Abstract
The microsymbiont was isolated from the root nodules of Sesbania cannabina (Wild.) Pers. Initially eleven strains were isolated and all were identified as Rhizobium sp. through different physiological and biochemical tests. Ascorbic acid (AsA) production by all the isolates was checked in glucose supplemented basal medium. All the strains had the capacity to produce AsA in the culture filtrate, however to different extent. The isolate S5 showed highest AsA production (160 μg mL−1) in the culture filtrate and hence subsequent experiments were carried out with S5 strain. Both the growth and AsA production phase were started simultaneously at the onset of inoculation, and reached maximum at 24 h. The optimum pH for the AsA production was 7.0. Production of AsA by the isolate was increased to a greater extent over control when the basal medium was supplemented individually with glucose (0.7 %), thiamine-hydrochloride (200 μg L−1), biotin (200 μg L−1), glycine (0.15 %) and sodium dodecyl sulfate (1.0 μg mL−1). AsA production was increased by 278.3 % at a time when all the individual best supplements were added to the basal medium. The results of the Basic Local Alignment Search Tool search of the 16S ribosomal ribonucleic acid gene sequences indicated that S5 isolate was closely related to Rhizobium sp. Moreover, it was found that root contained very negligible amount of AsA than the root nodule. The possible role of AsA production by the Rhizobium sp. in the legume-rhizobia symbiosis has been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leguminous plants are crops of major economic value as a protein source for human and animal consumption [1]. These are essential for sustainable agricultural systems because of their ability to form root nodule establishing nitrogen (N2)-fixing symbiosis with the soil bacteria, rhizobia. The nodule morphogenesis appears to be elicited by at least two distinct signals; one from Rhizobium and the second is generated within the plant tissue. Changes in the balance of endogenous level of plant hormones are likely to be involved in nodule morphogenesis [2, 3].
All aerobic biological systems, including the N2-fixing root nodules, are subjected to O2 toxicity that results from the formation of reactive intermediates such as H2O2 and free radicals of O2. H2O2 may be removed from root nodules in a series of enzymatic reactions involving ascorbate peroxidase, dehydroascorbate reductase and glutathione reductase [4]. Ascorbate acts as a substrate for this ascorbate peroxidase [5] and an enzyme cofactor for the biosynthesis of many important biochemicals. It is further known that ascorbate may also have a regulatory role in nodules as a major contributor to the redox state of cells. Production of ascorbic acid (AsA) and its role in scavenging oxidative stress of plant system is one of the recent thrust areas in the legume-root nodule symbiosis [6]. Legume nodules are at high risk of being damaged as a result of generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In general, ROS are potentially toxic and their uncontrolled production can result in oxidative damage to the cellular components [7]. However, some ROS such as superoxide radical and H2O2, at low concentrations, perform important roles in stress perception, photosynthesis regulation, pathogen recognition, programmed cell death (PCD) and plant development [8]. These ROS and RNS at specific concentrations, which are slightly controlled by antioxidant enzymes and metabolites, also play positive role as critical components of signal transduction cascades during nodule development and stress [9]. On the contrary, it was hypothesized that pea (Pisum sativum) nodules are unable to synthesize ascorbate and have to import it from the shoot or root [10]. The capacity to accumulate ascorbate was retained in young nodules but was lost during the course of development. In contrast, the last step of the d-Mannose/l-Galactose [11] and d-Galacturonic acid [12] pathways for ascorbate synthesis catalyzed by l-galactono-1,4-lactonedehydrogenase (GalLDH), was found in mitochondrial membrane of bean nodules [13]. The expression of five genes of the Smirnoff-Wheeler pathway of AsA biosynthesis was also detected in Lotus japonicas bean nodules [6, 13] lending further support to the functionality of ascorbate biosynthetic pathway in nodules. Moreover, GalLDH expression (mRNA and activity) and ascorbate concentration were detected in the infected zone of both types of nodules [13]. Though nodule host cells make their own metabolite-glutathione (GSH), some of the critical antioxidants need to be produced by the bacterial partner to achieve optimal nitrogen fixation, which is further supported by the observation that rhizobia deficient in glutathione synthase (GSHS) formed nodules with early senescence and diminished symbiotic performance [14, 15]. Hence, AsA produced by Rhizobium sp. in nodule plays an important role in different metabolic functions particularly in stress condition [16].
