vit P, Pedro SRM, Roubik DW (eds) Pot-honey: a legacy of stingless bees. Springer, New York, pp 3–17
Sakagami SF (1978) Tetragonula stingless bees of the continental Asia and Sri Lanka (Hymenoptera, Apidae). J Fac Sci Hokkaido Univ Ser VI Zool 21:165–247
Rasmussen C (2008) Catalog of the Indo-Malayan/Australasian stingless bees (Hymenoptera: Apidae: Meliponini). Zootaxa 1935:1–802
Rasmussen C, Cameron SA (2007) A molecular phylogeny of the Old World stingless bees (Hymenoptera: Apidae: Meliponini) and the non-monophyly of the large genus Trigona. Syst Entomol 32:26–39
Rasmussen C, Cameron SA (2010) Global stingless bee phylogeny supports ancient divergence, vicariance and long distance dispersal. Biol J Linn Soc 99:206–232
Rathore VS, Rasmussen C, Saini MS (2013) New record of the stingless bee Tetragonula gressiti from India (Hymenoptera: Apidae: Meliponini). J Melittol 7:1–5
Abrol DP (2012) Pollination biology: biodiversity conservation and agricultural production. Springer, Berlin, p 792
Couvillon MJ, Wenseleers VL, Fonseca VLI, Nogueira-Neto P, Ratnieks FLW (2007) Comparative study in stingless bees (Meliponini) demonstrates that nest entrance size predicts traffic and defensivity. J Evol Biol 21:194–201
Rahman A, Das PK, Rajkumari P, Saikia J, Sharmah D (2015) Stingless bees (Hymenoptera:Apidae: Meliponini): diversity and distribution in India. Int J Sci Res 4(1):77–81
Cubero OF, Crespo A, Fatehi J, Bridge PD (1999) DNA extraction and PCR amplification method suitable for fresh herbarium-stored, lichenized and other fungi. Plant Syst Evol 216:243–249
Michener CD (2007) The bees of the world, 2nd edn. Johns Hopkins University Press, Baltimore, p 992
Ruttner F (1988) Biogeography and taxonomy of honey bees. Springer, Berlin, p 284
Sakagami SF, Inoue T (1987) Stingless bees of the genus Trigona (subgenus Trigonella) with notes on the reduction of spatha in male genitalia of subgenus Tetragonula (Hymenoptera: Apidae). Kontyû 55:610–627
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc Lond [Biol] 270:313–321
Chaudhary OP, Singh J (2007) Diversity, temporal abundance, foraging behaviour of floral visitors and effect of different modes of pollination on coriander (Coriandrum sativum L.). J Spices Aromat Crops 16:8
Gupta RK, Charan SK, Tiwari P (2011) Forage plant of Tetragonula iridipennis (Smith), a stingless bee (Hymenoptera, Apoidea, Apidae, Meliponini), in the desert of Thar in Rajasthan. J Environ Bio Sci 25:171–174
Pavithra N, Reddy Shankar M, Jayaprakash (2013) Nesting pattern preferences of stingless bee, Trigona iridipennis Smith (Hymenoptera: Apidae) in Jnanabharathi Campus, Karnataka, India. Int Res J Biol Sci 2(2):44–50
Mohan R, Devanesan S (1999) Dammer bees, Trigona iridipennis Smith. (Apidae: Meliponinae) in Kerala. Insect Environ 5(2):79
Premila K, Devanesan S, Arthur Jacob J, Shailaja K (2007) Foraging plants of stingless bee Trigona iridipennis Smith and physico-chemical characteristics of its honey. Abstr No 183. In: 40th Apimondia, International Apicultural Congress, Melbourne, Australia. Sept 9–14, p 129
Bomfim IGA, Bezerra ADM, Nunes AC, Aragao FAS, Freitas BM (2014) Adaptive and foraging behavior of two stingless bee species (Apidae: Meliponini) in greenhouse mini watermelon pollination. Sociobiology 61(4):502–509
Pessarakli M (2016) Handbook of cucurbits, 1st edn. CRC Press, Boca Raton, p 561
Acknowledgments
The authors extend sincere thanks to Dr. (Ms) Debjani Dey, Incharge, Insect Identification Service, National Pusa Collection, Division of Entomology, IARI, New Delhi and Dr. Rajiv K. Gupta, Professor and Former Head, Department of Zoology, Jai Narain Vyas University, Jodhpur for confirming the identity of the bee specimens.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Makkar, G.S., Chhuneja, P.K. & Singh, J. Stingless Bee, Tetragonula iridipennis Smith, 1854 (Hymenoptera: Apidae: Meliponini): Molecular and Morphological Characterization. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 285–291 (2018). https://doi.org/10.1007/s40011-016-0757-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0757-4