Abstract
Pharmacokinetics of enrofloxacin was studied after intravenous and oral bolus administration at 10 mg/kg in healthy emus aged between 18 and 24 months. Blood samples were collected from jugular vein at predetermined time intervals after drug administration. Enrofloxacin and its active metabolite ciprofloxacin in plasma were determined by HPLC. Plasma concentrations versus time were analyzed by a non-compartmental analysis. For i.v. and oral bolus dose of administration, elimination half-life (t1/2β) was 4.364 ± 0.179 and 4.125 ± 0.361 h, respectively; AUC0–∞ was 20.085 ± 3.493 and 16.056 ± 1.436 µg h/mL, respectively; mean residence time (MRT) was 5.105 ± 0.216 and 6.616 ± 0.475 h, respectively; volume of distribution was 3.921 ± 1.005 and 3.171 ± 0.296 L/kg, respectively and total body clearance was 0.629 ± 0.164 and 0.507 ± 0.003 L/h kg, respectively. Mean absolute bioavailability for enrofloxacin after oral administration was 79.941 ± 7.147 %. The metabolite ciprofloxacin could be detected from 15 min to 24 h following i.v. and oral administration of enrofloxacin. The ratio of AUC0–tcipro/AUC0–tenro was 7.764 and 9.031 %, respectively for i.v. and oral administration of enrofloxacin. The t1/2β and MRT of the metabolite were longer than those of parent substance. From the PK/PD indices such as Cmax/MIC, AUC/MIC and Cmax/MPC, AUC/MPC study, the recommended doses of enrofloxacin in emu birds were 10 mg/kg body weight once daily for i.v. and oral routes against organisms susceptible to 0.25 and 0.125 μg/mL, respectively. Taking the PAE and active metabolite ciprofloxacin into consideration, it is recommended that enrofloxacin could be used at the dose rate of 10 mg/kg at every 24 h even against the organisms susceptible to 0.5 µg/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enrofloxacin is a fluoroquionolone antimicrobial agent developed solely for use in animals. The relative safety of enrofloxacin, its low minimum inhibitory concentrations (MIC), broad spectrum of activity, long post antibiotic effect (PAE), good tolerance and rapid absorption after parenteral and oral administration resulting in high blood and tissue concentrations have encouraged its use in veterinary medicine. Although enrofloxacin itself is an active antimicrobial, biotransformation to active metabolite ciprofloxacin may occur in some species [1].
Pharmacokinetic studies offer highly relevant information on the time course of the drugs, their metabolites facilitate the computation of optimal dosage regimens of drugs to maintain their therapeutic concentration at the biophase [2]. The pharmacokinetic behaviour of enrofloxacin has been investigated in various animal and bird species including wild animals and aquatic species. But, pharmacokinetics of enrofloxacin remains less well understood in emu birds.
Emu (Dromaius novaehollandiae) belongs to ratite group of birds. Bacterial infections are important causes of morbidity and mortality in domestic emu birds [3]. E. coli and Salmonella sp. was isolated in emu birds reared in India by Kumar et al. [4]. Since research on antimicrobial therapies in ratite birds has been minimal, the determination of some drug doses for these birds is strictly empirical or based on metabolic scaling. Because drug disposition differs among species, extrapolation of dosages from domestic animals may result in sub-therapeutic or toxic level of drug [5]. The computation of an optimal dosage regimen depends on the understanding of the drugs in the target species. Hence, in the current study, it was proposed to investigate the disposition kinetics of enrofloxacin in emus following intravenous and oral bolus dose administration.
Material and Methods
Drugs and Chemicals
Enrofloxacin hydrochloride and ciprofloxacin hydrochloride purchased from Himedia Laboratories Pvt. Ltd., India were utilized for the study. For HPLC analysis, HPLC grade acetonitrile, methanol, triethyl amine and phosphoric acid were purchased from Merck Specialities Ltd., India. Water for HPLC obtained by Millipore water purification system was utilized. All solvents and solutions for HPLC analysis were filtered through 0.2 μ HNN nylon membrane filter (Nupore) and degassed using sonicator. All other chemicals and solvents were of analytical reagent grade and were used without further purification.
