Abstract
In the present study, spatial variation of groundwater quality parameters in Udhampur district, Jammu and Kashmir, India, has been evaluated for drinking water purposes using geographic information system techniques. In total, 211 GW samples were collected from different sources, i.e. dug wells, springs and tube wells covering the entire district during the pre- and post-monsoon seasons. The GW samples were analysed for various physico-chemical parameters like pH, total hardness, calcium, magnesium, iron, fluoride, sulphate, nitrate, potassium, chloride, sodium, bicarbonate and total dissolved solids using the standard methods. Inverse distance-weighted interpolation technique has been applied for predicting the spatial distribution of the GW parameters. The Canadian Council of Ministers for the Environment Water Quality Index, generated to assess the spatial variability of the groundwater quality, revealed that the groundwater in the area is of good quality falling within the permissible limits and therefore potable during both the pre- and post-monsoon seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The sustenance of water resources is an important indicator of health and socio-economic status of many nations worldwide [1, 2]. The quality of water determines its use for human, animal, agricultural and industrial purposes. In view of the depleting surface water resources in the Himalayas [3, 4], GW becomes an important supplement to supply water for various human uses. According to the UNEP [5] estimates, approximately one-third of the world population uses groundwater for drinking purposes. However, recent studies have shown that anthropogenic activities, urbanization and industrialization are affecting the quality of groundwater [6,7,8,9,10,11]. Poor quality of groundwater poses a severe threat to human health, plant growth and socio-economic development [12, 13]. About 80% of all diseases in developing countries, including India, are directly related to the unhygienic conditions and the poor quality of drinking water [14]. A number of geochemical processes and biophysical factors govern the physico-chemical characteristics of the groundwater. The quality of recharging water, precipitation, unscientific disposal of human and industrial wastes, reckless use of fertilizers and pesticides, the chemical composition of parent rock, residence time, rock–water interactions, reactions with aquifer minerals, etc. have great impacts on the quality of groundwater [15,16,17,18,19]. It hence becomes imperative to evaluate the spatiotemporal patterns of groundwater quality parameters and understand the processes that affect the GW potability and sustainable exploitation of the GW resources.
Geographic information system (GIS) is used to precisely determine spatiotemporal variability and suitability of groundwater quality (GWQ) for drinking, industrial and agricultural purposes by generating seamless surfaces from a limited number of sampled observations [20,21,22,23]. GIS has been efficiently used for assessing the seasonal variability of groundwater quality in subtropical Siwaliks [24]. Similarly, the concept of the water quality index (WQI), in which the presence or absence of certain chemical parameters in water or their presence beyond the standard threshold is used as an indicator of the potability of the water, is now being used worldwide [25,26,27]. WQI is a managing tool that summarizes a large amount of complex data into an index that yields easily interpretable statistics for reporting to policy makers and common masses. WQI assists in determining the overall water quality at a specific location and period, thus determining the appropriateness of water for various uses [28,29,30]. Keeping this in view, the Canadian council of ministers of the environmental water quality index (CCME WQI), a well-established and frequently acknowledged model for the calculation of WQI [31,32,33], was used in this study for assessing the potability of the groundwater in Udhampur, the study area.
The previous studies on groundwater in Udhampur district have focussed on the general assessment of the groundwater quality or groundwater levels or GW zonation using a limited number of samples over a part of the district. [34,35,36]. Furthermore, the use of GIS technology for determining the spatial distribution of physico-chemical parameters of groundwater is missing in the previous studies. However, in the present study spatial variation in groundwater quality based on the physico-chemical parameters of a large number of spatially well-distributed groundwater samples collected during pre-monsoon (PRM) and post-monsoon (POM) seasons was studied and evaluated for drinking water purposes in accordance with the BIS and WHO standards. Besides, spatial interpolation of GWQ parameters and CCME WQI techniques in GIS environment are used to generate the spatial variability of GW parameters and assess the groundwater suitability for drinking purposes. Therefore, keeping in view the above facts, this study is an improvement in terms of the number of samples, use of GIS and WQI and would lead to better understanding of the groundwater quality and distribution in this data-scarce mountainous Himalayan region.
2 Study Area
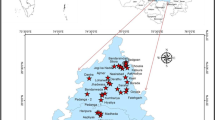
Udhampur district is located in the south-eastern part of the Jammu and Kashmir, India. It is surrounded on the west by Rajouri district, in the north by Anantnag district, in north-east by Doda district, in the south-east by Kathua district and in the south-west by Jammu district (Fig. 1). Geographically, it lies between 32° 39′0″N to 33°31′ 20″N latitudes and 74° 35′ 20″E to 75° 40′ 30″E longitudes, spreading over an area of 4500 km2 approximately. Udhampur district has a varied topography ranging from hilly tracts, intermountain valleys and lofty mountain ranges. The region is famous for various tourist destinations like Patnitop, Sudh Mahadev, Sanasar and Latti. Farming is the main occupation of the populace. The district is drained by four major rivers, namely Chenab, Tawi, Basantar and Ujh. The climate of the district is the alpine type, with monsoon having a significant impact. Mean temperature varies and ranges between 12 and 28 °C (1985–2015) in the region due to the wide altitudinal range (1000–4500 m). Precipitation is mostly in the form of rainfall, but higher reaches also receive snowfall in winter. Geologically, the major portion of the area is characterized by Murree, Salkhala and Siwalik formations. Limestones, Ramsu formation and Panjal volcanics are other geological formations, but they occupy only a small portion of the district [34]. Groundwater exists under confined as well as unconfined conditions in the underlying rocks of the Siwaliks and alluvial formation, respectively. Perennial springs having good discharge are abundant in the area forming the primary source of water supply in the area. The movement of water in the region is controlled by joints and bedding planes of the hard rocks. Due to the high density of joints and the presence of bedding planes, the groundwater percolates to greater depths in the region. The depth to groundwater in the area ranges from 0.10 to 11.50 m below the ground level [37].
