Abstract
The effect of phosphate-solubilizing bacteria (PSB), Bacillus subtilis KF279045, identified on the basis of 16S rRNA gene sequencing was investigated on plant growth and diosgenin content in Dioscorea alata in pots containing tricalcium phosphate (TCP) supplemented soil. The PSB were isolated from the rhizosphere of D. alata plants and tested for P-solubilization ability and indole acetic acid production. High performance thin layer chromatography method was used for quantitative determination of diosgenin in different samples of D. alata. In glasshouse study, treatment of PSB resulted in increased plant growth and diosgenin content. The stimulatory effect was observed with PSB + TCP treatment in plant growth parameters and diosgenin content was compared to the uninoculated plants. This is the first study documenting the stimulatory effect of PSB on diosgenin content and it suggests the establishment of synergistic interactions between B. subtilis and D. alata.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dioscorea alata L. (Dioscoreaceae) is an important tuber plant for food and medicinal use to promote health and longevity in Chinese traditional medicine [1]. Steroidal saponins were reported to be the major physiologically active constituents in Dioscorea sp. [2]. Dioscorea is a potent source of diosgenin, a steroidal saponin having significant pharmaceutical importance. Diosgenin is an important precursor of semi-synthetic steroids such as corticosteroids, sex hormones (e.g., progesterone) and other steroidal drugs in pharmaceutical industry [3, 4]. Diosgenin has also been reported against cancer [5], cardiovascular disease [6], diabetes [7], Alzheimer’s disease [8], osteoporosis [9] and skin aging [10].
The amount of active principles depends on total biomass, which further depends on climatic factors, agro-techniques, water management and fertilizer applications. Recently, the integrated application of microbial inoculants to agro-technologies for the cultivation of medicinal plants is being promoted for improving their productivity in terms of biomass and biochemical constituents [11–14]. Phosphate solubilizing bacteria (PSB) are well known to promote plant growth because of their ability to convert insoluble form of P to soluble form that can be readily taken up by the plant roots. Usually the soils are supplemented with inorganic P in the form of chemical fertilizers. A large proportion of the applied P gets fixed in the soil as phosphates of iron, aluminum and calcium [15]. This fixed form of P is not efficiently taken up by the plants and known to cause many environmental problems like eutrophication and soil salinity [16]. The use of PSB as biofertilizers could decrease the environmental problems associated with conventional chemical fertilizers. In addition to P-solubilization, PSB may also improve plant productivity by synthesizing other secondary metabolites. Several evidences related to plant growth promotion exhibited by PSB via production of IAA make it more suitable as biofertilizer [17, 18].
The effect of PSB on medicinal plants is gaining momentum, as evidenced by an increase in the number of reports [11, 19, 20]. However, there are no information related to studying the effect of PSB on the growth and diosgenin content in D. alata. Therefore, the objective of the present study was to isolate the PSB from the rhizosphere of Dioscorea plants and examine their effect on plant growth, availability of P in soil, P uptake by plants and diosgenin yield from D. alata. Moreover, diosgenin contents in the aerial and underground parts of different plant samples have been analyzed by high performance thin layer chromatography (HPTLC). Further, the PSB was screened and characterized with molecular biological tools.
Material and Methods
Isolation and Screening of PSB
Rhizospheric soil samples of D. alata plants growing in organic farms (loamy soil, without any input of chemical fertilizers) were collected in sterile plastic bags from the herbal garden of Lovely Professional University, Phagwara, Punjab, India in the month of January, 2013. Plant material was identified by the authors following the identification key [21]. A voucher specimen (DA 001) was preserved at the Department of Biotechnology, Lovely Professional University for future reference. Samples were processed on the same day on which the soil was collected. One gram soil of each sample was suspended separately in 9.0 ml of phosphate buffer saline of pH 7.2. The serial dilutions (1:10) were made and spread on Pikovskaya’s (PVK) agar plates containing 0.5 % tricalcium phosphate (TCP) and incubated at 30 °C for 72 h. Colonies showing zone of solubilization indicating microbial P-solubilizing ability, were streaked on nutrient agar plates to check their purity and stored for further studies. P-solubilization was estimated in a PVK liquid medium amended with 0.5 % TCP. The isolates were grown in a 100 ml liquid medium at 30 °C on a rotary shaker (130 rev min−1) and the pH was adjusted to 7.0. The 5.0 ml culture was taken out at regular intervals of 24 h, for 6 days, centrifuged (10,000×g for 10 min) and soluble-P content of culture supernatant was estimated by colorimetric chlorostannous reduced molybdophosphoric acid blue method [22].