The above information led the authors to undertake the present study to check the status of AsA in the root nodules of a leguminous plant S. cannabina which has immense economic importance. This plant gained popularity as a green manure crop for rice, sugarcane and cotton. The plant is also useful in reclamation of saline and alkaline lands [17]. Bioproduction of AsA by the isolated symbiont was also checked to get an explanation of nodular AsA. Moreover, the medium for AsA production by the isolated Rhizobium was further optimized by supplementation of different chemicals.
Material and Methods
Collection of Root Nodules, Isolation and Scanning Electron Microscopy (SEM) Imaging of Micro-Symbionts
The leguminous herb S. cannabina, was selected as the experimental material and the plants were grown and collected from the experimental field of Vivekananda Institute of Biotechnology, Nimpith, West Bengal. The plants were grown in field condition for 40–45 days during autumn (temperature: 25–30 °C and relative humidity: ~80 %). The mature, fresh root nodules and young roots were collected from the plant to perform the experiments.
Fresh, healthy and pink coloured mature nodules were taken for isolation of microsymbionts. The nodules were surface sterilized by 95 % ethanol and 0.1 % HgCl2 followed by washing (3 times) with sterile distilled water. The nodules were cut open and crushed between two sterile glass slides and the fluid coming out of the crushed nodules was streaked aseptically on yeast extract mannitol agar (YEMA) plates which were incubated at 30 ± 1 °C. The bacterial growth obtained from the streaks was diluted serially in order of 10 using sterile distilled water. From the dilution tubes of higher order, 0.1 mL aliquots were plated on YEMA plates. From the dilution plate, 11 individual colonies were isolated separately on YEM slants. The bacteria were numbered as S1–S11 randomly to designate well. All the individual isolates were identified as Rhizobium sp. following the methods described in Manual of Microbiological Methods [18] and also by Jordan [19]. The bacteria were routinely observed in microscope to check its purity and the purified cultures were maintained in YEMA slants.
SEM of the symbiont was studied following Rigaud and Puppo [20]. Bacteria of different phases from culture broth were centrifuged at 8000g for 10 min and the pellets of bacterial cells were collected. The bacteria were then washed with phosphate buffer (0.2 M, pH 7.2) and subsequently centrifuged at 8000g for 10 min. After that, cells were fixed with glutaraldehyde for 3 h at 16 °C, centrifuged and washed with same concentration of phosphate buffer (pH 7.2) and centrifuged again. The pellets of the bacteriods were dehydrated with different concentrations of ethanol up to 100 %. The samples were washed with acetone and were ready for grid preparation to enable the study of symbiont under SEM (Model No. HITACHI S-530) (Fig. 1).
AsA Production Medium and Growth Conditions
To estimate AsA productivity by the isolates (S1–S11), a culture medium formulated by Ghosh et al. [21] was used. The composition of the medium was; glucose: 5 g, K2HPO4: 500 mg, MgSO4·7H2O: 200 mg, NH4Cl: 2 g, thiamine hydrochloride: 100 μg and d-biotin: 200 μg per liter of distilled water at pH 7.0. The bacteria grown in YEM medium were transferred (10 % inoculum, from exponential growth phase) in 20 mL AsA production medium in 100 mL Erlenmeyer flasks with three replicates at 30 ± 1 °C on a rotary shaker at 150 rpm for 24 h (optimum time for growth and AsA production). The strain S5, among the tested eleven isolates, showed highest level of AsA production in the medium and was selected for further experiments. S5 strain was grown individually in YEM medium and the growth was measured turbidometrically using a spectrophotometer at 540 nm.