3 Methodology
3.1 Physico-Chemical Analysis of Groundwater Samples
Spatially well-distributed groundwater samples were taken from dug wells, springs and tube wells based on soil, lithology and land-use pattern of the region. Care was taken to safeguard that the samples taken are representative of the system sampled. A total of 211 water samples were collected for various physico-chemical tests during 2012–2013 and samples were subcategorized into PRM and POM in order to check the variability of GWQ during both the seasons and the sampling was done using the standard procedures [38]. Stratified sampling scheme was utilized for groundwater sampling sites. Samples were collected in clean plastic bottles fitted with screw caps to avoid unpredictable changes and contamination of physico-chemical characteristics. Parameters like pH and conductivity were measured in situ by using a calibrated pH meter and conductivity meter, respectively. The cations (Na+, K+, Ca2+ and Mg2+) and anions (Cl−, HCO3− and SO42−) were determined in the laboratory using standard methods given by the American Public Health Association [38].
3.2 Spatial Interpolation of Physico-Chemical Parameters
The sampling site coordinates were obtained during the fieldwork using the Trimble hand-held GPS (Juno SB), and subsequently, the coordinates were imported into the GIS environment. Spatial distribution of GWQ parameters like pH, Ca2+, Cl−, F−, Fe2+, Na+, HCO3−, Mg2+, NO3−, TH and TDS was determined using the inverse distance-weighted (IDW) spatial interpolation [39] technique in GIS. The parameter values were classified according to the BIS [40] and WHO [41] standards for drinking water. IDW is a poignant average interpolator that is frequently used for the interpolation of highly variable data like the physico-chemical parameters. IDW algorithm assumes that every input point has a native impact that reduces with distance. IDW is given by:
where Ai is the value of known point, dij is the distance to the known point, Aj is the unknown point and n is a user-selected exponent [42].
3.2.1 Conceptual Framework of CCME WQI
Water quality assessment based on the water quality index (WQI) developed by the Canadian Council of Ministers of the Environment (CCME WQI) was conducted, and the spatial variability of the groundwater quality in the region was plotted. The CCME WQI provides a mathematical framework to evaluate the quality of water in combination with a set of conditions representing quality criteria or limits. CCME WQI contains three factors and is a well-recognized indicator for water quality CCME [43].
3.2.2 Factor 1: F1 (Scope)
It assesses the proportion by which the variables deviate from their objectives. It has been adopted directly from the British Columbia Water Quality Index and is expressed as:
where the variables indicate those water quality parameters whose objective values (threshold limits) are specified and observed values at the sampling sites are available for the index calculation.
3.2.3 Factor 2: F2 (Frequency)
It is the percentage of failed tests and is represented as:
3.2.4 Factor 3: F3 (Amplitude)
The amount by which the goals are not met (amplitude), which symbolizes the quantity by which the unsuccessful test values do not meet their goals, is designed in three steps. The number of intervals by which an individual concentration is greater than (or less than, when the objective is a minimum) the objective is termed an “excursion” and is expressed as follows. When the test value does not go beyond the objective:
For the cases in which the test value must not fall below the objective:
The total amount, by which the individual tests are out of obedience, is calculated by summing the excursions of individual tests from their objectives and then dividing the sum by the total number of tests. This variable referred to as the normalized sum of excursions (nse) and is calculated as:
F3 is then calculated by an asymptotic function that scales the normalized sum of the excursions from objectives (nse) to yield a value between 0 and 100.
The CCME WQI is finally calculated as:
The factor of 1.732 has been introduced to scale the index from 0 to 100. Since the individual index factors can range as high as 100, it means that the vector length can reach a maximum of 173.2 as shown below:
The above design yields a value between 0 and 100 and gives a statistical value to the state of water quality. A zero (0) value signifies very poor water quality, whereas a value nearby 100 signifies excellent water quality. The assignment of CCME WQI values to different categories is a relatively subjective process and stresses skilled decision and public’s beliefs about water quality. The water quality is ranked in the following five categories:
-
1.
Excellent: (CCME WQI values 95–100)
-
2.
Good: (CCME WQI values 80–94)
-
3.
Fair: (CCME WQI values 60–79)
-
4.
Marginal: (CCME WQI values 45–59)
-
5.
Poor: (CCME WQI values 0–44)
In the present study, the WQI using all the samples was calculated at the individual level and stored in GIS environment for classification of groundwater quality using IDW spatial interpolation technique for better understanding of the groundwater quality distribution in the region. The index provides an appropriate means of summarizing complex water quality data that can be easily understood by the public, organizers, water distributors, administrators and policy makers.