Preparation of Drug Solution
Enrofloxacin hydrochloride was dissolved in sterile distilled water to prepare 1 % solution for oral administration and 5 % solution for i.v. administration. For all the treatments drug solution was prepared freshly.
Experimental Design
The study was conducted in apparently healthy 8 emu birds (4 male + 4 female) aged 18–24 months with a mean (±SE) body weight of 38.20 ± 1.03 kg. The birds were under uniform conditions of housing (semi intensive system) and feeding, according to the birds requirements. Birds were offered feed and water ad libitum. Before the start of the experiment, the birds were examined clinically to rule out the possibility of any disease. No antibiotics and anthelmintics were administered 2 months prior to the start of experiment. The use of the birds and experimental design was approved by Institutional Animal Ethics Committee (IAEC), TANUVAS, Chennai.
Emu birds were randomly divided into two treatment groups. Using cross over design, the i.v. and oral bolus pharmacokinetics of enrofloxacin in emu birds was determined at a dose of 10 mg/kg body weight. Enrofloxacin was administered intravenously (bolus dose) through the jugular vein. Blood samples (2 mL) were drawn by jugular venipuncture into heparinized tubes immediately before and at 0.083, 0.167, 0.25, 0.50, 0.75, 1, 1.5, 2, 3, 4, 8, 12, 18, 24 and 36 h after dosing. After 2 weeks wash out period, the same birds were administered with the same dose of enrofloxacin orally directly using a thin plastic tube attached to a syringe. Then, 2 mL of blood samples were drawn by same method at 0.25, 0.50, 0.75, 1.5, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48 and 60 h after dosing.
The collected blood samples were centrifuged at 950×g for 20 min to separate the plasma. The plasma samples were stored at −40 °C until assay.
Drug Assay
Determination of enrofloxacin and ciprofloxacin was performed by high performance liquid chromatography (HPLC). The method developed by Kung et al. [6] was followed.
The HPLC system comprised of LC-20 AD double plunger pump, Rheodyne manual loop injector with a 20 μL loop, column oven CTO-10 AS vp, SPD-M20A diode array detector and a software LC Solution for data analysis. The compound separation was achieved using a reverse phase C18 column (Hibar 250-4, 6 RP-18 endcapped, Particle size 5 μm, 4.6 × 250 mm, Merck, Germany) as a stationary phase. The column was protected with 2 to 8 mm Phenomenax guard column (KJO-4282). The mobile phase consisted of a mixture of acetonitrile, methanol and water (containing 0.4 % phosphoric acid and adjusted to pH 3.0 using triethylamine) in the ratio of 17:3:80 (v/v/v). The flow rate of mobile phase was 1 mL/min and samples were analyzed for 10 min at 40 °C. The scan range of PDA was 220–400 nm, and the detection wavelength was 278 nm. The mean (±SE) retention times for ciprofloxacin and enrofloxacin were 5.65 ± 0.003 and 7.16 ± 0.006 min, respectively.
Enrofloxacin and ciprofloxacin from the plasma were subjected to liquid–liquid extraction according to the method of Nielsen and Hansen [7]. To 0.5 mL of plasma, 0.75 mL of acetonitrile was added in the ratio of 1:1.5. The mixture was vortex-mixed for 15 s and centrifuged for 15 min at 4 °C at a speed of 900×g. The clear supernatant was thus obtained (0.5 mL) and twice the volume of HPLC grade water (1 mL) was added in the ratio of 1:2. The aliquot was then filtered through 0.2 μ HNN nylon membrane filter and 20 μL of filtrate was injected into the HPLC system.
Working standards of enrofloxacin (0.01, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, and 10 µg/mL) and ciprofloxacin (0.01, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, and 10 µg/mL) were prepared from respective stock solutions after diluting with plasma collected from emus. Standard calibration curves were prepared from plasma samples containing known concentrations of enrofloxacin and ciprofloxacin separately.