Quantitative Estimation of Indole Acetic Acid (IAA)
IAA was assayed by the colorimetric method using ferric chloride-perchloric acid reagent (FeCl3–HClO4) [23]. PSB were inoculated in the minimal medium (g l−1): KH2PO4, 1.36; Na2HPO4, 2.13; MgSO4·7H2O, 0.2, pH 7.0, amended with 5.0 mM l-tryptophan solution (g 100 ml−1: glucose, 10; tryptophan, 1.0; yeast extract 0.1; filtered through sterile 0.2 μm Millipore membrane filter) [24]. Flasks were incubated at 30 °C on a rotary shaker (130 rev min−1). Cultures were withdrawn after 48 h intervals and were centrifuged (10,000×g for 10 min). The 2.0 ml of Salper’s reagent was added to 1.0 ml of culture supernatant and samples were incubated in dark for 30 min. Development of pink color was assayed by a spectrophotometer at 530 nm. The concentration of IAA (in μg ml−1) was determined from a standard curve of IAA (0–10 μg ml−1).
Physiological Characterization and Molecular Identification of PSB
PSB isolates were characterized based on colony morphology, Gram-staining and biochemical testing of catalase [25], oxidase [26] and lactose fermentation [27]. The very first step executed was isolation of DNA from the culture. Hence, evaluation of the quality was done on 1.2 % agarose gel and a single band of high-molecular weight DNA (representing single clone of the PSB chosen for the studies) was observed following standard protocol. Fragment of 16S rDNA gene was amplified by PCR from the isolated DNA. A single discrete PCR amplicon band of 1500 bp was observed when resolved on agarose gel (Fig. 1). Forward and reverse DNA sequencing reaction of PCR amplicon were carried out with 27F and 1492R primers using BDT v3.1 Cycle sequencing kit on ABI 3730xl Genetic Analyzer. Consensus sequence of 1457 bp rDNA gene was generated from forward and reverse sequence data using aligner software (Fig. 2). The 16S rDNA gene sequence was used to carry out BLAST with the nrdatabase of NCBI genbank database (http://www.ncbi.nlm.nih.gov). Based on maximum identity score, first ten sequences were selected and aligned using multiple alignment software program Clustal W. Distance matrix was generated using RDP database and a phylogenetic tree was constructed using MEGA 4 (Fig. 3).
Phylogenetic tree based on 16S rRNA gene sequences, showing the relationships among selected PSB isolates (shown in bold letters) and representatives of other related taxa with validly published names. The 16S rRNA gene accession numbers are given within brackets. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree with the sum of branch length = 0.97398246
Pot Experiments
PSB was grown in the nutrient broth at 30 °C in an orbital shaker (150 rev min−1) for 24 h. The culture was centrifuged in 50 ml sterile plastic tubes at 6000×g for 15 min. The pellets were resuspended in PBS and optical density (OD) was adjusted to get a final concentration of colony forming units, i.e. 108 CFU ml−1. This liquid culture of PSB was used for inoculation during pot experiments.