4 Results and Discussion
The physico-chemical characteristics of the GW samples, collected during the pre-monsoon and post-monsoon seasons, were analytically assessed in the laboratory. The statistical values like maximum, minimum, average and standard deviation of the WQ parameters are given in Table 1. To determine the distribution patterns of the concentration of different groundwater quality parameters, maps for various elements were generated in the GIS environment. The spatial distribution map was prepared for each parameter for the PRM and POM seasons, and the concentration values were evaluated according to the BIS and WHO standards fixed for drinking water purposes. The concentration values were categorized into desirable, permissible, non-potable, highest desirable, maximum permissible and the values exceeding maximum permissible are termed as not permissible (NP). Further, the GW samples were categorized on the basis of surface geology. It was found that majority of the samples (89) fall in Murree, Dharamsala lithology, followed by Salkhala/Haimanta/Tanwal formation (36), Siwalik (30), Sirban, Bilaspur Riasi formation (15), biotite–muscovite granite (13), Mandi, Permian and Triassic of Kashmir (9), Panjal volcanics (8), Subathu (8), Ramsu formation (2), Fenestella shale and Syringothyris limestone (1). Lithology-wise values of the maximum, minimum, average and standard deviation of the WQ parameters are given in Table 2. From the analysis of the GW parameters on the basis of surface geology, it is observed that from biotite–muscovite granite formation concentration of HCO3− at three sampling sites is above the permissible limits of BIS and WHO standards. From Murree, Dharamsala lithology, the concentration of F− at Dehahri village, concentration of Fe2+ at five sampling sites and concentration of NO−3 at four sampling sites are above the permissible limits of BIS and WHO standards. Further, from Murree, Dharamsala lithology, the concentration of HCO3− at twenty sampling sites is found above the permissible limits set under the two standards. From Salkhala/Haimanta/Tanwal formation, the concentration of Fe2+ at Chenani village and concentration of HCO3− at twelve sampling sites is above the permissible limits of both BIS and WHO standards. From Sirban, Bilaspur Riasi formation, the concentration of Fe2+ at four sampling sites, concentration of NO3− at three sampling sites and concentration of HCO3− at seven sampling sites are above the permissible limits of BIS and WHO standards. From Siwalik formation, the concentration of Fe2+ and NO3− at Chowki Chuora village and concentration of HCO3− at eight sampling sites are above the maximum allowable limits of BIS and WHO standards. From Subathu formation, the concentration of NO3− at four villages is above the maximum allowable limits of BIS and WHO standards. From Mandi, Permian and Triassic of Kashmir formation, the concentration of HCO3− at Chakras village is above the permissible limits of BIS and WHO standards. From Panjal volcanics, the concentration of HCO3− at Nunkhel village is above the permissible limits set under the two standards. Similarly, from Fenestella shale and Syringothyris limestone formations, the concentration of HCO3− at Khaur village is above the maximum permissible limits of BIS and WHO standards.
4.1 General Ion Chemistry
The concentration values of pH in the groundwater samples collected from the study area varied during PRM and POM. The pH ranges from 6.5 to 8.4 and 6.5 to 8.3 during PRM and POM. The pH concentration was found well within the desirable limits and the maximum permissible limits of BIS and WHO standards, respectively (Table 3). The spatial distribution of general ions of groundwater samples is shown in Fig. 2. Spatial distribution of pH during the PRM season revealed that the lowest pH values of the parameter (6.5) was observed mostly around the villages of Ramnagar taluka and a few villages of Udhampur, Gool and Chenani talukas. The highest pH value (8.4) was observed in the Seen Brahma village of Udhampur taluka. During POM season, the lowest pH (6.5) was observed mostly around the villages of Gool followed by Udhampur, Ramnagar and few villages from Chenani taluka and highest concentration pH (8.3) was observed in Kashirah village of Udhampur taluka. Total dissolved solids (TDS) of the groundwater samples vary from 360 to 740 mg/l during the PRM and 360 to 720 mg/l during the POM. Spatial distribution of TDS concentration revealed that during PRM season, the lowest values (360 mg/l) are observed mostly around the villages of Ramnagar taluka followed by Reasi, Gool and Udhampur talukas. The highest values of TDS (740 mg/l) are observed only near the Guli gali village of Ramnagar taluka. However, during the POM season, the lowest TDS concentration are observed mostly around the villages of Reasi taluka and to some extent in a few villages of Udhampur, Chenani, Ramnagar and Gool talukas (360 mg/l). The highest values of TDS during the POM are observed in Narsu village of Ramnagar taluka (720 mg/l), respectively. TDS concentration ranges well within the desirable and permissible limit under the BIS and within the maximum allowable limits under WHO standards. To ascertain the suitability of groundwater for various purposes, the groundwater samples were classified on the basis of TDS values [44], the details of which are given in Table 4.
Total hardness (TH) ranges from 10 to 355 mg/l with an average value of 62 mg/l during the PRM and 10 to 405 mg/l with an average value of 89 mg/l during the POM. Spatial distribution of TH concentration during PRM season showed that the lowest values are observed mostly around the villages of Ramnagar taluka and in a few villages of Chenani, Gool, Udhampur and Reasi talukas (10 mg/l). The highest values are found in the Krul village of Ramnagar taluka (347 mg/l). During the POM season, the lowest TH concentration was observed mostly around the villages of Gool taluka, and a few villages of Chenani, Udhampur, Ramnagar and Reasi talukas (10 mg/l). The highest values of the parameter were observed in Kanthan village of Reasi taluka (405 mg/l). The TH values of all the samples fall within the desirable and permissible limits set under the BIS and the maximum allowable concentration limits provided under the WHO standards. In determining the suitability of groundwater for domestic and industrial purposes, hardness is an important criterion, as it is involved in making the water hard. Water hardness would affect water supplying schemes, cause excessive soap consumption and calcification of arteries and cause urinary concretions, diseases of kidney, bladder and stomach disorder, and even some evidence indicates its role in heart disease [45, 46]. The classification of groundwater based on TH shows that all the groundwater samples fall in the desirable category. TH in mg/l is determined by the following equation [47].
