Abstract
Metal–oxide–semiconductor field-effect transistors (MOSFETs) have significant role on producing the electronic devices. The producers try to make the chips in smaller size so they need materials with high dielectric constant. Here, in this research, Al/Sn/La2O3 will introduce as a material with higher dielectric constant and less defections. In this research, Al/Sn/La2O3 nanostructure was prepared by sol–gel and spin-coating methods. Lanthanum chloride (LaCl3·7H2O), cetyltrimethylammonium bromide, ammonia (25%), aluminum tri-sec-butylate (C12H27AlO3), acetyl acetone (C2H5O8), isopropyl alcohol (C3H8O), Tin (II) chloride (SnCl2) and H2O were used to synthesize Al/Sn/La2O3. Structural properties and surface morphology of nanocrystallites were investigated by X-ray diffraction, differential scanning calorimetry, scanning electron microscopy, atomic force microscopy and Fourier-transform infrared radiation, respectively. Electrical properties were determined with metal–dielectric–semiconductor through capacitance–voltage (C–V), the hysteresis curve and current density–voltage (J–V). The conduction mechanism was measured in the temperature range of 330 K < T < 410 K and in the electrical field < 0.12 MV cm−1, and the results showed ohmic emission. A thermal excited model was proposed to explain the mechanism of ohmic conduction current. The highest value of dielectric constant (k) for Al/Sn/La2O3 structure was 33 at T = 250 °C with almost amorphous structure. The result showed that \( \frac{{I_{\text{on}} }}{{I_{\text{off}} }} \) of Al/Sn/La2O3 structure was ~ 1.3. The leakage current and capacitance–voltage of Al/Sn/La2O3 nanocomposite for the sample calcined at T = 250 °C showed that this sample can be good enough for using as a gate dielectric of MOSFETs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The metal–oxide–semiconductor field-effect transistors (MOSFETs) have an important role on ultra-large-scale integration (ULSI) that is used in electric products, for example: personal computers, digital cameras and mobile phones [1]. Geometrical size of MOSFETs with high k-dielectric constant has an important role on processing the speed and electrical power dissipation [2]. Gate dielectric has major role to the MOSFETs performance [3]. Now, silicon dioxide (SiO2) with a few atomic layers thick is used in MOSFETs as a gate dielectric [4]. In recent years, high leakage currents and tunneling currents, high power dissipation and boron diffusion become critical issues of SiO2 gate dielectric. Minimizing the size of MOSFETs can solve these issues [4]. Lately, many researchers suggest the materials with lower leakage and tunneling currents and lower boron diffusion with high k-dielectric [5]. The results of researches show that uncommon earth oxides like lanthanum oxide (La2O3) can be appropriate for MOSFETs because of having high k-dielectric constant [6,7,8]. Nanomaterials with amorphous structure and flat surface can make tunneling currents, leakage currents and boron diffusion less in MOSFETs gates [9, 10]. La2O3 has high k-dielectric ~ 27 [11], but its crystalline temperature is above 400 °C [12]. La2O3 shows p-type semiconducting properties because its resistivity decreased with increasing temperature [13].
Al can make composites more amorphous [14] so we can use Al to make an amorphous structure. In this research, we use SnO because of its appropriate effects such as thermal stability [15,16,17].
In this research, first, we synthesized Al/La2O3 nanostructure and Sn/La2O3 nanostructure with sol–gel method then we synthesized Al/Sn/La2O3 nanostructure with Al-30% and Sn-15%. Next, the samples were investigated with several techniques such as X-ray diffraction (XRD), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), atomic force microscopy (AFM) and Fourier-transform infrared radiation (FTIR). Capacitance–voltage curve (C–V) and current density–voltage curve (J–V) were used for investigating electrical properties of samples. The hysteresis curve was shown for Al/Sn/La2O3 nanostructure, too.
2 Materials and Methods
In this research, lanthanum chloride (LaCl3·7H2O), cetyltrimethylammonium bromide (CTAB), ammonia (25%), aluminum tri-sec-butylate (C12H27AlO3), acetyl acetone (C2H5O8), isopropyl alcohol (C3H8O), Tin (II) chloride (SnCl2) and H2O were used. All these chemicals were of analytical reagent grade purchased from Merck (Darmstadt, Germany) unless otherwise stated. The sol–gel and then spin-coating methods were used in this paper.