Experiments were conducted in a glasshouse during February–April, 2013, in the Department of Biotechnology, Lovely Professional University. Unsterile loamy soil (pH, 7.6; available N, 46.2 mg kg−1; available P, 5.3 mg kg−1; available K, 14.2 mg kg−1; total Ca, 6.2 m Equiv. kg−1; total Mg, 1.3 m Equiv. kg−1; total organic carbon, 0.13 %) were mixed thoroughly and passed through 2 mm sieve to remove large particulate matters following which the mixture was autoclaved. Plastic pots (10 cm × 20 cm), were filled with 1.0 kg of soil. The cuttings of D. alata with nodes were surface-sterilized by dipping in 2 % NaOCl solution for 10 min and then washed thrice with distilled water. The surface-sterilized nodal cuttings were dipped in the desired culture inoculums (≈108 CFU ml−1) for 15 min and were planted in three different sets of treatment with five replications of each. These sets included: (1) unamended soil (2) soil + TCP (100 mg kg−1 soil); (3) soil + TCP + B. subtilis. Experiments were performed in completely randomized block design.
Analysis of Available P Content in Soil and P Uptake in Plants
After the harvesting of plants, the P content of soil and dried plant parts were analyzed. Available P content in soil was determined by colorimetric sodium bicarbonate-extractable P method [28]. P uptake in plant parts was determined using vanado-molybdo-phosphoric yellow color method [29].
HPTLC Analysis of Diosgenin Content in the Aerial and Underground Parts of Different Samples of D. alata
Dioscorea alata roots and aerial shoots were dried in an oven at 40 °C for 5 days (prolonged drying period was used to make it completely moisture free before going for the extraction process). The 50 mg of completely dried and powdered samples were taken in 25 ml conical flasks. Samples were extracted with 10 ml methanol for 24 h and then filtered through Whatman filter paper in a 50 ml distillation flask. The extract was completely dried and dissolved in 2 % H2SO4 in water for hydrolysis of saponins. The extract was dried and chloroform was added to the sample. Chloroform soluble fraction containing diosgenin was used for HPTLC quantification. Instrument used for HPTLC analysis was a CAMAG TLC system comprising of a Linomat-5 applicator and CAMAG TLC densitometer scanner. Stationary phase used was silica gel 60 F254, 20 × 10 cm TLC plate obtained from Merck. Solvents toluene and ethyl acetate (EA) were of analytical grade procured from Himedia. For each run, the factors kept constant were (1) solvent front: 8 cm, (2) time of run: 12–15 min, (3) developing reagent: methanol and conc. H2SO4, (4) time of development: 15 min in oven at 60 °C with air circulation, (5) densitometry scan: 430 nm. Working standard of diosgenin (50 μg ml−1) (Sigma-Aldrich, 95 % pure) prepared in methanol was taken for the study. The calibration curve from 100 to 500 ng spot−1 was prepared and checked for reproducibility, linearity and validating the method. The method was validated in terms of linearity range, accuracy, limit of detection (LOD) and limit of quantification (LOQ).
Statistical Analysis
Statistical analysis was conducted using one-way analysis of variance (ANOVA) by Minitab 15. Comparisons of means were performed by the LSD test at P ≤ 0.05.
Results and Discussion
Isolation and Screening of PSB and Indole Acetic Acid (IAA) Production by the Isolates
Only one out of 3 PSB isolated from the rhizosphere of Dioscorea plants showing P-solubilizing zone >5 mm on the Pikovskaya’s (PVK) agar medium was screened. In liquid PVK medium, the P-solubilizing ability of the organism was 325 μg ml−1. The selected bacterial isolates were further tested for IAA production. Isolates, DK1 (tentatively named before identification) produced IAA (15.3 μg ml−1) in the highest amount. Other two isolates DK2 and DK3 produced 13.7 and 13.1 μg ml−1 IAA respectively.