The classification of the groundwater based on total hardness [44] is presented in Table 5. Seventy-six samples fall under the soft category, 6 samples fall under moderate hardness, 16 samples fall in the hard and 3 samples fall under very hard class during the PRM season. In the POM season, 65 samples fall under the soft category, 16 samples fall under moderate hardness, 25 samples fall in the hard and 4 samples fall under very hard class. This reveals that study area experiences a mixed groundwater hardness ranging from soft to very hard category during both the seasons. However, the percentage of soft water category samples is much more than that of hard and very hard category samples observed during both the seasons.
Though the region has a good groundwater potential, it was observed during the fieldwork that the boreholes and tube wells are pervasive all over the region which is leading to the high rate of groundwater extraction to meet the rising water demands of the rapidly growing population and increasing industrialization. The extraction of groundwater, if continued unsustainably, might put severe pressure on the groundwater resources in the region and would, in the long run, make the aquifer vulnerable to over-exploitation and water quality deterioration.
4.2 Major Cation Chemistry
Dominant cations analysed from the GW samples fall in the order; Ca2+ > Mg2+ > Na+ > K+ and Ca2+ > Na+ > Mg2+ > K+ during the PRM and POM seasons, respectively. Figure 3 shows the spatial distribution of major cations during the PRM and POM seasons. Among the major cations, calcium is dominant during both the seasons. Therefore, among cations, calcium and magnesium play a dominant role in the geochemistry of the groundwater in this Himalayan region during PRM.
Calcium concentration is found to vary between 10 and 92 mg/l with an average value of 38 mg/l during the PRM and 10–84 mg/l with an average value of 37 mg/l during the POM season. Spatial distribution of calcium concentration during the PRM season showed that the lowest and the highest values of the parameter are observed in Chhapar and Chuna villages of Reasi taluka (10 mg/l) and Pata-khu village of Ramnagar taluka (92 mg/l), respectively. During the POM season, the lowest and the highest calcium concentration was observed in Kakri (10 mg/l) and Kanthan (84 mg/l) villages of Reasi taluka, respectively. The desirable limits and the maximum allowable limit of calcium ion concentration in groundwater is 200 mg/l as per the BIS and WHO standards, and all the water quality parameters fall well below the limit. Magnesium concentration ranges from 7 to 50 mg/l with an average value of 23 mg/l during the PRM and 1 to 69 with an average value of 22 mg/l during the POM. Spatial distribution of magnesium concentration during PRM season revealed that the lowest and the highest values of the parameter are observed in Kardoh (7 mg/l) and Jharog gali (50 mg/l) villages of Gool taluka, respectively. During the POM season, the lowest and the highest magnesium concentration was observed in Meer village of Chenani taluka (1 mg/l) and Tanori village of Reasi taluka (69 mg/l), respectively. All the parameters fall well within the maximum allowable and the desirable limits of magnesium ion concentration in groundwater with the values of 150 mg/l and 100 mg/l as per the WHO and BIS standards, respectively.
Potassium concentration ranges from 0.1 to 9 mg/l with an average value of 2.77 mg/l during the PRM and 1 to 9 mg/l with an average value of 4.40 mg/l during the POM. Spatial distribution of potassium concentration during the PRM season showed that the lowest values of the parameter are observed mostly around the villages of Ramnagar and a few villages of Chenani, Udhampur and Reasi talukas (0.1 mg/l). The highest values of the parameter are mostly around the villages of Gool taluka and a few villages of Reasi taluka (9 mg/l). During the POM season, the lowest potassium concentration was observed mostly around the villages of Udhampur taluka and a few villages of Reasi, Ramnagar and Gool talukas (1 mg/l). The highest concentration of the parameter was observed mostly in the villages of Ramnagar, Gool, Udhampur and Reasi talukas (9 mg/l). As per the WHO and BIS standards, the maximum allowable limit for potassium is 10 mg/l. Potassium concentration in all the GW samples falls well within the limits of the desirable and maximum allowable limits under the BIS and WHO standards. Sodium concentration ranges from 4 to 96 mg/l with an average value of 23 mg/l during the PRM and 3 to 82 mg/l with an average value of 23 mg/l during the POM. Spatial distribution of sodium concentration revealed that during the PRM season, the lowest and the highest values are observed in Kardoh (4 mg/l) and Suka (96 mg/l) villages of Gool taluka. During the POM season, the lowest values of sodium concentration was observed mostly around the villages of Udhampur and Reasi talukas (3 mg/l) and the highest values of the parameter was observed in Muhur, Trilla and Ghugot villages of Udhampur taluka (82 mg/l). All the WQ samples fall within the maximum allowable limits and the desirable limits of Mg as per the WHO and BIS water quality standards.
Iron concentration ranges from 0.01 to 1.65 mg/l with an average value of 0.22 mg/l during the PRM and 0.01 to 3.0 mg/l with an average value of 0.2 mg/l during POM. Spatial distribution of iron concentration revealed that during the PRM season, the lowest values are observed in Kardoh village of Gool taluka (0.1 mg/l) and the highest value of the parameter was observed in the Chowki Chuora village of Reasi taluka (1.65 mg/l). During the POM season, the lowest and the highest values of iron concentration was observed mostly around the villages of Chenani taluka (0.01 mg/l) and Kotli village of Chenani taluka (3 mg/l), respectively. The maximum allowable limit of iron concentration in groundwater is 1.5 and 0.3 mg/l as per WHO and BIS, respectively. As per WHO standards 1 sample during PRM and 2 samples during POM were above the maximum allowable limits. Furthermore, according to BIS standards, 5 samples during both PRM and POM seasons were falling above the maximum allowable limits.