3 Experimental
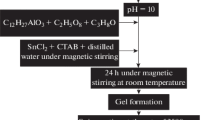
The synthesis of La2O3 nanostructure was carried out as follows: First, 0.91 g of cetyltrimethylammonium bromide (CTAB) was put into 102.0 ml distilled water under magnetic stirring at room temperature. Then, 2.0 g lanthanum chloride (LaCl3·7H2O) was added with stirring to form a homogeneous transparent solution; then, 3.0 ml ammonia 25% was added drop wise to the above solution to adjust the pH value of the solution to 10.0. Along with the addition of ammonia, the solution was turned translucent colloidal right now. After that 0.3 ml aluminum tri-sec-butylate (C12H27AlO3) was dissolved in 0.5 ml isopropyl alcohol (C3H8O) and 0.5 ml acetyl acetone (C2H5O8) under magnetic stirring at room temperature (Al-30%). This example was repeated with 0.15 ml aluminum tri-sec-butylate (C12H27AlO3) (Al-15%) and 0.05 ml aluminum tri-sec-butylate (C12H27AlO3) (Al-5%). Simultaneously, 0.8 g Tin (II) chloride (SnCl2) with 0.1 g CTAB was dissolved in 10.0 ml distilled water under magnetic stirring at room temperature (Sn-15%). This example was repeated with 0.54 g Tin (II) chloride (SnCl2) (Sn-5%). At last, all the samples were added to La2O3. Then, according to the results of XRD analysis, we choose two samples of them (Al-30% and Sn-15%) for mixing with each other to synthesis Al/Sn/La2O3 nanostructure. After stirring for 24 h under magnetic stirring, the gel was coated on Si substrate at 2500 rpm to form Al/Sn/La2O3/Si structure. After that Al/Sn/La2O3 nanostructure was calcined at T = 250 °C. Figure 1 shows the schematic flowchart of synthesis process. The sample’s characteristics are shown in Table 1.
4 Results and Discussion
The XRD patterns of the samples are shown in Fig. 2. According to Fig. 2, it is clear that with increasing Al in Al/La2O3 nanostructure, the samples became more amorphous in structure. Al/Sn/La2O3 nanostructure was calcined at T = 250 °C which has relatively amorphous structure because the Al can make the composite more amorphous [18]. Amorphous structures have lower tunneling and leakage currents and boron diffusion than crystalline structures in MOSFETs gates [19]. In Fig. 2, it was evidenced that with increasing Sn, the composite became more amorphous, too. According to these results, we chose the samples with 15% Sn and 30% Al for preparing the Al/Sn/La2O3 nanostructure. The miller indices of the samples are shown in Fig. 2 [19].
The X-powder software was used to calculate the size of nanocrystallite, and the results are shown in Fig. 3. The sizes of nanocrystallite were about 45, 42 and 41 nm of Al/La2O3 nanostructure [for the peak with miller indices of (110)] with Al-5%, Al-15% and Al-30% and about 34 and 19 nm for Sn/La2O3 nanostructure with Sn-5% and Sn-15%. We could see that these sizes were decreased with increasing the amounts of Al and Sn.
The relation between yield and nanocrystallites’ size is described mathematically by the Hall–Petch equation [20]:
In Eq. (1), σy is the yield stress, σ0 is a material constant for the starting stress for dislocation movement (or the resistance of the lattice to dislocation motion), ky is the strengthening coefficient (a constant unique to each material), and D is the average nanocrystallites’ diameter. So, it is evidenced that with reducing the nanocrystallites’ size, the yield strength and yield stress of the samples decreased so they became more stable, which can prevent light atom penetration and leakage current. Al/Sn/La2O3 nanostructure which was calcined at T = 250 °C had relatively amorphous structure with crystallite size ~ 22 nm that it was calculated by using X-powder software or correct Scherrer.
The DSC curve of Al/Sn/La2O3 nanostructure (sample 6) is shown in Fig. 4. This figure showed the various of decomposition stages of Al/Sn/La2O3 nanostructure with respect to temperature. An endothermic peak till T = 200 °C could be due to removal of adsorbed water, and the exothermic peaks in these area could be due to the heat of evaporations. A broad endothermic peak at around T = 230 °C could be due to brake of previous junctions, and the exothermic peaks at T = 300 °C, T = 500 °C and T = 600 °C could be due to form of amorphous structure in sample 6.
