Abstract
Reaction of 4-R-benzaldehyde thiosemicarbazone (denoted as HL-R; where H stands for the dissociable acidic proton and R (R = OCH3, CH3, H, Cl and NO2) for the substituents) with [Ru(CO)2Cl2]n in toluene in the presence of triethylamine affords a group of yellow complexes of the type [Ru(CO)2(L-R)2]. Structure of [Ru(CO)2(L-NO2)] has been determined by X-ray crystallography. In [Ru(CO)2(L-R)2] complexes, the thiosemicarbazone ligands are bound to the metal center as monoanionic bidentate N,S-donor forming four-membered chelate ring. All the complexes are diamagnetic, and show characteristic 1H NMR signals. The [Ru(CO)2(L-R)2] complexes show intense absorptions in the visible and ultraviolet regions, which have been analyzed by DFT calculations. Cyclic voltammetry on the complexes shows two irreversible oxidations near 0.87 and 1.28 V versus SCE, and an irreversible reduction around −1.24 V versus SCE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mixed-ligand ruthenium carbonyl complexes have been receiving considerable current attention, mainly because of their catalytic and biological applications [1–15]. Reactivity of such complexes is dictated by both the carbonyl and the other ancillary ligands. Binding of ligands of selected types to the metal center in the Ru(CO)n fragment is of significant importance for modulation of the properties of such mixed-ligand complexes. For the present study, which has originated from our interest in the chemistry of mixed-ligand ruthenium carbonyl complexes [16–20], we have selected a group of five 4-R-benzaldehyde thiosemicarbazones, abbreviated in general as HL-R, where H stands for the dissociable acidic proton and R for the substituent in the phenyl ring. Though the thiosemicarbazone complexes are cultivated usually for their bioinorganic relevance [21–23], we have been exploring the chemistry of transition metal complexes of the thiosemicarbazones mainly because of the variable binding mode displayed by these ligands in their complexes [16, 24–34]. For example, from our earlier studies we have found that the chosen thiosemicarbazones can bind to a metal center as N,S-donor forming four-membered chelate ring (I), as N,S-donor forming five-membered chelate ring (II), as C,N,S-donor forming (III), and as N,S-donor forming five-membered chelate ring with a change in geometry around the pre-existing C=N bond (IV) [24–34]. We were interested to examine the mode of binding of the selected thiosemicarbazones to ruthenium with carbonyl as the co-ligand. As the source of ruthenium, we chose [Ru(CO)2Cl2]n, as it can readily provide the Ru(CO)2 fragment and also because of its demonstrated ability to undergo facile reaction with organic ligands of different types [19]. Herein, we wish to report our findings on the formation of a group of new complexes through interaction of the selected thiosemicarbazones with [Ru(CO)2Cl2]n, their structures, and their spectral and electrochemical properties.

2 Experimental
2.1 Materials and methods
Ruthenium trichloride was purchased from Arora Matthey, Kolkata, India. [Ru(CO)2Cl2]n was prepared by following a reported procedure [35]. Thiosemicarbazide was procured from Spectrochem, Mumbai, India. The 4-R-benzaldehyde thiosemicarbazones (HL-R; R = OCH3, CH3, H, Cl and NO2) were prepared by reacting equimolar amounts of thiosemicarbazide and the respective 4-R-benzaldehyde in 1:1 ethanol–water mixture. All other chemicals and solvents were reagent grade commercial materials and were used as received. Tetrabutylammonium hexaflurophosphate (TBHP), obtained from Aldrich, and AR grade acetonitrile, procured from Merck, India, were used for electrochemical work.
2.2 Physical Measurements
Microanalyses (C, H, N) were performed using a Heraeus Carlo Erba 1108 elemental analyzer. Magnetic susceptibilities were measured using a Sherwood MK-1 balance. 1H NMR spectra recorded in CDCl3 solution on a Bruker Avance DPX 300 NMR spectrometer using TMS as the internal standard. IR spectra were obtained on a Perkin Elmer Spectrum Two spectrometer with samples prepared as KBr pellets. Electronic spectra were recorded on a JASCO V-630 spectrophotometer. Electrochemical measurements were made using a CH Instruments model 600A electrochemical analyzer. A platinum disc working electrode, a platinum wire auxiliary electrode and an aqueous saturated calomel reference electrode (SCE) were used in the cyclic voltammetry experiments. All electrochemical experiments were performed under a dinitrogen atmosphere. All electrochemical data were collected at 298 K and are uncorrected for junction potentials. Optimization of ground-state structure and energy calculation were carried out by density functional theory (DFT) method using the Gaussian 03 (B3LYP/SDD-6-31G) package [36].
2.3 Synthesis of Complexes
All the five [Ru(CO)2(L-R)2] complexes were prepared by following a general procedure. Specific details are given below for a particular complex.
[Ru(CO)2(L-OCH3)2]
To a solution of 4-methoxy-benzaldehyde thiosemicarbazone (101 mg, 0.48 mmol) in warm toluene (40 ml), triethylamine (49 mg, 0.48 mmol) was added. To this solution [Ru(CO)2Cl2]n (50 mg, 0.22 mmol) was added.Footnote 1 The mixture was refluxed for 3 h to yield an orangish-yellow solution. The solvent was evaporated and the solid mass thus obtained was subjected to purification by thin layer chromatography on a silica plate. With acetonitrile:benzene (1:2) as the eluant, a yellow band separated, which was extracted with acetonitrile. Evaporation of the acetonitrile extract gave [Ru(CO)2(L-OCH3)2] as a yellow crystalline solid. Yield: 61 %. Anal. calcd. for C20H20N6O4S2Ru: C, 41.88; H, 3.49; N, 14.66. Found: C, 41.96; H, 3.44; N, 14.68 %. 1H NMR (300 MHz, CDCl3)Footnote 2 δ: 3.84 (OCH3), 5.74 (s, NH2), 6.97 (d, J = 7.0, 2H), 7.58 (d, J = 8.0, 2H), 8.78 (s, azomethine). IR (cm−1): 3443, 3332, 2045, 1978, 1602, 1585, 1507, 1462, 1441, 1420, 1369, 1324, 1306, 1206, 1170, 1112, 1096, 1054, 1028, 953, 931, 873, 856, 831, 812, 775, 735, 722, 657, 583, 572, 526.