The standard curves of enrofloxacin and ciprofloxacin were linear in the range of 0.01–10.0 µg/mL. The calibration curve for enrofloxacin was characterized by its regression coefficient (r2 = 0.999), slope (19,070) and intercept (13,182), and was used to determine the analyte concentrations in the sample. The calibration curve for ciprofloxacin was characterized by its regression coefficient (r2 = 0.998), slope (14,777) and intercept (6507.4), and was used to determine the analyte concentrations in the sample.
The concentrations of enrofloxacin and ciprofloxacin in the plasma samples were determined by substituting the respective peak areas/peak heights in the linear regression formula after calibration of standard curves.
Absence of change in the retention time was considered as the method which was found specific and selective. The mean absolute recovery was within the range of 97.778–107.45 % for plasma and the coefficient of variation (CV) was 2.129–7.676 % suggesting the suitability of the method for analysis of enrofloxacin and ciprofloxacin in emu plasma. The intra-day and inter-day CV were within the limits (<10 %) specified (enrofloxacin: 5.307–8.827 %, ciprofloxacin; 4.757–8.632 %) and hence the method was suitable for assay of both enrofloxacin and ciprofloxacin in emu plasma. The limit of detection and quantification were 0.01 and 0.025 µg/mL for enrofloxacin and 0.025 and 0.05 µg/mL for ciprofloxacin, respectively.
Pharmacokinetic Analysis
Pharmacokinetic parameters were derived from concentration vs. time curves obtained for each bird after single i.v. and p.o. administration. Non-compartmental pharmacokinetic analysis was used to describe the pharmacokinetics of enrofloxacin and ciprofloxacin using pharmacokinetic software PK function [8].
The elimination rate constant (β) was calculated from the log-linear portion of the elimination curve using linear regression analysis. The elimination half-life (t1/2β) was calculated according to t1/2β = ln2/β, where, ln2 − 0.693. The area under the plasma concentration–time curve (AUC) and the area under the first moment curve (AUMC) were calculated using the trapezoidal rule and extrapolated to infinity by means of the elimination rate constant. The mean residence time (MRT = AUMC/AUC), total body clearance (CLB = Dose/AUC), volume of distribution to steady state (Vdss = CLB × MRT) and apparent volume of distribution (Vdarea = Dose/β × AUC0–∞) were calculated after i.v. administration. Comparing the corresponding oral and i.v. route of administration the bioavailability (F) after oral administration was calculated as F = AUC0–∞.(oral)/AUC0–∞ (i.v.) × 100; mean absorption time as MAT = MRToral−MRTi.v.; total body clearance as CLB = Dose × F/AUC0–∞; apparent volume of distribution as Vdarea = Dose × F/β × AUC0–∞).
Pharmacokinetic/Pharmacodymanic (PK/PD) Integration
The ratios Cmax/MIC and AUC/MIC; Cmax/MPC and AUC/MPC were calculated for hypothetical MIC90 (0.05, 0.125, 0.25 and 0.5 µg/mL) and MPC (0.2, 0.5, 1.0 and 2 µg/mL) values using the means of Cmax and AUC obtained in this study.
Statistical Analysis
Statistical analysis of the data was performed by using SPSS 17.0 software. The results were expressed as mean ± SE. Harmonic mean was used with data not distributed normally. Test of significance such as t test and analysis of variance (one way ANOVA) were applied to find out difference between and among various groups respectively [9]. Comparison of the means of the different subgroups was performed by Duncan‘s multiple range tests as described by Kramer [10].
Results and Discussion
Inter-species differences in the pharmacokinetics behaviour of enrofloxacin existed even within the ratite group at different dosage. From the available published data, it is difficult to decide the proper dosage of enrofloxacin in emus for the different routes of administration. Moreover, the data on pharmacokinetic characteristics of enrofloxacin after oral administration has not been published for emus. Hence, in the present study, pharmacokinetic parameters of enrofloxacin obtained after i.v. and oral administration are used to deduce recommendations for dosages.