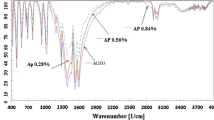
The FTIR technique was used for characterizing the purity and quality of conjunctions. The results of FTIR spectra of the samples (samples 3, 5 and 6) are shown in Fig. 5. In Fig. 5, it was evidenced that the junctions of dipoles such as C=C in 700 cm−1 wave number of sample 3 (Al-30%) were the widest. The sample with Al-30% had the strongest metal-O and metal-O-metal junctions, and Al/Sn/La2O3 had the weakest junctions of metal-O and metal-O-metal because the Sn was added into this composite; Sn’s junctions were the weakest between these three samples (see Fig. 5).
The surface morphology of the dielectric layer was studied by the SEM technique. SEM images of Al/La2O3 (sample 3), Sn/La2O3 (sample 5) and Al/Sn/La2O3 nanostructure (sample 6) are shown in Fig. 6. As they were shown in SEM images, the structure phases had been changed before and after mixing with each other. These images showed that the uniform structure was formed at T = 250 °C with almost amorphous structure.
Moreover, statistical properties of the surface such as surface roughness were described with AFM technique. An area of 10 μm × 10 μm of the samples was used for obtaining each AFM image. The surface roughness was described with topography spectra (Fig. 7) and DME software images. The opposite of roughness is smoothness. The roughness causes light dispersion and absorption. The surface roughness parameters (see Table 2) such as the roughness average (Sa), the mean value (Sm) and the root mean square (Sq) of the Al/Sn/La2O3 nanostructure were determined with using DME software.
DME software reaches different amounts of roughness parameter (see Table 2). In this research, the Al/Sn/La2O3 nanostructure showed much decrease in root-mean-square roughness (Sm). According to amount of average roughness (Sa), an almost uniform surface was observed for Al/Sn/La2O3 nanostructure. These measurements showed that this sample had a flat and smooth surface morphology and it made the Al/Sn/La2O3 nanostructure more stable to local shear so lower roughness and crack free with relatively amorphous structure made this sample more stable. The reason was that fewer cracks could reduce the leakage current and light atom penetration through the gate materials in MOSFETs.
Capacitance–voltage curve (C–V) is affected by capacitive response of interface traps and oxide charges. Figure 8 shows C–V characteristics of Al/La2O3 (sample 3), Sn/La2O3 (sample 5) and Al/Sn/La2O3 nanostructure (sample 6) with 16.3 nm thick of thin films which were taken at 1 MHz. The variations of capacitance (C) with gate voltage (VG) were measured from − 3.0 to 3.0 V. This figure indicated that the capacitance of Al/Sn/La2O3 nanostructure was lower than Al/La2O3 and higher than the capacitance of Sn/La2O3. The capacitance of the samples decreased with increasing gate voltage because of high leakage current density. The accumulation capacitance (Cacc) of sample 6 (Al/Sn/La2O3) was ~ 2024 nF with relatively amorphous structure. The maximum amount of accumulation capacitance was used for measuring the EOT and the dielectric constant. According to this measurement, the dielectric constant (k) was ~ 33. According to Eq. (2), EOT is the thickness of SiO2 layer to achieve the same density of capacity (capacity per unit area) with regard to high k-material [21]:
where ɛr,SiO2 is dielectric constant of silicon, ɛr,high-k is the Al/Sn/La2O3 dielectric constant, and Dhigh-k is the Al/Sn/La2O3 gauge. The corresponding equivalent oxide thickness (EOT) value of Al/Sn/La2O3 was about 1.3 nm.
The variations of dielectric constant versus frequency of Al/Sn/La2O3 nanostructure (sample 6) were investigated to see its response to an applied low AC voltage in the frequency range of 100 Hz to 1 MHz. The variations of dielectric constant versus frequency are shown in Fig. 9. It was observed that dielectric constant (k) decreased with increasing frequency. The highest value of k in 100 Hz was ~ 32 for sample 6.
J–V curves of the samples under positive bias and in the temperature range of 330 K < T < 410 K are shown in Fig. 10. Current density versus voltage (J–V) trace was obtained in a substrate injection mode. In Fig. 10, it was evidenced that the biggest amount of current density of sample 6 (Al/Sn/La2O3 nanostructure) was ~ 6.9 μA cm−2 at 5 V bias voltage. The J–V curves confirmed that tunneling process was the dominant transport mechanism because they displayed the same weak temperature dependence. Mathematically, the expression for current density according to Poole–Frenkel model [22] can be written as follows
where K is the dielectric constant of the film, d is electrode spacing in Å, φt is the depth of the trap potential well, and A is the Richardson constant having a value of 120 A cm−2 K−2.