Biochemical and Molecular Characterization of PSB
Best PSB (DK1) was chosen for biochemical and molecular characterization. DK1 PSB isolates were Gram-positive unicellular rods (Table 1). The results of the BLAST search of the 16S rRNA gene sequences indicated DK1 isolates being closely related to Bacillus subtilis strain BAB-2441. Based on the Neighbor-Joining (NJ) phylogenetic tree done with the 16S rDNA similarity (%), the nearest taxon of PSB isolates was identified as B. subtilis KF279045 for DK1. The evolutionary history was inferred using the NJ method [30]. The optimal tree with the sum of branch length as 0.97398246 is shown. The evolutionary distances were computed using the Kimura 2-parameter method [31] and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (complete deletion option). There was a total of 1360 positions in the final dataset. Phylogenetic analysis was conducted in MEGA4 [32] (Fig. 3).
Effect of PSB on Plant Growth, P Content in Soil and P Uptake by Plant
The PSB treatments increased all parameters of D. alata than control. It significantly (P ≤ 0.05) increased shoot length by 25 %, root length by 32 %, total number of leaves by 26 % over control while PSB + TCP amended soil showed increase in shoot length by 90 %, root length by 57 %, total number of leaves by 106 % over control (Table 2). Inoculation with PSB increased the available P content of the soil (Table 3). The results were again more pronounced in the presence of the bacteria than in the control. Soil with the inoculated bacteria had available P content 2.67-fold higher than the control. The TCP amended soil, had available P with an increase of 4.56-fold as compared to the control. The increase in soil available P, increased the P uptake by the plants (Table 3). The maximal increase in P uptake was shown by the plants inoculated with the bacteria + TCP (58 % in roots and 97 % in shoots respectively).
HPTLC Studies on Different Samples of D. alata
The method was developed on TLC aluminum plates precoated with silica gel 60F254 using solvent system toluene: ethyl acetate (7:3 v/v), which gave compact spot for diosgenin (R f value 0.56 ± 0.02) (Fig. 4). Densitometric analysis of diosgenin was carried out in the absorbance mode at 430 nm after spraying with methanolic sulphuric acid (Fig. 5). The linear regression analysis data for the calibration plots (y = 315.3 + 5.929x) showed good linear relationship with r = 99.13 for diosgenin with respect to peak area, in the concentration range of 100–500 ng spot−1 (Fig. 6). The limit of detection (LOD) and limit of quantification (LOQ) for diosgenin were 20 and 60 ng spot−1 respectively (Table 4). Method validation, recovery study and intra and inter day precision studies by the proposed HPTLC method were tabulated in Tables 4, 5 and 6. Statistical analysis proved that the method is repeatable, selective and accurate for the estimation of diosgenin in D. alata.
Effect of PSB on Diosgenin Content
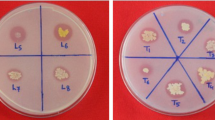
Dioscorea plants grown in TCP amended soil, showed higher diosgenin content than plants grown in soil without TCP amendment (Fig. 1). The PSB treated plants grown in TCP amended soil showed maximum yield of diosgenin and also the highest increase in the diosgenin content plant−1. The increase was 1.61-fold diosgenin plant−1 on the dry weight basis (Table 3).
Diosgenin is traditionally produced by acid hydrolysis of Dioscorea zingiberensis tubers producing various byproducts causing serious hazards [33]. Enzymatic saccharification and microbial transformation (by Trichoderma reesei) were used to produce diosgenin from D. zingiberensis tubers yielding 42.4 % higher diosgenin as compared to the production from direct bioconversion of raw tubers [34]. The microbial system was reported as clean, effective and more environmentally acceptable method for diosgenin production as compared to the traditional acid hydrolysis [35]. Moreover, a strain of Gibberella intermedia WX12 (the sexual stage of Fusarium proliferatum) was used to achieve three times higher diosgenin production when an optimized conversion media was applied [36]. The effect of phosphate-solubilizing microorganisms on growth of medicinal plants and their biosynthesis of specific drugs is poorly known [13, 37]. In the present report, the authors have studied the effect of PSB on D. alata, grown in different soil conditions (soil alone, soil + TCP) concerning plant growth and diosgenin content. The addition of TCP to soil increased the soil available P, which enhanced plant growth and increased the content of biochemical constituents. Statistically significant increase was found for plant growth and diosgenin content in D. alata inoculated with PSB grown in both soils. The highest stimulatory effects were observed with a bacterial strain + TCP, confirming similar results for terpinene-4-ol in Majorana hortensis L. inoculated with A. brasiliense + A. chroococcum + B. polymyxa as compared to single inoculations [38]. This may be due to a more effective dissolution of P [39] and synergistic effects of PSB on plant roots [40]. Moreover, these bacteria are also reported as endophytes [41] which efficiently solubilize and assimilate the phosphates [42].