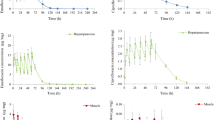
The mean (±SE) plasma concentrations of enrofloxacin and its active metabolite ciprofloxacin after i.v. and oral administration of enrofloxacin at 10 mg/kg are depicted graphically in Fig. 1. After i.v. administration, enrofloxacin could be detected up to 18 h in one bird while in seven birds the drug was detected up to 24 h. The highest mean (±SE) concentration was 14.756 µg/mL at 5 min and lowest was 0.054 µg/mL at 24 h. The mean (±SE) values of plasma concentration of enrofloxacin following oral administration of enrofloxacin rapidly increased from 0.591 ± 0.073 µg/mL at 15 min to 2.207 ± 0.098 µg/mL within 1.5 h and then declined to 0.004 ± 0.004 µg/mL at 36 h. Detectable concentrations of enrofloxacin were found up to 24 h in seven birds while in one bird the drug was detected up to 36 h. The plasma concentration of the active metabolite ciprofloxacin was observed from 15 min to 24 h for the both routes of i.v. and oral administration of enrofloxacin.
Comparatively rapid absorption and excellent bioavailability was observed after oral administration in emu birds. The mean (±SE) bioavailability (79.941 ± 7.147 %) after oral administration recorded in this study is in agreement with Bugyei et al. [11] who found 80.1 % in chicken. As compared to the present study, Anadon et al. [12] in domestic fowl (64 %) and Dimitrova et al. [13] in turkeys (69.20 %) reported lesser bioavailability and Dorrestein [14] in pigeons (92 %) found higher bioavailability at the same dose of enrofloxacin. Differences in the anatomy of the digestive system are known to cause marked differences in the rate and extent of drug absorption from the oral route [14]. Retention time of particulate matter in the digestive tract of emus was 5.5 h [15], although some food items were retained commonly for one to 2 days, sometimes over 1 week [16]. Thus relatively slow intestinal transit and comparatively long intestinal tract may be the factors that could increase the absorption of orally administered drugs. The excellent bioavailability noted after oral administration was more favorable to i.v. injection.
After i.v. administration, enrofloxacin showed AUC0–∞ of 20.085 ± 3.493 µg h/mL with large apparent volume of distribution (3.921 ± 1.005 L/kg) (Table 1). The slower elimination half-life (4.364 ± 0.179 h) was observed with the total body clearance (ClB) of 0.629 ± 0.164 L/h.kg. After oral administration, enrofloxacin peak plasma concentration (Cmax) of 2.397 ± 0.052 µg/mL was achieved at (tmax) 2.167 ± 0.279 h with high bioavailability of 79.941 ± 7.147 %. After i.v. and oral administration of enrofloxacin, the ciprofloxacin AUC0–t was 7.764 and 9.031 % of enrofloxacin AUC0–t, respectively (Table 1). The elimination half life (t1/2) and MRT of the metabolite after i.v. and oral administration of enrofloxacin were longer than those of parent substance. The clearance of the active metabolite recorded in this study was faster as compared to the enrofloxacin.
The t1/2β of enrofloxacin recorded in this study was similar to values reported for greater rheas [17] and emus [18], but much slower than the results obtained in ostrich [19], whereas, Anadon et al. [12] in chickens, Dimitrova et al. [13] in turkey and Bailey et al. [20] in houbara bustard observed longer elimination t1/2β. The t1/2β obtained in the present study indicates that emu tends to eliminate enrofloxacin slower than ostrich and faster than chickens and turkeys. The elimination half-life had the negative correlation with the body weight for all drugs studied [21]. It might be the reason that emus had the slower t1/2β in the current study. Differences between species in elimination and protein binding are other possible explanations. Baert and De-Backert [21] observed that the half-life, as the most robust parameter for interspecies scaling and point to the risk of extrapolating doses and treatments from one species to another without suitable pharmacokinetic data.