In Fig. 11, the plot of log (J) versus 1000/T was shown. This measurement was taken at applied voltage below 0.2 V and in the temperature range of 330–410 K. Ohmic emission is considered for studying the conduction mechanism at higher temperature and lower electric field because of the strong dependence on electric field and applied temperature. Current density equation for ohmic conduction can be written as follows [23]:
where J is the current density, q is the electron charge, and µ is the electron mobility in insulator. ∆Eac is the electron activation energy that it can be obtained from the fitted curve value of the log (J) versus 1000/T plot of Fig. 11. And
is the effective density of states in the conduction band [24], where \( m^{*} \) is electron effective mass, \( m^{*} \) can be assumed as \( m^{*} = 0.3m_{0} \), \( m_{0} \) is free electron mass, k is Boltzmann constant, and T is the absolute temperature. Following equation can obtain the hopping electron concentration [25]:
In Table 3, all above parameters (at T = 330 K) were calculated and listed.
According to Table 3, the activation energy decreased with increasing electric field and so the hopping electron concentration increased.
As it was clear, the electron mobility decreased with increasing electric field that it could be due to increase in density of electron cloud overlap. Electrons can easily move along electric field when the overlapping of adjacent atoms is more.
At the electric field of 0.12 MV/cm, the hopping electron concentration was 38.7 × 1021 cm−3, so in the lower conduction current of Al/Sn/La2O3, lower concentration and carrier mobility were resulted.
The hysteresis curve of the sample 6 (Al/Sn/La2O3 nanostructure) is shown in Fig. 12. In a certain period, gate voltage (VGS) changed from negative bias to positive and then it returned again. At this time, the transistor reached an off current (Ioff) in 0.0 V gate voltage, and after that we increased the gate voltage to positive amount to turn on the transistor again (Ion). As \( \frac{{I_{\text{on}} }}{{I_{\text{off}} }} \) is an important parameter; when this parameter became less, the current shocks became less in transistors, too. For this sample, \( \frac{{I_{\text{on}} }}{{I_{\text{off}} }} \) was about 1.3.
5 Conclusions
In this paper, first, we synthesized Al/La2O3 and Sn/La2O3 with sol–gel method, and then, we coated them with spin-coating method on Si to form Al/Sn/La2O3 nanostructure. The XRD analysis showed relatively amorphous structure for the Al/Sn/La2O3 nanostructure at T = 250 °C with 19 nm nanocrystallites’ size that it was calculated with X-powder software. The surface topography of the samples was investigated by AFM technique, and it was found that the roughness values for the sample 6 (Al/Sn/La2O3 nanostructure) were the best of all three samples for using as a gate dielectric materials. One reason can be existence of Al and Sn together. It was found that with both of them, we can have almost good roughness parameter for the gate dielectric because this reduced the grain boundaries in the conduction channel for higher mobility of MOSFET devices. The results of analysis (such as the results of C–V and J–V) showed that the Al/Sn/La2O3 nanostructure had the fewest leakage currents and the highest amount of capacitance because of almost amorphous structure. The highest value of dielectric constant was ~ 33 for sample 6. The current leakage density was about 6.9 μA cm−2 at 5 V. The conduction mechanism was found to be ohmic emission. The lowest concentration and carrier mobility in the low conduction current were for Al/Sn/La2O3 nanostructure. The results showed that Ion/Ioff was ~ 1.3. These properties can also prevent leakage current, tunneling current and boron diffusion through the thin Al/Sn/La2O3 structure. At last, we can introduce the Al/Sn/La2O3 nanostructure which was calcined at T = 250 °C as a good gate dielectric candidate for MOSFETs devices.