There are a few reports on the influence of microbial P-solubilizers on Aloe vera. An increase in shoot length, total leaf length, number of leaves, N and P concentrations in Aloe plants upon inoculation with Glomus clarum and Gigaspora decipiens has been reported [43]. Increased barbaloin content (51.7 %) in A. vera by dual inoculation with arbuscular mycorrhizal (AM) fungi (Glomus mossae) and Azotobacter along with rock phosphate in different soil types has also been reported [12]. Positive correlation was found between aloin-A biosynthesis and increased P uptake in plants treated with PSB. The treatment of plants with individual PSB or mixtures increased available P in soil, P uptake in plants and plant growth. Increase in aloin-A content due to higher plant biomass and unit biomass production was 673, 294, 276, 119 and 108 % in plants treated with a PSB consortium, P. synxantha, S. marcescens, B. gladioli, and E. hormaechei in TCP amended soil respectively [14]. The stimulatory effect was observed with consortium treatment in plant growth parameters (shoot length by 22.5 %; root length by 14.7 %; leaf dry weight by 89.0 %; stem dry weight by 76.3 % and shoot biomass by 82.5 %) and glycoside contents (ST, 150 % plant−1 and R-A, 555 % plant−1) as compared to the uninoculated plants. Among individual PSB treatments, B. gladioli 10,216 showed most promising response in majority of the parameters studied [13]. Positive correlation was found between aloin-A biosynthesis (mg g−1) and increased P uptake in plants treated with PSB. Similar observations have been reported on the effect of PSB on the phenolic contents of banana plants [44]. The increased P uptake may have stimulated anabolic pathways of phenolic compounds as observed in leaves of Salvia officinalis stimulated by P fertilization [45]. The PSB solubilizes inorganic phosphates by several mechanisms, including the production of (1) organic acids like gluconic, ketogluconic, oxalic, succinic etc. [46], (2) polysaccharides [47]; and (3) phosphatase enzymes such as acid phosphatases [48]. Moreover, B. subtilis induced IAA production and its effect on sprouting of Dioscorea rotundata has been reported by Swain et al. [49]. Here, for the first time, stimulation of growth and diosgenin biosynthesis by a PSB in the D. alata is being reported suggesting synergistic interactions between B. subtilis and D. alata.
Conclusion
The present study clearly indicates the potential of P solubilizers on D. alata plant growth and diosgenin content under glasshouse conditions. The effect of an inoculation by a B. subtilis KF279045 was more pronounced than TCP and untreated soil for all the mentioned parameters in both soil conditions. This study also shows that increased P uptake in plants directly correlates with enhanced diosgenin production. The significant increase in the diosgenin content of PSB treated plants emphasizes the potential of an economical and eco-friendly mean of achieving higher levels of diosgenin. However, further research is needed to understand the specific mechanisms involved in the positive effects of PSB on the biosynthesis of diosgenin content and to verify these results under field conditions.