4.3 Major Anion Chemistry
Dominant anions are in the order of HCO3− > SO42− > NO3− > Cl− > F− observed in the GW samples during both the POM and PRM seasons. Among the major anions, bicarbonates play a dominant role in governing the groundwater chemistry. Figure 4 shows the spatial distribution of major anions during the PRM and POM seasons.
Bicarbonate concentration ranges from 85 to 480 mg/l with an average value of 247 mg/l during the PRM and 79 to 445 mg/l with an average value of 237 mg/l during the POM. Spatial distribution of bicarbonate concentration revealed that during PRM season the lowest and the highest values are observed from Battal Ballian village of Udhampur taluka (85 mg/l) and Chakras village of Gool taluka (480 mg/l), respectively. During POM season, the lowest value was observed in Simalari village of Udhampur taluka (79 mg/l) and the highest value was observed in Kermun village of Ramnagar taluka (445 mg/l). Maximum allowable limit of bicarbonates according to the WHO and BIS standards is 300 mg/l. Twenty-eight samples during PRM and 31 samples during POM seasons were found having values above the maximum allowable limits. The increase in HCO3− content in the groundwater samples is due to the agricultural return flow where dissolution of the precipitated carbonate minerals in the soil due to the high evaporation rates is a common process.
Chloride concentration ranges from 7.10 to 71 mg/with an average value of 15 mg/l during the PRM and 3.5 to 71 mg/l with an average value of 13 mg/l during the POM. Spatial distribution of chloride concentration revealed that during PRM season the lowest values are observed from Sail dabri, Numbal villages of Ramnagar taluka and Snwari village of Udhampur taluka (8 mg/l) and the highest values are observed from Panora village of Ramnagar taluka (71 mg/l). During the POM season, the lowest chloride concentration was observed in Narsu village of Chenani taluka (3.5 mg/l) and the highest concentration of the parameter was found in Panora village of Ramnagar taluka (71 mg/l). All the samples were found to have values within the maximum allowable limits of the WHO and BIS standards during both the seasons. Fluoride concentration varies from 0.07 to 0.60 mg/l with an average value of 0.28 mg/l during the PRM and 0.01 to 1.9 mg/l with an average value of 0.3 mg/l during the POM. Spatial distribution of fluoride concentration revealed that during the PRM season the lowest values are observed mostly around the villages of Ramnagar and a few villages of Reasi taluka (0.07 mg/l) and the highest value of the parameter was observed in Chachwal village of Chenani taluka (0.60 mg/l). During the POM season, the lowest fluoride concentration was observed in Kuh village of Udhampur taluka (0.01 mg/l) and the highest concentration of the parameter was observed in Dehahri village of Ramnagar taluka (1.9 mg/l). Allowable fluoride concentrations in potable waters as per the WHO and BIS standards is 1.5 mg/l. Except for one sample from Dehahri village from POM season, all other samples are having values within the maximum allowable limits during both the seasons. Concentrations higher than this can cause dental fluorosis, mild skeletal fluorosis and crippling skeletal fluorosis [48].
Nitrate concentration ranges from 10 to 76 mg/l with an average value of 23 mg/l during the PRM and 2.5 to 72 mg/l with an average value of 24 mg/l during the POM season. Spatial distribution of nitrate concentration showed that during the PRM season, the lowest values of the parameter are observed mostly around the Udhampur taluka, a few villages of Ramnagar, Reasi and Gool talukas (10 mg/l) and the highest value of the parameter was observed in Chowki Chuora village of Reasi taluka (76 mg/l). During the POM season, the lowest and the highest nitrate concentration was observed in Kud (2.5 mg/l) and Mauri (72 mg/l) villages of Chenani taluka. Five sampling sites were showing the nitrate values above the maximum allowable limits during the POM season, while nitrate values of all the GW samples during the PRM fall within the maximum allowable limits under WHO standards. High nitrate concentration in drinking water may cause methemoglobinemia or blue baby syndrome in infants. Continuous intake of nitrates in high concentration may lead to gastric problems and an increased risk of cancer [49,50,51]. However, according to the BIS standards, the nitrate values of all the samples during both the seasons fall within the portable water limits. Principle sources of nitrates in the GW are exposed disposal of human and animal waste, industrial trash related to food processing and sites where handling and accidental spills of nitrogenous materials may accumulate [52, 53]. Another potentially anthropogenic source of nitrogen contamination of groundwater is the use of nitrogen-rich fertilizers in farming [54].
Sulphate concentration in the samples ranges from 8 to 98 mg/l with an average value of 24 mg/l during the PRM and 6 to 98 mg/l with an average value of 25 mg/l during the POM. The spatial distribution of sulphate concentration revealed that during the PRM season the lowest values of the parameter are observed mostly around the villages of Reasi and Chenani talukas (8 mg/l) and the highest value of the parameter was observed in Panora village of Ramnagar taluka (98 mg/l). During the POM season, the lowest and the highest values of sulphate were observed in the Mauri village of Chenani taluka (6 mg/l) and Budhan village of Ramnagar taluka (98 mg/l), respectively. The concentration of sulphate is likely to adversely affect human organs if the value exceeds the maximum allowable limit of 400 mg/l and will cause a laxative effect on a human system with the excess magnesium in groundwater [55]. However, all the samples were found having values within the maximum allowable limits according to both the WHO and BIS standards.