The AUC values reported in ostrich [19] and greater rheas [17] were lower while in broiler chicken [22] and houbara bustard [20] were higher as compared to the present study. The differences might be due to the difference in anatomy, dosages and species. The mean residential time reported in the present study is comparatively higher than the values found in greater rheas [17], ostrich [19] and lower than broiler chicken [12] and turkeys [13]. From these data, it appears that the persistence of enrofloxacin is longer in emus as compared to other ratite species.
The large volume of distribution obtained after i.v. and oral dosing in this study indicated good tissue penetration of enrofloxacin in emus. It is in agreement with Walker [23] who explained fluroquinolones, in general, have excellent tissue penetration as reflected by high Vdarea in the present study. Tissue distribution studies in domestic fowls and pigeons have shown that most of organs contained higher concentrations of enrofloxacin than the corresponding blood concentrations [12, 24, 25]. Ultimately, tissue distribution studies in emus would be needed to complement plasma pharmacokinetic investigations in order to assess the distribution of enrofloxacin to the major organs. As compared to the present study value of Vdarea, Abd-El-Aziz et al. [22] found lesser values (2.17 L/kg) in chicken while De-Lucas et al. [17] observed higher values (5.01 L/kg) in greater rheas. Bugyei et al. [11] suggested that this variability might be due to differences in protein binding. As compared to the total body clearance (ClB) recorded in this study, faster clearance of enrofloxacin was found in ostrich (76 mL/kg.min; at a dose of 5 mg/kg) by De-Lucas et al. [19] and in greater rheas (3.95 L/kg h) by De-Lucas et al. [17]. The ClB values for chickens (4.8 mL/min kg); [12] and houbara bustard (5.7 mL/min kg); [20] are comparatively lesser than the result obtained in the present study. Cox et al. [26] explained that the clearance and volume of distribution were proportional to body weight. In agreement with this statement, the clearance and volume of distribution obtained in this study are high as compared to other avian species with less body weight.
The degree of metabolism varies considerably across species [26]. In the present study, ciprofloxacin AUC0–t was lower than 10 % of enrofloxacin AUC0–t after i.v. and oral administration of enrofloxacin. Similar results were obtained by De-Lucas et al. [19] in ostrich. Helmick et al. [18] reported that the plasma concentration of metabolite ciprofloxacin was not consistent in emus. However, Anadon et al. [12] observed a high hepatic conversion of enrofloxacin to ciprofloxacin in the chicken. The difference between the ratio of AUC0–t cipro/AUC0–t enro after i.v. and oral administration were 7.764 and 9.031, respectively which indicated limited, but rapid conversion of ciprofloxacin in the liver of emu birds.
The PK/PD integration parameters of Cmax/MIC and AUC0–24/MIC were calculated from the obtained PK parameters are presented in Table 2. The pharmacodynamic ratios of mutant prevention concentration (Cmax/MPC and AUC0–24/MPC) were also determined from the obtained PK parameters which are given in Table 3.
Enrofloxacin are concentration-dependent killing agents. In addition to this, enrofloxacin exert long PAE, could well be one of the guiding factors in the optimization of dosage schedule. Fluoroquinolones exert a PAE of 4–8 h against a number of strains including E. coli, Klebsiella pneumoniae and Pseudomonas aeruginosa [27] and PAE in vivo is generally longer than PAE in vitro due to post-antibiotic sub-MIC effect (PASME) and the post-antibiotic leukocyte enhancement (PALE) exerted in vivo [23].
Taking the above factors into consideration, several workers have proposed that AUC/MIC and Cmax/MIC ratios are the best indicators for good clinical outcome. It is well established that plasma Cmax/MIC > 8 and AUC/MIC > 100 are required for efficient and optimal pharmacotherapy of enrofloxacin [28]. In the current study, the Cmax/MIC and AUC/MIC ratios suggested that the enrofloxacin administration at 10 mg/kg through i.v. route was effective against the organisms susceptible to MIC of 0.25 µg/mL while, the oral dosing was effective against the organisms susceptible to MIC of 0.125 µg/mL.