References
Liu H, Chou GX, Wang JM, Ji LL, Wang ZT (2011) Steroidal saponins from the rhizomes of Dioscorea bulbifera and their cytotoxic activity. Planta Med 77:845–848
Hu K, Dong AJ, Yao XS (1996) A new pregnane glycoside from Dioscorea collettii var. hypoglauca. Planta Med 62:573–575
Oncina R, Botia JM, Del Rio JA, Ortuno A (2000) Bioproduction of diosgenin in callus cultures of Trigonella foenum-graecum L. Food Chem 70:489–492
Qin Y, Wu X, Huang W, Gong G, Li D, He Y, Zhao Y (2009) Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis CH Wright in rodents. J Ethnopharmacol 126:543–550
Raju J, Mehta R (2009) Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr Cancer 61:27–35
Vasanthi HR, ShriShriMal N, Das DK (2012) Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem 19:2242–2251
Pari L, Monisha P, Mohamed Jalaludeen A (2012) Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin induced diabetic rats. Eur J Pharmacol 691:143–150
Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T (2012) Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Sci Rep 2:535
Higdon K, Scott A, Tucci M, Benghuzzi H, Tsao A, Puckett A, Cason Z, Hughes J (2001) The use of estrogen, DHEA, and diosgenin in a sustained delivery setting as a novel treatment approach for osteoporosis in the ovariectomized adult rat model. Biomed Sci Instrum 37:281–286
Tada Y, Kanda N, Haratake A, Tobiishi M, Uchiwa H, Watanabe S (2009) Novel effects of diosgenin on skin aging. Steroids 74:504–511
Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Gopi R, Somasundaram R, Panneerselvam R (2007) Pseudomonas fluorescens enhances biomass yield and ajmalicine production in Catharanthus roseus under water deficit stress. Colloids Surf B Biointerfaces 60:7–11
Pandey DK, Banik RM (2009) The influence of dual inoculation with Glomus mossae and Azotobacter on growth and barbaloin content of Aloe vera. Am Eurasian J Sustain Agric 3:703–714
Mamta Rahi P, Pathania V, Gulati A, Singh B, Bhanwra RK (2010) Stimulatory effect of phosphate-solubilizing bacteria on plant growth, stevioside and rebaudioside—a contents of Stevia rebaudiana Bertoni. Appl Soil Ecol 46:222–229
Mamta G, Kiran S, Gulati A, Singh B, Tewari R (2012) Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin—a biosynthesis of Aloe barbadensis Miller. Microbiol Res 67:358–363
Altomare C, Norvell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphates and micronutrient by plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai. Appl Environ Microbiol 65:2926–2933
Del Campillo SE, Van der Zee S, Torrent J (1999) Modelling long term phosphorus leaching and changes in phosphorus fertility in excessively fertilized acid sandy soils. Eur J Soil Sci 50:391–399
Patten CL, Glick BR (2002) Role of Pseudomonas putida indole acetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Shahab S, Ahmed N, Khan NS (2009) Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. Afr J Agric Res 4:1312–1316
Jaleel CA, Gopi R, Panneerselvam R (2009) Alterations in non-enzymatic antioxidant components of Catharanthus roseus exposed to paclobutrazol, gibberellic acid and Pseudomonas fluorescens. Plant Omics J 2:30–40
Ghorbanpour MNM, Hosseini S, Rezazadeh M, Omidi KK, Etminan A (2010) Hyoscyamine and scopolamine production of black henbane (Hyoscyamus niger) infected with Pseudomonas putida and P. fluorescens strains under water deficit stress. Planta Med 76:167
Prain D (1903) Bengal plants. Botanical Survey of India, Calcutta
Jackson ML (1973) Methods of chemical analysis. Prentice Hall of India (Pvt.) Ltd, New Delhi
Gordon SA, Paleg LG (1957) Observations on the quantitative determination of indole acetic acid. Plant Physiol 10:39–47
Frankenberger WT, Poth M (1988) L-Tryptophan transaminase of a bacterium isolated from the rhizosphere of Festuca octoflora (Gramineae). Soil Biol Biochem 20:299–304
Graham PH, Parker CA (1964) Diagnostic features in characterization of the root nodule bacteria of the legumes. Plant Soil 20:383–386