4.4 Hydro-chemical Facies
The analysed GWQ data were plotted on a Piper trilinear diagram to understand and identify the hydro-geochemical regime during both the seasons (Fig. 5). Facies are recognizable parts of different characters fitting to any hereditarily interrelated system and highlight distinct zones that possess cation and anion concentration classes [56]. These plots include two triangles: one for plotting cations and other for plotting anions. The cation and anion fields are combined to show a single point in a diamond-shaped field, from which implications are drawn on the basis of hydro-geochemical facies concept [57]. Piper trilinear diagram is suitable for bringing out chemical relationships among groundwater samples in more definite terms compared to other conventional plotting methods. Ca–HCO3, Mg–HCO3, Ca–Mg–HCO3 and Na–HCO3 are the most common hydro-geochemical facies observed during the PRM. In the POM period, Ca–HCO3, Na–HCO3 and Ca–Mg–HCO3 facies predominate. From both the diagrams, it is evident that lithology and anthropogenic activities play a major role in governing the groundwater facies in the region. The Ca–Mg–HCO3 water type is regarded as recharged groundwater from sources connected to atmospheric precipitation and dissolution of carbonates. Na–HCO3 type is a strong indicator of cation interchange process [58, 59]. From the analysis of the data, it is evident that there is not a significant difference in the chemical properties of groundwater during the PRM and POM seasons.
4.5 Findings from Previous Work
Kanwar and Khanna [35] carried interpretation of hydro-chemical analysis and assessment of groundwater samples in accordance to the BIS standards of Udhampur–Dun Terrace belt and reveals that the most of the groundwater samples are suitable for drinking. The study concluded that the dominance of cations and anions in the area is in the order of Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42− > NO−3 . Similarly, NIH [34] studied the surface and groundwater quality evaluation in parts of Udhampur district, Jammu and Kashmir, during PRM and POM seasons in 1999. The study revealed that almost all the sampling sites fall under the Ca2+ > Mg2+ >HCO3− hydro-chemical facie during PRM and POM seasons. Further, assessment of groundwater quality with special emphasis on fluoride ions of Udhampur district, Jammu and Kashmir, was carried out by Kour and Kour [36] and revealed that most of the groundwater samples were in permissible limits and there is no dire need of defluoridation required. The findings of the current study are mostly in concurrence with that of the above-cited studies.
4.6 Spatiotemporal Variability of Water Quality Index
The CCME WQI was used to evaluate groundwater quality for drinking purposes and services. Canadian Water Quality Guidelines were applied to the CCME WQI calculator to assess spatial changes in the groundwater quality in the region. Figure 6 shows the spatial distribution of CCME WQI of groundwater samples during the PRM and POM seasons. The values of CCME WQI are categorized into four classes, viz. excellent, good, fair and marginal, during both the seasons. It was found that no sample falls in the poor class according to the CCME WQI. The WQI categories suggest that the groundwater in the major part of the study area falls under good category during both the PRM and POM seasons. A few of the sampling sites fall under the marginal WQI category during the PRM season like Katra (SID-34), Kirmichi (SID-45) and Baghad (SID-61). During the POM season, the sites falling the marginal WQI category are Baba Agarjito (SID-169), Mangruli (SID-203) and Udhampur (SID-173). These areas are highly populated, industrialized and tourism hubs in the Udhampur district [34]. Nala Ghoran (SID-151) sampling site falls under the excellent WQI category during the POM season. Malhad (SID-29), Dughaj (SID-83), Sukhwalgali (SID-17), Chachwal (SID-16), Dhaleran (SID-18), Seen Brahma (SID-50), snwari (SID-49), Kharkain (SID-78), Leha (SID-60), SayadPathri (SID-12) and Sangat (SID-75) sampling sites fall under the fair WQI category during the PRM season. Lakhniser (SID-125), areas around the Udhapmur town (SID-173), Chamba (SID-172), Kud (SID-190), Chenani (SID-168), Kotli (SID-165), Gool (SID-144), Arnas (SID-210), Chinkah (SID-187), Kakri (SID-199), Khera Lahir (SID-200) and Reasi (SID-178) sampling sites fall under fair WQI category during the POM season.
5 Conclusions
Most of the parameters, analysed from 211 GW samples collected during the PRM and POM seasons, were found to be within the permissible limits of the existing WHO and BIS standards. On the basis of the hardness, most of the region falls under the soft water category except a few samples. The major cation, calcium played a dominant role in determining the groundwater quality during the PRM and POM seasons. Among the major anions, bicarbonates played a dominant role in determining the GWQ during the PRM and POM seasons. The abundance sequence of cations is in the order of Ca2+ > Mg2+ > Na+ > K+ and Ca2+ > Na+ > Mg2+ > K+ during the PRM and POM seasons, respectively. Anions are represented in the order HCO3− > SO42− > NO3− > Cl− > F− during both the POM and PRM seasons. The Piper diagram revealed that the Ca–HCO3, Mg–HCO3, Ca–Mg–HCO3 and Na–HCO3 are the most common hydro-geochemical facies during the POM. However, during the PRM, Ca–HCO3, Na–HCO3 and Ca–Mg–HCO3 facies predominate. The use of GIS and CCME WQI provided valuable information about the spatial variability of groundwater parameters and WQI in the data-scarce mountainous Himalayan region. The groundwater quality evaluated in the district using CCME WQI mostly falls under good category during both the seasons. The CCME WQI helped to summarize the complex WQ data into a format that is easily comprehensible to the public, organizers, water distributors, administrators and policy makers. The increasing number of boreholes and tube well observed in the district is an indicator of the high rate of groundwater extraction to meet the rising demands of GW for various purposes. The extraction of groundwater, if done unsustainably, might put severe pressure on groundwater resources in the region and make the aquifer vulnerable to over-exploitation and water quality deterioration. Therefore, it is suggested that the groundwater extraction should be regulated and water quality analysis be carried out regularly to ensure sustainability of the depleting groundwater resources and to maintain the GWQ within the acceptable limits in this Himalayan region.