Extraction and Estimation of AsA and Estimation of Glucose
AsA was extracted and estimated following the method of Oser [22] with slight modifications. To estimate the nodular and root AsA content, fresh uninfected root or nodule of 1 g each was homogenized with 10 mL of 6 % trichloroacetic acid (TCA), centrifuged at 5000 rpm for 2 min to eliminate plant debris. In 5 mL filtrate, 3 mL of 2.5 % 2,4-dinitrophenyl hydrazine solution (prepared in 9 N H2SO4, to which 2–3 drops of 10 % thiourea solution in 70 % ethanol was added to check the oxidation of AsA) was added. The mixture was boiled for 25 min in a water bath, cooled to 0 °C and then 5 mL of 80 % H2SO4 was added and kept for 30 min following which the optical density (OD) was measured at 530 nm using a spectrophotometer and AsA was estimated using a standard curve prepared with authentic AsA following the above mentioned method. To estimate AsA in the bacterial culture filtrate, the bacteria S5 were grown for 24 h in AsA producing medium, centrifuged at 8000 rpm for 10 min, following which the filtrate was taken and treated in the same way as mentioned above. Glucose present in the culture filtrate as well as in root nodule and root was estimated by phenol–sulphuric acid method [23], as glucose serves as the precursor for AsA synthesis.
Optimization of AsA Production
During medium characterization for optimum production of AsA by the isolate, different concentrations (0.1, 0.3, 0.5, 0.7, 0.9, 1.0, 1.5 %) of glucose were used. Different chemicals viz. N2 sources (glycine, ammonium sulfate, l-glutamic acid, ammonium chloride, l-asparagine), vitamin sources (thiamine-HCl, d-biotin, riboflavin, nicotinic acid), cell wall affecting agents (SDS, EDTA Na- salt, triton × 100, penicillin) were then added individually to the basal medium and the individual effect of each chemical on AsA production was tested. To check the maximum production of AsA by the Rhizobium sp. in culture, the medium was enriched with supplements, which was found to increase AsA production individually.
PCR Amplification and 16S rRNA Sequencing
PCR amplification of the bacterial 16S rRNA fragments was done from genomic DNA (extracted following standard protocol from 24 h cultural broth of the isolate) using standard eubacterial primers namely: Fc27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and RC1492 (5′-TACGGCTACCTTGTTACGACTT-3′) (Lane 1991). Each PCR reaction consisted of 0.5 μL (~20 ng) DNA template, 2.5 μL 10× Taq buffer, 2.5 μL dNTPs (final concentration 0.2 mM), 1 μL MgCl2 (final concentration 2.0 mM), 0.25 μL each of the Fc27 and RC1492 primers (final concentration 0.5 μM), 0.5 μL BSA (1 mg mL−1), 0.25 μL Taq polymerase (5 U μL−1) (Fermentas) and nuclease free water to make a final volume of 25 μL. PCR conditions were as follows: initial denaturation at 95 °C for 5 min, 30 cycles of 95 °C for 1 min, 55 °C for 1 min, 72 °C for 3 min, and final extension at 72 °C for 10 min. Purification of the PCR product was done with Qiagen gel purification kit. Purified PCR product in one direction (forward direction) was subjected to DNA sequencing in an ABI Prism 3730 Genetic Analyzer based on BigDye Terminator chemistry. Generated chromatogram was checked for any ambiguity or error before BLASTn validation.
Statistical Analyses
Statistical analyses were done following Panse and Sukhatme [24] using three to five replicates.
Results and Discussion
The symbiont was isolated from healthy root nodules of S. cannabina in YEMA plates. Initially eleven strains were isolated and all the strains were identified to be a species of Rhizobium according to Conn et al. [18] and Jordan [19], but the species were not ascertained. The renodulation experiment confirmed the host. SEM imaging of the isolates is depicted in Fig. 1. Small rod shaped bacteria similar to other Rhizobium sp. were noted. AsA production was checked in AsA producing medium and it was found that all the strains had the capacity to produce AsA in culture filtrate. However, the strain number S5 produced highest amount of AsA (160 μg mL−1) in the medium (Table 1) and hence subsequent experiments were carried out with this strain. Strain S5 gave the following physiological and biochemical characteristics: positive tests for catalase, oxidase, acid from glucose, starch and urea hydrolysis, growth on Hofer’s alkaline broth (HAB), 8 % KNO3 tolerance and precipitation in calcium glycerophosphate experiments and negative tests for growth on glucose peptone agar (GPA), growth on 0.1 % methylene blue, growth on 0.1 % gentian violet, citrate utilization, gelatin hydrolysis etc.