The mutant prevention concentration (MPC), a concept meant to face the increased prevalence of antibiotic resistance was used to calculate Cmax/MPC and AUC/MPC. According to Drlica [29] the Cmax/MPC90 and AUC/MPC90 ratios for enrofloxacin were 1.4 and 39, respectively, were found protective against the selection of resistant mutants of E. coli. From this Cmax/MPC and AUC/MPC ratios of present study, administration of enrofloxacin through i.v. route was most useful in preventing resistance as compared to oral route of administration.
From these PK/PD results, it is obvious that use of enrofloxacin administration at 10 mg/kg through i.v. and oral route is able to produce an ideal clinical outcome against pathogens susceptible to 0.25 and 0.125 µg/mL, respectively. However, these derived values do not take into account the contribution made by the active metabolite ciprofloxacin, and therefore underestimate enrofloxacin efficacy.
Conclusion
From the present study, it can be concluded that enrofloxacin pharmacokinetic parameters after i.v. and oral bolus administration in emus at 10 mg/kg are characterized by high volume of distribution, slower terminal elimination half life and high bioavailability. Based on the PK/PD study, the recommended doses of enrofloxacin in emu birds were 10 mg/kg body weight once daily for i.v. and oral routes against organisms susceptible to 0.25 and 0.125 μg/mL, respectively. Taking the active metabolite ciprofloxacin and PAE into consideration, it is recommended that enrofloxacin could be used at the dose rate of 10 mg/kg at every 24 h even against the organisms susceptible to 0.5 µg/mL.
Abbreviations
- β:
-
Elimination rate constant
- AUC0–t :
-
Area under the concentration vs. time curve 0 to time
- AUC0–∞ :
-
Area under the concentration–time curve 0 to infinity
- AUMC0–t :
-
Area under the first moment curve from 0 to time
- AUMC0–∞ :
-
Area under the first moment curve from 0 to infinity
- MRT:
-
Mean residence time
- MAT:
-
Mean residence time
- Vd area/F:
-
Apparent volume of distribution after oral administration
- Vd area :
-
Apparent volume of distribution
- Vdss/F:
-
Volume of distribution at steady-state after oral
- CLB :
-
Total body clearance
- CLB/F:
-
Total body clearance after oral administration
- t1/2β :
-
Elimination half life,
- Cmax :
-
Maximum (peak) plasma concentration
- tmax :
-
Time of maximum observed concentration in plasma
- AF:
-
Absolute bioavailability
References
Boothe DM (1994) Enrofloxacin revisited. Vet Med 89:744–753
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel-Dekker Inc., New York
Sales J (2007) The emu (Dromaius novaehollandiae): a review of its biology and commercial products. Avian Poult Biol Rev 18:1–20
Kumar SP, Jagatheesan PNR, Ananth AM, Arivuchelvan A (2014) Mortality pattern in emu (Dromaius novaehollandiae) birds. Indian Vet J 91(6):23–26
Jensen JM (1998) Current ratite therapy. Vet Clin North Am Food Anim Pract 14:484–502
Kung K, Riond JL, Wanner M (1993) Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin after intravenous and oral administration of enrofloxacin in dogs. J Vet Pharmacol Ther 16:462–468
Nielsen P, Hansen NG (1997) Bioavailability of enrofloxacin after oral administration to fed and fasted pigs. Pharmacol Toxicol 80:246–250
Usansky JI, Desai A, Tang-Liu D (2011) PK functions for Microsoft Excel. Department of Pharmacokinetics and Drug Metabolism. Allergan, Irvine, CA 92606, USA
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Ames