Kovaks N (1956) Identification of Pseudomonas pyocyanea by the oxidation reaction. Nature 178:703
Ronald MA, James WS (2006) Handbook of media for clinical microbiology. Taylor and Francis, New York
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorous in soils by extraction with sodium bicarbonate. USDA Circ 1:939
Koeing RA, Johnson CR (1942) Colorimetric determination of phosphorus in biological materials. Ind Eng Chem Anal Ed 14:155–156
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Liu L, Dong YS, Qi SS, Wang H, Xiu ZL (2010) Biotransformation of steriodal saponins in Dioscorea zingiberensis CH Wright to diosgenin by Trichoderma harzianum. Appl Microbiol Biotechnol 85:933–940
Zhu YL, Huang W, Ni JR, Liu W, Li H (2010) Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation. Appl Microbiol Biotechnol 85:1409–1416
Zhu Y, Ni J, Huang W (2010) Process optimization for the production of diosgenin with Trichoderma reesei. Bioprocess Biosyst Eng 33:647–655
Zhang J, Li H, Li H, Lu Z, Shi J, Xu Z (2013) Screening and condition optimization of a strain for efficiently biotransformation of saponins in Dioscorea zingiberensis into diosgenin. Sheng Wu Gong Cheng Xue Bao 29:848–852
Kaymak HC, Yarali F, Guvenc I, Donmez MF (2008) The effect of inoculation with plant growth rhizobacteria (PGPR) on root formation of mint (Mentha piperita L.) cuttings. Afr J Biotechnol 7:4479–4483
Gharib FA, Moussa LA, Massoud ON (2008) Effect of compost and bio-fertilizers on growth, yield and essential oil of sweet marjoram (Majorana hortensis) plant. Int J Agric Biol 10:381–387
Kundu BS, Gaur AC (1982) Yield increases of wheat after inoculation with A. chroococum and phosphobacteria. Curr Sci 51:291–293
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1768
Rosenblueth M, Martinez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 19:827–837
Kuklinsky-Sobral J, Araujo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL (2004) Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6:1244–1251
Tawaraya K, TurJaman M, Ekamawanti HA (2007) Effect of arbuscular mycorrhizal colonization on nitrogen and phosphorus uptake and growth of Aloe vera L. HortScience 42:1737–1739
Mahmood M, Rahman ZA, Saud HM, Shamsuddin ZH, Subramaniam S (2010) Influence of rhizobacterial and agro-bacterial inoculation on selected physiological and biochemical changes of Banana cultivar, Berangan (AAA) plantlets. J Agric Sci 2:115–137
Nell M, Votsch M, Vierheilig H, Steinkellner S, Zitterl-Eglseer K, Franz C (2009) Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L.). J Sci Food Agric 89:1090–1096
Vazquez P, Holguin G, Puente M, Elopez Cortes A, Bashan Y (2000) Phosphate solubilizing microorganisms associated with the rhizosphere of mangroves in a semi arid coastal lagoon. Biol Fertil Soils 30:460–468
Goenadi DH, Sisweto I, Sugiarto Y (2000) Bioactivation of poorly soluble phosphate rocks with a phosphorus-solubilizing fungus. Soil Sci Soc Am J 64:927–932
Rodriguez H, Rossolini GM, Gonzalez T, Li J, Glick BR (2000) Isolation of a gene from Burkholderia cepacia IS-16 encoding a protein that facilitates phosphatase activity. Curr Microbiol 40:362–366
Swain MR, Naskar SK, Ray RC (2007) Indole-3-acetic acid production and effect on sprouting of Yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol J Microbiol 56:103–110
Acknowledgments
The authors acknowledge the Department of Biotechnology, Lovely Professional University, India for assistance regarding laboratory facilities and instrumentation. The authors also declare no conflict of interest for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, D.K., Nazir, A. & Dey, A. Isolation and Characterization of Phosphate Solubilizing Bacteria from Rhizosphere of Dioscorea alata Stimulating Growth and Diosgenin Production. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 87, 1143–1152 (2017). https://doi.org/10.1007/s40011-015-0670-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0670-2