Effect of Glucose on AsA Production
The growth and AsA production phase of the isolate started almost immediately after inoculation and reached its respective stationary phase at 24 h (Fig. 2). The level of AsA production decreased after the stationary phase and the level of glucose was decreased simultaneously with the increase in AsA production (Fig. 3). The optimum concentration of glucose in the medium for highest AsA production by the isolate was found to be 0.7 % for this particular strain (Fig. 4).
Effect of Different N Sources on AsA Production
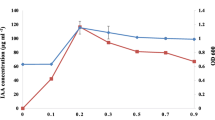
To check the effect of N2 sources on AsA production, five different N2 sources were added separately to the AsA medium omitting NH4Cl. From the Table 2, it was evident that, though the isolate could use all the tested N2 sources for growth and AsA production, glycine induced maximum production of AsA. The optimum concentration of glycine for maximum AsA production was 0.15 % (Fig. 5).
Effect of different concentration of glycine (optimum N2 source) on growth (open circle) and AsA production (closed circle) by the Rhizobium sp. (S5) in culture. Bacteria were grown in the basal medium for AsA production containing 0.7 % glucose and glycine at different concentrations for 24 h at 30 ± 1 °C in a rotary shaker at 150 rpm. Bars on the points indicate ±SE value up to ±5 %
Effect of Different Vitamin Sources on AsA Production
The effect of different vitamin sources was also checked for AsA production. From Table 2, it was noted that thiamine-HCl followed by d-biotin had most promotive effect for AsA production. The production enhanced synergistically by 258 % over control when thiamine-HCl, d-biotin and glycine (0.15 %) were supplemented in production medium (Table 3).
Effect of Different Cell Wall Affecting Chemicals on AsA Production
Further, the effect of different cell wall affecting chemicals on AsA production was also checked. From the Table 5, it was found that SDS (1.0 μg mL−1) had most promoting and penicillin had most inhibiting effect on AsA production (Table 4).
Synergistic Effect of Combined Supplements on AsA Production
To check the maximum AsA production by the Rhizobium sp. in culture, the supplements, which individually increased the AsA production to the most, were added to the medium. The isolated Rhizobium which initially produced 60.0 μg mL−1 AsA in glucose supplemented basal medium without any vitamin and nitrogen source was induced to produce 227.0 μg mL−1 of AsA, an increase of 278.3 % over control (Table 5). The root nodules of S. cannabina contained moderate amount of AsA (579 μg g−1 of fresh tissue) and glucose pool (910 μg g−1 of fresh tissue) than their corresponding uninfected roots which contain AsA (217 μg g−1 of fresh tissue) and glucose (380 μg g−1 of fresh tissue).
Molecular Characterization of the Bacteria
The results of the BLASTn search of the 16S rRNA gene sequences indicated that S5 isolates were closely related to Rhizobium sp. (99 % identity matches with Rhizobium sp. ORS 3441, Rhizobium sp. L28, Rhizobium L38 and Rhizobium sp. JNVU TP3) (length of the amplicon: 659 bp and query coverage: 100 %).
From the root nodules of S. cannabina, microsymbionts were isolated in the YEMA medium and eleven strains were isolated initially. All the strains were identified as species of Rhizobium according to Conn et al. [18] and also following Jordan [19], but the species were not ascertained. The strains showed similarities to the characters of a fast- growing Rhizobium sp. and give proper results for different biochemical and physiological tests.
AsA, a secondary metabolite and an important antioxidant, production capacity of which by all the isolates was checked in AsA producing medium. It was found that all the strains had the capacity to produce AsA in culture filtrate. However the strain number S5 produced highest amount of AsA in the medium. Secondary metabolites are generally produced in the stationary phase. However, though AsA is a secondary metabolite, it was produced from the very beginning of growth of the bacteria, because this secondary metabolite being an antioxidant, has a role in protecting the cell from oxidative damage by scavenging the ROS from the very beginning [25]. ROS are continuously produced in response to biotic and abiotic stresses [26]. There is a decreasing trend in AsA production after the stationary phase is reached. Similar trends were also noticed during AsA production [20] and indole acetic acid (IAA) production, another secondary metabolite [27] by other Rhizobium sp. For AsA production, d-glucose can serve as a precursor molecule [12] and the level of glucose was also decreased simultaneously with the increase in AsA production by the selected S5 strain. The bacterium preferred an optimum concentration of glucose in the medium for maximum AsA production.
