Abstract
A reverse-transcription loop mediated isothermal amplification (RT-LAMP) was developed for rapid diagnosis of infectious bronchitis (IB) in poultry by targeting the spike protein 2 gene (S2). RT-LAMP primers were designed for IBV-S2 targets and optimized to run at 60 °C for 45 min. As compared with RT-PCR, RT-LAMP was 100 times more sensitive for IBV-S2 gene. RT-LAMP showed specific amplification with IB viral genome but not with other avian respiratory pathogens due to their mismatching with IBV-S2-RT-LAMP primers. RT-LAMP reaction products were visually detected by the addition of propidium iodide stain. Out of 102 field samples tested for detection of IBV, RT-LAMP detected IBV in 12 samples for S2 gene whereas RT-PCR detected IBV in six samples for S2 gene. The sensitivity of the RT-LAMP was 100 % and the specificity was 94 % for S2 gene. Since the developed RT-LAMP to detect IBV is simple, rapid, sensitive and specific, it can be a useful diagnostic tool for detection of IB in poultry in less equipped laboratories and in field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poultry are kept in most areas of the world and it provides an acceptable form of animal protein to most people throughout the world. During the last decade, many developing countries have adopted intensive poultry production in order to meet the demand for this form of animal protein [1]. It also increases the incidence of several infectious diseases which affect the economy widely throughout the world. Out of these, infectious bronchitis (IB) is an acute, highly contagious viral disease of chicken [2] caused by avian infectious bronchitis virus (IBV). It is listed as notifiable disease by the office of international des epizooties [3], because of its domestic and international importance.
Chicken of all ages are susceptible to IB. IBV belongs to the group three genus Coronavirus, family Coronoviridae, of order Nidovirales [4]. It is enveloped and pleiomorphic with single stranded positive sense RNA genome, approximately 27.6 kb in length. It encodes four structural proteins; the spike (S), the membrane (M) glycoprotein, the internal nucleoprotein (N) and small envelope (E) protein [5].
Conventionally IB can be diagnosed by virus isolation (VI), virus neutralization test (VNT), immunofluorescence assay (IFA), immunoperoxidase assay (IPA), enzyme linked immune sorbent assay (ELISA), agar-gel precipitation test (AGPT), reverse transcriptase-polymerase chain reaction (RT-PCR) [6], restriction fragment length polymorphism (RFLP) [7] and real-time PCR [8, 9]. There are several limitations with these techniques such as VI is laborious and time consuming, antibody based techniques require specific monoclonal antibodies for each strain, and real-time PCR is expensive and requires expertise. RT-PCR is the most widely used molecular technique to detect the IB viral genome either in allantoic fluids from embryonated chicken eggs (ECE) inoculated with field samples or directly from the tissue samples. However, it requires an expensive thermal cycler to carry out the reaction. Several authors used different targets like the nucleoprotein gene (N-gene) [10–12], Spike protein 2 (S2) [13–15], Spike protein 1(S1) [16, 17], M [18] and 3′-UTR [19] to diagnose IB by RT-PCR.
To overcome the difficulties of PCR-based techniques, Notomi et al. [20] developed a novel technique called loop-mediated isothermal amplification (LAMP). This technique requires four different primers, Bst DNA polymerase to displace DNA strands and isothermal conditions for the amplification of target DNA. The technique has been applied for the detection of several poultry pathogens [21–24]. The advantages of this technique are that it requires only 30–60 min to complete the reaction and can be performed at a single temperature ranging from 60 to 65 °C. The reaction can be carried out in a laboratory water bath or a dry heat block and the results can be read with the naked eye.
RT-LAMP for detection of IB has been reported by Chen et al. [25] targeting N gene and Luo et al. [26] targeting 1a gene. Here the authors have reported RT-LAMP for detection of IB by targeting S2 gene with increased sensitivity and visual detection of RT-LAMP by using propidium iodide dye.