References
Morgan P, Bahari A, Robenhagen U, Anderson J, Hansen K, Pederson M, Rao G, Li Z (2005) Reads to ultrathin silicon oxide. J Vac Sci Tech A 23:201–207
Bahari A, Ebrahimzadeh M, Gholipur R (2014) Structural and electrical properties of zirconium doped yttrium oxide nanostructures. Int J Mod Phys B 28:1450102–1450113
Okamura K, Nasr B, Brand RA, Hahn H (2012) Solution-processed oxide semiconductor SnO in p-channel thin-film transistors. R Soc Chem 32:1–19
Khaing AM, Win TT, Maung YM, Kyaw Soe KK (2014) Characterization and output characteristics of Al (6 mol%) doped PbTiO3 thin film transistors. Res J 6:15–20
Bahari A, Ramzannejad A (2012) Nanostructural properties of La2O3/HfO2 gate dielectrics. Int J Mod Phys B 26:1250080–1250089
Bahari A, Morgan P, Li Z (2008) Ultra thin silicon nitrite films on Si(100) studied with core level photoemission. Surf Sci 602:2315–2323
Bahari A, Morgan P, Li Z (2006) Valence band studies of the formation of ultrathin pule silicon nitride film on Si(100). Surf Sci 600:2966–2972
Bahari A, Gholipur R (2012) Investigation of ZrLaO2 nano crystallites in metal- high k oxide-silicon-type nonvolatile memory devices I. J Mod Phys B 26:1250191–1250212
Chowdhury HM, Mannan AM, Mahmood AS (2010) High-k dielectrics for submicron MOSFET. Emerg Tech Sci Eng 2:1–8
Bahari A, Roodbari M (2013) Langevin treatment of sub diffusing phenomena in ultra thin film. J Man Proc 4:1–6
Kale S, Jadhav K, Patil P, Gujar T, Lokhande C (2005) Characterizations of spray-deposited lanthanum oxide (La2O3) thin films. Mat Lett 59:3007–3024
Aun JN (2006) A study on mobility of MOSFETs with La2O3 gate dielectric. Doctorial Thesis of Engineering
Ng JA, Kuroki Y, Sugii N, Kakushima K, Ohmi SI, Tsutsui K, Hattori T, Iwai H, Wong H (2005) Effects of low temperature annealing on the ultrathin La2O3 gate dielectric; comparison of post deposition annealing and post metallization annealing. Microelectron Eng 80:206–209
Alizadeh Eslami A, Haghighi M, Rahemi N, Nasiri Laheghi S (2010) Sol–gel synthesis and characterization of Si/Al2O3 anode catalysts doped with Co and Cu. In: Proceedings of the international conference on the advanced materials processing technology, pp 1297–1299
Batzill M, Diebold U (2005) The surface and materials science of tin oxide. Prog Surf Sci 79:147–154
Okamura K, Nasr B, Brand RA, Hahn H (2012) Solution-processed oxide semiconductor SnO in p-channel thin-film transistors. R Soc Chem 32:1–19
Thanachayanont C, Yordsri V, Boothroyd C (2011) Microstructural investigation and SnO nanodefects in spray-pyrolyzed SnO2 thin films. Mater Lett 65:2610–2613
Li YL, Chen LQ (2006) Temperature-strain phase diagram for BaTiO3 thin films. Appl Phys Lett 88:072905–072908
Roodbari M, Rezaee M, Shahtahmasbi N (2011) Nano structural properties of Al2O3/SiO2/Si in integrated electronic systems. Int J Chem Tech Res 3:1681–1684
Hansen N (2004) Hall–Petch relation and boundary strengthening. Scrip Mater 51:801–806
Yamaguchi T, Satake H, Fukushima N, Toriumi A (2000) Band diagram and carrier conduction mechanism in ZrO/sub2//Zr-silicate/Si MIS structure fabricated by pulsed-laser-ablation deposition. Int J Electron Dev Meet 63:19–23
Bahari A, Gholipur R (2012) Electrical and optical properties of ZrxLa1−xOy nanocrystallites as an advanced dielectric for the next FET devices. J Mater Sci Mater Electron 24:674–686
Sze SM (1981) Physics of semiconductor devices, 2nd edn. Wiley, New York
Houssa M, Tuominen M, Naili M, Afanas V, Stesmans A, Haukka S, Heyns M (2000) Trap-assisted tunneling in high permittivity gate dielectric stacks. J Appl Phys 87:8615–8620
Mahapatra R, Lee JH, Maikap S, Kar GS, Dhar A, Hwang NM, Kim DY, Mathur BK, Ray SK (2003) Electrical and interfacial characteristics of ultrathin ZrO2 gate dielectrics on strain compensated SiGeC/Si heterostructure. Appl Phys Lett 82:2320–2322
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebrahimzadeh, M., Nakhaei, M., Padam, M. et al. Nanostructural and Electrical Properties of Al/Sn/La2O3 Nanocomposite as a Gate Dielectric of MOSFETs. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 90, 637–646 (2020). https://doi.org/10.1007/s40010-019-00637-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-019-00637-0