As nitrogen is the main component of proteins necessary in cell metabolism which comprises up to 8–14 % of the cell dry mass of bacteria [28], the effect of different nitrogen sources on AsA production was checked. Among different nitrogen sources, in the present study, the bacteria used all such sources for AsA production, with a preference for glycine. Earlier it was reported that Rhizobium sp. could use several nitrogen sources for growth [29]. However, in another study, both the growth promoting and growth retarding effects of different nitrogen sources were exhibited by a Rhizobium sp. isolated from Crotalaria retusa [30].
Vitamins, especially the B-group, are known to be essential for growth of Rhizobium sp. [31]. Among the tested vitamins, thiamine-HCl followed by d-biotin had most promotive effect. Similar promoting effect was also reported for IAA production [30] and AsA production [21]. Furthermore, permeability may be increased by affecting cell wall or membrane which yielded an increased amount of amino acids in the medium [32]. Among the effect of different cell wall affecting chemicals on AsA production, it was found that SDS (1.0 μg mL−1) had the most promoting and penicillin had the most inhibiting effect. Using all the different supplements viz. optimum carbon source, glycine as nitrogen source, optimum vitamin sources and cell wall affecting chemicals such as SDS in combination in medium formulation, the isolated Rhizobium sp. (S5) was induced to produce 227.0 μg mL−1 of AsA, an increase in 278.3 % over control, which serves as a great achievement for industrial production of AsA using Rhizobium sp. in vitro. The root nodules of S. cannabina contained moderate amount of AsA and glucose pool than their corresponding uninfected root which contained AsA and glucose in much little quantity. The root nodule of Phaseolus mungo also contained higher amount of AsA when compared to the root [5]. Presence of AsA in the root nodules was also reported earlier [13]. Ascorbate is an antioxidant, a direct scavenger of most ROS and is the substrate of ascorbate peroxidase (APX). Cytosolic APX has been purified from soybean nodules [13]. Efficacy of N2 symbiotic fixation in legume nodules is controlled via various metabolic pathways and needs suitable environmental conditions [33]. Antioxidants were reported immensely critical for the protection and optimal performance of N2 fixation in the nodules [34]. Involvement of ROS and antioxidant defense was also noted in key steps of nodule formation, infection thread development and nodule meristem development [25]. The thioltripeptide GSH is also an abundant metabolite found in plants which functions as an antioxidant. However, in some legumes, homoglutathione may partially or completely replace GSH. Homoglutathione is the major tripeptide found in root nodules of several leguminous plants [13]. However, ascorbate and thiols are also present at high concentration in the nodule apex, which is an indicative that they fulfill additional roles in the symbiosis [13]. Several lines of evidence show that ascorbate-GSH pathway is critical for nodule functioning [35]. There are further indications of the importance of the ascorbate-GSH pathway for N2 fixation. The ascorbate-GSH pathway involves four enzymes operating in concert to remove H2O2 at the expense, ultimately, of the reducing power of NADH or NADPH. This pathway is operative in nodule compartment [5]. In addition, direct infusion of ascorbate into stems of soybean plants leads to an increase in leghemoglobin content, a fourfold increase in rates of N2 fixation, and a substantial delay in nodule senescence [36]. Furthermore, ascorbate in the infected zone of legume plant is primarily involved in the protection of host cells against peroxide damage [37]. In addition, ascorbate is required for the progression of cell cycle and for elongation which play a critical role in nodule development. In nodules, the high mitotic activity in zone I (meristem) and the growth of infection threads in zone II (invasion) are therefore expected to require ascorbate and thiols. Moreover, it is reported that, the low concentration of ascorbate and thiol compounds in the senescent zone are consistent with the proposed relationship between the decrease in antioxidant defenses and the onset of nodule senescence [13]. Even in non-leguminous crop such as Brassica, alleviation of osmotic stress tolerance was attributed to the application of ascorbic acid [38]. Thus a combination of multiple factors including ROS may be involved in the control of the events leading to the rupture of the symbiotic interaction [25].