Material and Methods
Viruses and Bacteria
IB vaccine (Mass 41) from M/s. Venkateswara Hatcheries Ltd., Pune, India was used to optimize the RT-LAMP. The vaccine strains of Newcastle disease virus (Lasota) and positive samples for avian leukosis virus and reo virus were used to study the specificity of developed RT-LAMP for detection of IB. Samples such as trachea, lungs and kidneys from birds suspected for IB were collected from poultry farms in Namakkal and Coimbatore districts of Tamil Nadu state in India.
Primers
The primers for RT-LAMP for S2 gene was designed by using Primer explorer software programme (http://primerexplorer.jp/e/). S2 gene sequences of Mass 41 strain (GenBank accession No. FJ904720) was used to design the primers. The details of binding sites of LAMP primers viz., F3 (outer forward primer), B3 (outer reverse primer), FIP (F1c + F2) (inner forward primer), BIP (B2 + B1c) (inner reverse primer) on the template were given in Fig. 1. The IBV LAMP primer sequences were screened by using BLAST (National Centre for Biotechnology Information; NCBI). The details of primer sequences used in RT-PCR and RT-LAMP were given in Table 1.
Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA from tissue samples and allantoic fluids of vaccine virus were extracted by Trizol-LS (M/s. Invitrogen, USA) as per the manufacturer’s instructions. One step RT-PCR was carried out in a 0.2 ml PCR tube by adding one step 2X RT-PCR master mix (M/s. GeNet Bio, Korea), appropriate primers (10 pmol each forward and reverse primers per reaction), 3 µl (1 µg) of RNA and nuclease free water to make up the volume to 25 µl. The RT-PCR cycling conditions for S2 gene were initial denaturation of 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 15 s, annealing at 60 °C for 1 min and extension at 72 °C for 2 min and a final extension of 72 °C for 7 min.
Optimization of RT-LAMP for Detection of S2 Gene of IB Viral Genome
RT-LAMP was optimized by following the method of Notomi et al. [20]. The RT-LAMP reaction was carried out in a water bath by mixing 10 pmol each of F3 and B3 primers, 40 pmol each of FIP and BIP primers, 1.4 mM of each dNTPs, 40 U of Reverse transcriptase, 8 U of Bst DNA polymerase with 1X Thermopol buffer, 8 mM of Magnesium sulphate, 0.8 M betaine and 3 µl (1 µg) of total RNA. Initially, RT-LAMP reaction was carried out at different temperatures (60, 62 and 64° C) for 90 min with termination temperature of 80° C for 5 min. The reaction temperature that showed clear ladder-like pattern was used in subsequent reactions and incubated at different reaction times (45, 60 and 90 min) to select the optimum reaction time. At the end of the reaction, an aliquot of RT-LAMP product (5 µl) was subjected to electrophoresis on 2 % agarose gel.
Analytical Sensitivity and Specificity of RT-LAMP to Detect S2 Gene of IB Viral Genome
The analytical sensitivity of RT-PCR and RT-LAMP using RNA for S2 gene target was assessed in terms of the lowest EID50 of allantoic fluid of IB vaccine from which the RNA extracted gave a positive amplification in RT-PCR and RT-LAMP. To assess the specificity of the RT-LAMP and RT-PCR primers of S2 gene, RNA from Newcastle disease virus, DNA extracted from reo virus and avian leukosis virus samples were used in RT-LAMP and RT-PCR.
Detection of RT-LAMP Products by Propidium Iodide Staining
The RT-LAMP products were detected by the addition of 2 µl of fluorescent DNA intercalating dye, propidium iodide solution (1 mg/ml) at the end of the reaction and then visualized by naked eye or under UV-transilluminator.
IB-RT-LAMP for Field Samples
Total RNAs isolated from the field samples (n = 102) (Table 2) were subjected to RT-LAMP. At the end of the reaction, these samples were analyzed by agarose gel electrophoresis and by adding propidium iodide. RT-LAMP positive samples were further confirmed by inoculating the samples into the allantoic cavity of 9–11 days old ECE from Poultry Research Station, Tamil Nadu Veterinary and Animal Sciences University, Chennai, Tamil Nadu and incubated at 37 °C for 48 h with humidity for three passages and then in fourth passage, the eggs were incubated for five days. At the end of the fifth day, the eggs were chilled in a cold room over night and next day, the eggs were opened to observe the embryo lesions such as dwarfing of embryos and curled toes suggestive of IB. Uninoculated embryos were used as control.