Kramer CY (1957) Extension of multiple range tests to group correlated adjusted means. Biometrics 13:13–18
Bugyei K, Black WD, McEwen S (1999) Pharmacokinetics of enrofloxacin given by the oral, intravenous and intramuscular routes in broiler chickens. Can J Vet Res 63:193–200
Anadon A, Martinez-Larranaaga MR, Diaz MJ, Bringas P, Martinez MA, Fernandez-Cruz ML, Fernandez MC (1995) Pharmacokinetics and residues of enrofloxacin in chickens. Am J Vet Res 56:501–506
Dimitrova DJ, Lashev LD, Yanev SG, Pandova B (2007) Pharmacokinetics of enrofloxacin in turkeys. Res Vet Sci 82:392–397
Dorrestein GM (1993) Antimicrobial therapy drug use in veterinary medicine in pet birds. In: Prescott JF, Baggot JD (eds) Antimicrobial therapy in veterinary medicine. Iowa State University Press, Ames, pp 490–506
Herd RM, Dawson TJ (1984) Fibre digestion in the emu (Dromaius novaehollandiae), a large bird with a simple gut and high rate of passage. Physiol Zool 57:70–84
Wilson MF (1989) Gut retention times of experimental pseudoseeds by emus. Biotropica 21:210–213
De Lucas JJ, Rodrıguez C, Martellab MB, Labaque MC, Navarro JL, San Andre´s MI (2005) Pharmacokinetics of enrofloxacin following intravenous administration to greater rheas: a preliminary study. Res Vet Sci 78:265–267
Helmick KE, Boothe DM, Jensen JM (1997) Disposition of single-dose intravenously administered enrofloxacin in emus (Dromaius novaehollandiae). J Zoo Wildl Med 28:43–48
De Lucas JJ, Rodrıgueza C, Waxman S, Gonzalez F, DeVicente ML, San-Andre MI (2004) Pharmacokinetics of enrofloxacin after single intravenous and intramuscular administration in young domestic ostrich (Struthio camelus). J Vet Pharmacol Ther 27:119–122
Bailey TA, Sheen S, Silvanose C, Samour JH, Garner A, Harro DWG (1998) Pharmacokinetics of enrofloxacin after intravenous, intramuscular and oral administration in houbara bustard (Chlamydotis undulata macqueenii). J Vet Pharmacol Therap 2:288–297
Baert K, De Backert P (2003) Comparative pharmacokinetics of three non-steroidal anti-inflammatory drugs in five birds species. Comp Biochem Phys C 134:25–33
Abd-El-Aziz MI, Aziz MA, Soliman FA, Afify NA (1997) Pharmacokinetic evaluation of enrofloxacin in chickens. Br Poult Sci 38:164–168
Walker RD (2000) The use of fluoroquinolones for companion animal antimicrobial therapy. Aust Vet J 78:84–90
Kietzmann M, Knoll U, Glunden G (1997) Pharmacokinetics of enrofloxacin and ciprofloxacin in broiler chicken. J Vet Pharmacol Ther Supplement 1:202–215
Scheer M (1987) Concentrations of active ingredient in the serum and tissues after oral and parenteral administration of Baytril. Rev de Méd Vét 2:104–118
Cox SK, Cottrell MB, Smith L, Papich MG, Frazier DL, Bartges J (2004) Allometric analysis of ciprofloxacin and enrofloxacin pharmacokinetics across specie. J Vet Pharmacol Ther 27:136–146
Brown SA (1996) Fluoroquinolones in animal health. J Vet Pharmacol Ther 19:1–14
Turnidge J (1999) Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs 58:29–36
Drlica K (2003) The mutant selection window and antimicrobial resistance. J Antimicrob Chemother 52:11–17
Acknowledgments
Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Chennai is gratefully acknowledged.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P.S., Arivuchelvan, A., Jagadeeswaran, A. et al. Pharmacokinetics of Enrofloxacin in Emu (Dromaius novaehollandiae) Birds After Intravenous and Oral Bolus Administration. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 85, 845–851 (2015). https://doi.org/10.1007/s40011-015-0525-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0525-x