References
Yidana SM, Ophori D, Banoeng-Yakubo B (2008) A multivariate statistical analysis of surface water chemistry data—the Ankobra Basin, Ghana. J Environ Manage 86(1):80–87
Saka D, Akiti TT, Osae S, Appenteng MK, Gibrilla A (2013) Hydrogeochemistry and isotope studies of groundwater in the Ga West Municipal Area, Ghana. Appl Water Sci 3(3):577–588
Romshoo SA, Dar RA, Rashid I, Marazi I, Ali N, Zaz S (2015) Implications of shrinking cryosphere under changing climate on the streamflows in the lidder catchment in the Upper Indus Basin, India. Arct Antarct Alp Res 47(4):627–644
Murtaza KO, Romshoo SA (2016) Recent glacier changes in the Kashmir Alpine Himalayas, India. Geocarto Int 32(2):188–205
UNEP (1999) Dioxin and furan inventories-National and regional emission of PCDD/PCDF. United Nations Environmental Programme
Abbasi SA, Khan FI, Sentilvelan K, Shabudeen A (2002) Modelling of Buckingham canal water quality. Indian J Environ Health 44(4):290–297
Jagdap J, BhushanK Deshpande L, Kelkar P (2002) Water quality assessment of the Purnariver for irrigation purpose in Buldana District, Maharastra. Indian J Environ Health 44(3):47–257
Gupta SK, Deshpande RD, Agarwal M, Raval BR (2005) Origin of high fluoride in groundwater in the North Gujarat-Cambay region, India. Hydrogeol J 13(4):596–605
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50(7):1025–1039
Fantong WY, Satake H, Ayonghe SN, Aka FT, Asai K (2009) Hydrogeochemical controls and usability of groundwater in the semi-arid Mayo Tsanaga River Basin far north province, Cameroon. Environ Geol 58(6):1281–1293
Rather MI, Rashid I, Shahi N, Murtaza KO, Hassan K, Yousuf AR, Romshoo SA, Shah IY (2016) Massive land system changes impact water quality of the Jhelum River in Kashmir Himalaya. Environ Monit Assess 188(3):1–20
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeastern Europe. Desalination 213(1):159–173
Corcoran E (ed) (2010) Sick water? The central role of wastewater management in sustainable development: a rapid response assessment. UNEP/Earthprint
Balakrishnan P, Saleem A, Mallikarjun ND (2011) Groundwater quality mapping using geographic information system (GIS): a case study of Gulbarga City, Karnataka, India. Afr J Environ Sci Technol 5(12):1069–1084
Jeong CH (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253(1):194–210
Efe SI, Ogban FE, Horsfall MJ, Akporhonor EE (2005) Seasonal variations of physico-chemical characteristics in water resources quality in western Niger Delta region, Nigeria. J Appl Sci Environ Manag 9(1):191–195
Banoeng-Yakubo B, Yidana SM, Nti E (2009) Hydrochemical analysis of groundwater using multivariate statistical methods—the Volta Region, Ghana. KSCE J Civil Eng 13(1):55–63
Shankar PV, Kulkarni H, Krishnan S (2011) India’s groundwater challenge and the way forward. Econ Polit Wkly 46(2):37
Dar RA, Rashid I, Romshoo SA, Marazi A (2014) Sustainability of winter tourism in a changing climate over Kashmir Himalaya. Environ Monit Assess 186(4):2549–2562
Goodchild MF (1993) The state of GIS for environmental problem-solving. Environmental modeling with GIS 8-15
Lo CP, Yeung AK (2003) Concepts and techniques in geographic information systems: laboratory manual. Pearson Prentice Hall, Upper Saddle River
Naghibi SA, Pourghasemi HR, Dixon B (2016) GIS-based groundwater potential mapping using boosted regression tree, classification and regression tree, and random forest machine learning models in Iran. Environ Monit Assess 188(1):1–27
Shukla SM (2014) Spatial analysis for groundwater potential zones using GIS and Remote Sensing in the Tons basin of Allahabad district, Uttar Pradesh, (India). Proc Natl Acad Sci, India, Sect A Phys Sci 84(4):587–593
Romshoo SA, Dar RA, Murtaza KO, Rashid I, Dar FA (2017) Hydrochemical characterization and pollution assessment of groundwater in Jammu Siwaliks, India. Environ Monit Assess 189(3):122
Machiwal D, Jha MK, Mal BC (2011) GIS-based assessment and characterization of groundwater quality in a hard-rock hilly terrain of Western India. Environ Monit Assess 174(4):645–663
Selvam S, Manimaran G, Sivasubramanian P, Balasubramanian N, Seshunarayana T (2014) GIS-based evaluation of water quality index of groundwater resources around Tuticorin coastal city, South India. Environ Earth Sci 71(6):2847–2867
Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5(4):335–343
Lermontov A, Yokoyama L, Lermontov M, Machado MAS (2009) River quality analysis using fuzzy water quality index: ribeira do Iguape river watershed, Brazil. Ecol Ind 9(6):1188–1197
Venkatramanan S, Chung SY, Lee SY, Park N (2014) Assessment of river water quality via environmentric multivariate statistical tools and water quality index: a case study of Nakdong River basin, Korea. Carpathian J Earth Environ Sci 9(2):125–132
Chung SY, Venkatramanan S, Kim TH, Kim DS, Ramkumar T (2015) Influence of hydrogeochemical processes and assessment of suitability for groundwater uses in Busan City, Korea. Environ Dev Sustain 17(3):423–441