Results and Discussion
IB is among the most common and difficult of all poultry diseases to control. It causes significant economic loss in commercial broiler, layer and breeder chicken [2]. IBV is characterized by worldwide distribution and many different variants appear continuously despite the use of vaccines. The nucleotide sequences of S2 subunit of spike gene is highly conserved among different strains of IBV and among different members of genus Coronavirus. Hence, S2 gene was targeted for IB viral genome detection in the field samples [13–15].
In the optimization experiments, the LAMP reaction was carried out using the total RNA extracted from vaccine IBV (Mass 41) as the template to determine the optimal reactions, temperature and time combination. The optimum volume of components in the reaction mixture included 10 pmol each of F3 and B3 primers, 40 pmol each of FIP and BIP primers, 2.5 mM dNTP, 10 mM MgSO4, 1 M betaine, 40 U of MMLV Reverse transcriptase, 8 U Bst polymerase and 3 µl of extracted template RNA (1 µg). At 60º C for 45 min, the product from LAMP was better than that in other temperatures and time (data not shown). Hence, 60 °C and 45 min were considered as the optimal temperature and time for IBV-S2 gene RT-LAMP respectively. Alternatively, visual inspection of RT-LAMP amplification products in the reaction tubes was carried out by using propidium iodide. A positive reaction tube containing IBV-specific amplicons exhibited a pink color, while the contents within the negative control tube showed the original orange red color and under the UV-transilluminator, the positive sample showed fluorescence and negative sample showed absence of fluorescence.
The lowest EID50 of viral genome in RT-PCR detectable upon gel electrophoresis was found to be 103 EID50 (Fig. 2a) and for RT-LAMP it was 10 EID50 for IBV-S2 gene (Fig. 2b). The results indicated that IBV-RT-LAMP assay is 100 times more sensitive for S2 gene than RT-PCR assay. Further, the sensitivity is similar to the results of the RT-LAMP for IB- N gene viral genome detection reported by Chen et al. [25] who reported a sensitivity of 10 EID50. Luo et al. [26] reported a sensitivity of 106 fold diluted RNA for RT-LAMP targeting 1a gene of IBV.
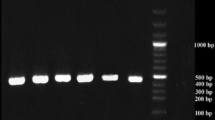
Analytical sensitivity of RT-PCR and RT-LAMP to detect S2 gene of IBV. a Analytical sensitivity of RT-PCR. b Analytical sensitivity of RT-LAMP. Lane M 100 bp DNA ladder, Lane 4 104 EID50, Lane 8 100 EID50, Lane 1 No template control, Lane 5 103 EID50, Lane 2 106 EID50, Lane 6 102 EID50, Lane 3 105 EID50, Lane 7 101 EID50
Primer sequences were verified with the BLAST programme and it was observed that the primers could detect all the important IB strains, which included H120 (GenBank accession no. GU393335), Connecticut (GenBank accession no. FJ904718), JMK (GenBank accession no. GU393338), Iowa97 (GenBank accession no. GU393337), Holte (GenBank accession no. GU393336), Gray (GenBank accession no. GU393334), Ark99 (GenBank accession no. GQ504721), VicS (GenBank accession no.JN983807), ITA/90254/2005 (GenBank accession no. FN430414). Moreover, these RT-LAMP primers were also blasted with complete genome of other avian related viruses which included avian Orthoreovirus (GenBank accession nos. from NC015123 to 35) and infectious laryngotracheitis virus (GenBank accession no. NC006623 and JQ083494) and the designed primer sequences were absent in these viruses. Both in RT-PCR and RT-LAMP reactions, the respective IBV- S2 gene primers could amplify only the RNA extracted from IB vaccine virus, but not in case of RNA extracted from NDV, DNA extracted from Reo virus and avian leukosis virus (Figs. 3a, b).