An important asset of antioxidant enzymes is expected in both the symbiotic partners [5]. The bacterial partner that differentiates to bacteroid to reduce atmospheric nitrogen also contains a large range of antioxidant systems including enzymatic and non-enzymatic defense. Several bacterial strains deficient in antioxidant defense exhibit an altered nitrogen fixation capacity without strong modifications of their growth efficiency in free living condition [25]. AsA might function as a growth factor for some bacteria. Nodular symbionts also help plants to protect themselves during the initial infection and further protecting from biotic stress in the rhizospheric area [39]. The higher amount of glucose in nodular tissue also supported the fact that nodule acts as reservoir for glucose in Rhizobium sp., serving as a precursor of AsA biosynthesis, which is supplied to the infected zone of plant tissue for further protection from stress [16]. The production of higher amount of AsA in nodule as compared to root was implicated to the presence of the enzyme GaILDH coded by a gene present in Rhizobium sp. The gene coding this enzyme is controlled by surrounding environmental condition of Rhizobium sp. as well as by biotic stress and different signaling of plant tissue [16].
Conclusion
In the present investigation, except cell wall affecting chemicals, all the supplements which increased AsA production in cultures may also be available for the bacteria within the nodule for enhanced AsA production. By such alteration in the supply of metabolites within the nodules, the host plant might induce the microsymbionts to produce more AsA helping to promote infection, enhance nodulation and also extend the life span of the nodule resulting delaying in nodule senescence. In the end, it can be suggested that legume-rhizobia symbiosis should be evaluated afresh in the light of AsA production, apart from N2 fixation and hormone production, which were thought earlier as the primary concern in this symbiosis. AsA production ability of Rhizobium sp., as a nodule microsymbiont or as in free living condition, with best medium supplements, can serve as a new approach for exploiting rhizobial source of AsA production in future.
References
Graham PH, Vance CP (2003) Legumes: importance and constraints to greater use. Plant Physiol 131:872–877
Hirsch AM (1992) Developmental biology of legume nodulation. New Phytol 122:211–237
Garg NG (2007) Symbiotic nitrogen fixation in legume nodules: process and signaling. A review. Agron Sustain Dev 27:59–68
Dalton DA, Hanus FJ, Russell SA, Evans HJ (1987) Purification, properties, and distribution of ascorbate peroxidase in legume root nodules. Plant Physiol 83:789–794
Matamoros MA, Dalton DA, Ramos J, Clementa MR, Rubio MC, Becana M (2003) Biochemistry and molecular biology of antioxidants in the rhizobia- legume symbiosis. Plant Physiol 133:499–509
Loscos J, Matamoros MA, Becana M (2008) Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural sensescence. Plant Physiol 146:1282–1292
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, UK
Mittler R, Vanderauwera S, Gollery M, VanBreusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Matamoros MA, Loscos J, Dietz KJ, Aparicio-Tejo PM, Becana M (2010) Function of antioxidant enzymes and metabolites during maturation of pea fruits. J Exp Bot 61:87–97
Groten K, Vanacker H, Dutilleul C, Bastian F, Bernard S, Carzaniga R, Foyer CH (2005) Theroles of redox processes in pea nodule development and senescence. Plant Cell Environ 28:1293–1304
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Valpuesta V, Botella MA (2004) Biosynthesis of l-ascorbic acid in plants: new pathways for anold antioxidant. Trends Plant Sci 9:573–577
Matamoros MA, Loscos J, Coronado MJ, Ramos J, Sato S, Testillano PS, Becana M (2006) Biosynthesis of ascorbic acid in legume root nodules. Plant Physiol 141:1068–1077
Harrison J, Jamet A, Muglia CI, Van de Sype G, Aguilar OM, Puppo A, Frendo P (2005) Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobiummeliloti. J Bacteriol 187:168–174
Muglia C, Comai G, Spegazzini E, Riccillo PM, Aguilar OM (2008) Glutathione produced by Rhizobiumtropici is important to prevent early senescence in common bean nodules. FEMS Microbiol Lett 286:191–198