Magesh NS, Chandrasekar N (2013) Evaluation of spatial variations in groundwater quality by WQI and GIS technique: a case study of Virudunagar District, Tamil Nadu, India. Arab J Geosci 6(6):1883–1898
Haldar D, Halder S, Das P, Halder G (2014) Assessment of water quality of Damodar River in South Bengal region of India by Canadian Council of Ministers of Environment (CCME) water quality index: a case study. Desalination Water Treat 57(8):3489–3502
Selvam S, Manimaran G, Sivasubramanian P, Balasubramanian N, Seshunarayana T (2014) GIS-based evaluation of water quality index of groundwater resources around Tuticorin coastal city, South India. Environ Earth Sci 71(6):2847–2867
NIH (2000) Surface and groundwater quality evaluation in parts of Udhampur district J&K. National Institute of Hydrology, Roorkee, Uttarakhand
Kanwar P, Khanna P (2014) Hydrogeochemical evaluation of groundwater in shallow aquifer system of Udhampur-Dun Terrace, J&K. India. Int J Sci Res 3(5):644–647
Kour S, Kour A (2016) Assessment of water quality with special emphasis on fluoride ions of Udhampur District, J&K, India: correlation with physico-chemical parameters. Indian Journal of Applied Research 2(1):655–658
Dullo SN (1994) Hydrogeology and groundwater potential of Udhampur district, J&K, North Western Region, CGWB, Chandigarh
APHA (2005) Standard methods for the examination of water and wastewater, vol 21. American Public Health Association, Washington, pp 258–259
Lu GY, Wong DW (2008) An adaptive inverse-distance weighting spatial interpolation technique. Comput Geosci 34(9):1044–1055
BIS (2012) Bureau of Indian Standards drinking water specifications. BIS 10500:2012
WHO (2004) Guidelines for drinking water quality, vol 1. Recommendations, 3rd edn. WHO, Geneva 515
Burrough PA, McDonnell RA (1998) Principles of geographical information systems. Oxford University Press, New York
CCME (2001) Canadian water quality guidelines for the protection of aquatic life: Canadian Water Quality Index 1.0 Technical Report. In: Canadian environmental quality guidelines, 1999. Winnipeg, Manitoba
Freeze AR, Cherrey JA (1979) Groundwater. Prentice-Hall, New Jersey
Schoeller H (1965) Qualitative evaluation of groundwater resources. Methods and techniques of groundwater investigations and development. UNESCO, pp 54–83
CPCB (2008) Guideline for water quality management. Central Pollution Control Board, Parivesh Bhawan
Todd DK (1959) Groundwater hydrology. Wiley, New York, p 336
Pandey HK, Duggal SK, Jamatia A (2016) Fluoride contamination of groundwater and it’s hydrogeological evolution in District Sonbhadra (UP) India. Proc Natl Acad Sci, India, Sect A Phys Sci 86(1):81–93
Gupta SK, Gupta RC, Gupta AB, Seth AK, Bassin JK, Gupta A (2000) Recurrent acute respiratory tract infections in areas with high nitrate concentrations in drinking water. Environ Health Perspect 108(4):363
Eichholzer M, Gutzwiller F (1998) Dietary nitrates, nitrites, and N-nitroso compounds and cancer risk: a review of the epidemiologic evidence. Nutr Rev 56(4):95–105
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Heal Perspect 112(14):1371–1374
Keeney D, Olson RA (1986) Sources of nitrate to ground water. Crit Rev Environ Sci Technol 16(3):257–304
Randall GW, Mulla DJ (2001) Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural practices. J Environ Qual 30(2):337–344
Rashid I, Romshoo SA (2013) Impact of anthropogenic activities on water quality of Lidder River in Kashmir Himalayas. Environ Monit Assess 185(6):4705–4719
Hoelen TP, Reinhard M (2004) Complete biological dehalogenation of chlorinated ethylenes in sulfate containing groundwater. Biodegradation 15(6):395–403
Sawyer C, McCarthy P (1967) Chemical and sanitary engineering, 2nd edn. McGraw-Hill, New York
Hanshaw BB, Back W, Rubin M (1965) Radiocarbon determinations for estimating groundwater flow velocities in central Florida. Science 148(3669):494–495
Ayuba R, Tijani MN, Omonona OV (2017) Hydrochemical characteristics and quality assessment of groundwater from shallow wells in Gboloko Area, central Nigeria. Glob J Geol Sci 15(1):65–76
Gibrilla A, Osae S, Akiti TT, Adomako D, Ganyaglo SY, Bam EP, Hadisu A (2010) Hydrogeochemical and groundwater quality studies in the northern part of the Densu River Basin of Ghana. J Water Res Prot 2(12):1071
Acknowledgements
This research work has been accomplished under a research Grant provided by the National Remote Sensing Centre (NRSC), ISRO, Hyderabad, for the Project titled “Rajiv Gandhi National Drinking Water Mission (RGNDWM)—Phase IV”. The authors express their gratitude to the funding agency for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murtaza, K.O., Romhoo, S.A., Rashid, I. et al. Geospatial Assessment of Groundwater Quality in Udhampur District, Jammu and Kashmir, India. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 90, 883–897 (2020). https://doi.org/10.1007/s40010-019-00630-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-019-00630-7