RT-PCR and RT-LAMP were used simultaneously for 102 samples that had been collected from several farms in Tamil Nadu for IB diagnostic purposes (Table 2). The IB viral genome was detected by RT-LAMP and RT-PCR in 12 and six samples for S2 gene respectively (Table 2). Among different tissue samples tested, more positive results were seen for kidney samples when tested by RT-LAMP (n = 6) than by RT-PCR (n = 3) whereas the results were similar in both the tests for trachea and caecal tonsils. The sensitivity of the RT-LAMP was 100 % and the specificity was 92 % for S2 gene. The analysis of field samples by RT-LAMP indicated increased percent positivity (13.7 %) when compared with the RT-PCR (5.9 %). This increased percent positivity is due to the higher sensitivity of RT-LAMP (10 EID50) than that of RT-PCR (103 EID50) for the detection of IB viral genome and this is in accordance with the results of Xu et al. [22] who showed that the RT-LAMP was 157 % sensitive than the RT-PCR in detecting IBD viral genome in field samples. RT-LAMP positive samples were further confirmed by using ECE as well as by sequencing the RT-PCR products which used F3 and B3 primers of RT-LAMP. The sequencing result showed that these samples were of M41 genotype. Thus, as the results indicate that the detection limit of RT-LAMP for IB viral genome is higher than the RT-PCR and the reaction time is 45 min only, this technique is more rapid than the RT-PCR. Moreover, the addition of dyes such as propidium iodide to the end product alleviates the use of electrophoresis. Hence, RT-LAMP for IB viral genome detection can be used as a routine diagnostic tool in avian disease diagnostic laboratories in which sophisticated equipments such as thermal cycler and electrophoresis apparatus are not available. In addition, RT-LAMP for IB viral genome detection can be used as a field diagnostic test.
Conclusion
RT-LAMP for rapid detection of IBV S2 gene has been optimized and the test was found more sensitive than the RT-PCR. It can be carried out without sophisticated instruments and can be a useful tool for the diagnosis of avian IBV.
References
Food and Agricultural Organization (2010). http://www.fao.org
Ignjatovic J, Sapats S (2000) Avian infectious bronchitis virus. Rev Sci Technol 19:493–508
OIE (2008) Avian infectious bronchitis. Manual of standards diagnostic tests and vaccine. Office International des Epizooties, Paris, pp 700–710
Enjuanes L (2000) Nidovirales. In: van Regernmortel MHV, Fauquet CM, Bishop DHL, Carsten EB, Estes MK, Lemon SM, Maniloff H, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB (eds) Virus taxonomy. Academic Press, New York, pp 827–834
Cavanagh D, Naqi SA (2003) Infectious bronchitis. In: Saif YM, Barnes JR, Glisson A, Fadly M, Mc Dougald LR, Swayne DE (eds) Diseases of poultry, 10th edn. Iowa state university press, Ames, pp 101–119
De Wit JJ (2000) Detection of infectious bronchitis virus. Avian Pathol 29:71–93
Kwon HM, Jackwood MW, Gelb J (1993) Differentiation of infectious bronchitis virus serotypes using polymerase chain reaction and restriction fragment length polymorphism analysis. Avian Dis 37:194–202
Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, Jackwood MW (2006) Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods 138:60–65
Meir R, Maharat O, Farnushi Y, Simanov L (2010) Development of a real-time TaqMan RT-PCR assay for the detection of infectious bronchitis virus in chickens and comparison of RT-PCR and virus isolation. J Virol Methods 163:190–194
Adzhar A, Shaw K, Britton P, Cavanagh D (1996) Universal oligonucleotides for the detection of infectious bronchitis virus by the polymerase chain reaction. Avian Dis 25:817–836