Ghosh P, Ghosh S, Saha P, Mayilraj S, Maiti TK (2012) Theascorbic acid production in root, root nodule and in culture by Rhizobium sp. isolated from the legume Cajanuscajan (L.) Millspaugh. J Pure Appl Microbiol 6:241–248
Ambasta SP (ed) (1986) The useful plants of India. Publication and information directorate. CSIR, New Delhi
Conn HJ, Jennison MN, Weeks OB (1957) Routine tests for the identification of bacteria. In: Conn HJ (ed) Manual of microbiological methods. Mc Graw Hill Book Co, New York, pp 140–168
Jordan DC (1984) Rhizobiaceae. In: Krieg NR, Holt JG (eds) Bergey’s manual of systematic bacteriology. Williams and Wilkins Co, Baltimore, pp 235–244
Rigaud J, Puppo A (1975) Indole-3-acetic catabolism by soybean bacteroids. J Gen Microbiol 88:223–228
Ghosh S, Maiti TK, Basu PS (2008) Bioproduction of ascorbic acid in root nodule and root of the legume pulse Phaseolus mungo. Curr Microbiol 56(5):495–498
Oser BL (1979) Hawk’s physiological chemistry. Mc Graw Hill, New Delhi
Dubois M, Gilles KA, Hamilton JK, Rebers RA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28(3):350–356
Panse VG, Sukhatme PV (1985) Statistical methods for agricultural workers, 4th edn. Indian Council of Agricultural Research, New Delhi
Chang C, Damiani I, Puppo A, Frendo P (2009) Redox changes during the legume-rhizobium symbiosis. Mol Plant 2(3):370–377
Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM (2008) Higher plant antioxidants and redox signaling under environmental stresses. Comput Rendus Biol 331:433–441
Bhattacharyya RN, Pati BR (1999) Growth behaviour and indole acetic acid (IAA) production by a Rhizobium isolated from root nodules of Alysicarpusvaginalis DC. Acta Microbiol Immunol Hung 47(1):41–51
Yodsuwan N, Owatworakit A, Ngaokla A, Tawichai N, Soykeabkaew N (2012) Effect of carbon and nitrogen sources on bacterial cellulose production for bionanocomposite materials. In: 1st Fah Luang University international conference, Thailand
Vincent JM (1974) Root-nodule symbiosis with Rhizobium. In: Quispel A (ed) The biology of nitrogen fixation. North-Holland Publishing Co, Amsterdam, pp 265–341
Bhattacharyya RN, Basu PS (1991) Studies of the root nodules of leguminous plants IV: production of indole acetic acid by a Bradyrhizobium sp. from the root nodules of a leguminous shrub, Crotalaria retusa L. Acta Biotechnol 11(5):439–447
Alexander M (1977) Introduction to soil microbiology, 2nd edn. Wiley Eastern Ltd, New Delhi
Sen K, Chatterjee SP (1983) Extracellular lysine production from hydrocarbons by Arthrobacterglobiformis. Folia Microbiol 28(4):292–300
Silveira JAG, Figueiredo MVB, Cavalcanti FR, Ferreira-Silva SL (2011) Legume nodule oxidative stress and N2 fixation efficiency. In: de Araújo ASF, Figueiredo MVB (eds) Microbial ecology of tropical soils. Nova Science Publishers Inc., New York, pp 49–78
Becana M, Dalton DA, Moran JF, Iturbe-Ormaetxe I, Matamoros MA, C Rubio M (2000) Reactive oxygen species and antioxidants in legume nodules. Physiol Plant 109:372–381
Dalton DA, Joyner SL, Becana M, Iturbe-Ormaetxe I, Chatfield JM (1998) Antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol 116(1):37–43
Bashor CJ, Dalton DA (1999) Effects of exogenous application and stem infusion of ascorbate on soybean (Glycine max) root nodules. New Phytol 142(1):19–26
Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, Foye CH (2005) Legumenodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165(3):683–701
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014) Alleviation of osmotic stress in Brassica napus, B. campestris, and B. juncea by ascorbic acid application. Biol Plant 58:697–708
Pastori G, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin c contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Acknowledgments
The authors acknowledge the effort of Sandip Das, Research scholar for his valuable inputs during drafting of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nath, A., Dey, A., Mukherjee, S. et al. Bioproduction of Ascorbic Acid and Its Optimization by a Rhizobium sp. from Root Nodules of Sesbania cannabina . Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 87, 1459–1467 (2017). https://doi.org/10.1007/s40011-016-0717-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0717-z