Farsang A, Ros C, Renstrom LHM, Baule C, Soos T, Belak S (2002) Molecular epizootiology of infectious bronchitis virus in Sweden indicating the involvement of a vaccine strain. Avian Pathol 31:229–236
Suresh Kumar K, Dhinakar Raj G, Raja A, Ramadass P (2007) Genotyping of avian infectious bronchitis viruses from India. Indian J Biotechnol 6:45–51
Lin Z, Kato A, Kudou Y, Ueda S (1991) A new typing method for the avian infectious bronchitis virus using polymerase chain reaction and restriction enzyme fragment length polymorphism. Arch Virol 116:19–31
Hung JL, Lee LH, Shih WL, Lin MY, Liao MH (2003) Detection of infectious bronchitis virus by multiplex polymerase chain reaction and sequence analysis. J Virol Methods 109:31–37
Kumanan K, Goodwin Jinesh G, Nachimuthu K (2003) A simple method of detection and typing of infectious bronchitis virus. Indian Vet J 80:1222–1224
Keeler CL Jr, Reed KL, Nix WA, Gelb J Jr (1998) Serotype identification of avian infectious bronchitis virus by RT-PCR of the peplomer (S-1) gene. Avian Dis 42:275–284
Okino CH, Montassier MFSM, Givisiez PEN, Furuyama CRAG, Brentano L, Montassier HJ (2005) Infectious bronchitis virus: detection and vaccine strain differentiation by semi-nested RT-PCR. Braz J Poultry Sci 7:59–66
Williams AK, Wang L, Sneed LW, Collisson EW (1993) Analysis of a hyper variable region in the 3′ non-coding end of the infectious bronchitis virus genome. Virus Res 28:19–27
Cavanagh D, Mawditt K, Britton P, Naylor CJ (1999) Longitudinal studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathol 28:593–605
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63
Hao-tai C, Jie Z, De-hui S, Li-na M, Xiang-tao L, Xue-peng C, Yong-sheng L (2008) Development of reverse transcription loop-mediated isothermal amplification for rapid detection of H9 avian influenza virus. J Virol Methods 151:200–203
Xu J, Zhang Z, Yin Y, Cui S, Xu S, Guo Y, Li J, Wang J, Liu X, Han L (2009) Development of reverse-transcription loop-mediated isothermal amplification for the detection of infectious bursal disease virus. J Virol Methods 162:267–271
Qing-mei X, Jun J, Tristan TP, Li-qin D, Yong-chang C, Hong-mei L, Lin-guo W, Jing-yun M, Ying-zuo B (2010) Rapid detection of infectious laryngotracheitis virus isolates by loop mediated isothermal amplification. J Virol Methods 165:71–75
Xiaoyun D, Xiaole Q, Yulong G, Yongqiang W, Liting Q, Honglei G, Li G, Xiaomei W (2010) Development of reverse transcription loop-mediated isothermal amplification for rapid detection of reticuloendotheliosis virus. J Virol Methods 168:82–86
Chen HT, Zhang J, Ma YP, Ding YZ, Liu XT, Cai XP, Ma LQ, Zhang YG, Liu YS (2010) Reverse transcription loop-mediated isothermal amplification for the rapid detection of infectious bronchitis virus in infected chicken tissues. Mol Cell Probes 24:104–106
Luo H, Zhu D, Xue C, Qio J, Chen F, Cao Y (2012) An improved reverse transcriptase loop-mediaed isothemal amplification assay for sensitive and specific detection of infectious bronchitis virus. J Animal and Vet Advances 11:2398–2402
Acknowledgments
The work was carried out under the Indian Council of Agricultural Research, New Delhi sponsored project on Niche Area of Excellence in Animal Biotechnology. The authors thank the Tamil Nadu Veterinary and Animal Sciences University, Chennai, TN, India for providing facilities to carry out the work. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrasekar, A., Raja, A., Dhinakar Raj, G. et al. Rapid Detection of Avian Infectious Bronchitis Virus by Reverse Transcriptase-Loop Mediated Isothermal Amplification. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 85, 815–820 (2015). https://doi.org/10.1007/s40011-015-0490-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0490-4