[Ru(CO)2(L-CH3)2]
Yield: 65 %. Anal. calcd. for C20H20N6O2S2Ru: C, 44.36; H, 3.69; N, 15.52. Found: C, 44.25; H, 3.71; N, 15.49 %. 1H NMR (300 MHz, CDCl3) δ: 2.35 (CH3), 5.77 (s, NH2), 7.19 (d, J = 6.6, 2H), 7.54 (d, J = 8.1, 2H), 8.79 (s, azomethine). IR (cm−1): 3441, 3335, 2044, 1974, 1603, 1584, 1507, 1411, 1367, 1325, 1310, 1291, 1227, 1211, 1178, 1111, 1098, 1054, 1018, 958, 935, 873, 854, 814, 774, 735, 711, 657, 583, 574, 513.
[Ru(CO)2(L-H)2]
Yield: 62 %. Anal. calcd. for C18H16N6O2S2Ru: C, 42.09; H, 3.12; N, 16.37. Found: C, 42.17; H, 3.15; N, 16.41 %. 1H NMR (300 MHz, CDCl3) δ: 5.78 (s, NH2), 7.35 (d, J = 8.0, 2H), 7.41 (d, J = 8.1, 2H), 7.67 (t, J = 7.0, H), 8.82 (s, azomethine). IR (cm−1): 3445, 3330, 2045, 1975, 1602, 1583, 1509, 1487, 1416, 1369, 1325, 1306, 1287, 1223, 1198, 1180, 1156, 1106, 1096, 1052, 1026, 950, 936, 869, 845, 808, 779, 755, 722, 691, 660, 626, 583, 574, 508.
[Ru(CO)2(L-Cl)2]
Yield: 61 %. Anal. calcd. for C18H14N6O2S2Cl2Ru: C, 37.11; H, 2.41; N, 14.43. Found: C, 37.20; H, 2.39; N, 14.46 %. 1H NMR (300 MHz, CDCl3) δ: 5.81 (s, NH2), 7.34 (d, J = 7.8, 2H), 7.59 (d, J = 8.1, 2H), 8.78 (s, azomethine). IR (cm−1): 3443, 3331, 2046, 1978, 1596, 1583, 1511, 1488, 1416, 1403, 1384, 1330, 1300, 1281, 1248, 1217, 1200, 1175, 1147, 1090, 1057, 1012, 956, 935, 866, 851, 824, 810, 741, 729, 710, 685, 667, 625, 592, 580, 511.
[Ru(CO)2(L-NO2)2]
Yield: 59 %. Anal. calcd. for C18H14N8O6S2Ru: C, 35.82; H, 2.32; N, 18.57. Found: C, 35.73; H, 2.29; N, 18.53 %. 1H NMR (300 MHz, CDCl3) δ: 5.92 (s, NH2), 7.79 (d, J = 8.7, 2H), 8.23 (d, J = 8.7, 2H), 8.88 (s, azomethine). IR (cm−1): 3444, 3332, 2042, 1974, 1600, 1590, 1513, 1489, 1409, 1384, 1331, 1298, 1256, 1225, 1197, 1173, 1149, 1104, 1055, 1011, 963, 934, 876, 853, 839, 804, 750, 690, 658, 626, 592, 579, 502.
2.3.1 X-Ray Crystallography
Single crystals of the [Ru(CO)2(L-NO2)2] complex were grown by slow evaporation of solvent from a solution of the complex in acetonitrile. Selected crystal data and data collection parameters are given in Table 1. Data were collected on a Bruker SMART CCD diffractometer using graphite monochromated MoKα radiation (λ = 0.71073 Å). X-ray data reduction and, structure solution and refinement were done using SHELXS-97 and SHELXL-97 programs [37]. The structure was solved by the direct methods.
3 Results and Discussion
3.1 Synthesis and Characterization
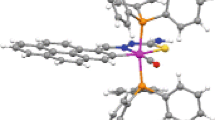
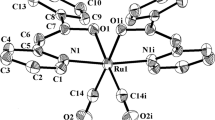
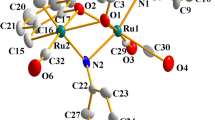
As delineated in the introduction, the initial aim of the present study was to study the interaction of the selected 4-R-benzaldehyde thiosemicarbazones (HL-R) with [Ru(CO)2Cl2]n and see how they bind to the metal center. Five different substituents in the thiosemicarbazone ligands, with different electron withdrawing properties, were chosen to study their influence, if any, on the redox properties of the resulting complexes. Reactions of the selected thiosemicarbazones (HL-R) with [Ru(CO)2Cl2]n proceeded smoothly in refluxing toluene to afford a group of yellow complexes of the type, viz. [Ru(CO)2(L-R)2], in decent yields. Preliminary characterization data (microanalysis, IR, NMR, etc.) of the complexes were found to be consistent with their compositions. In order to find out the stereochemistry of the complexes, as well as to ascertain coordination mode of the thiosemicarbazone ligands, structure of a representative member of this series, viz. [Ru(CO)2(L-NO2)2], was determined by X-ray crystallography. The structure is shown in Fig. 1 and some relevant bond distances and bond angles are listed in Table 2. The structure shows that both the thiosemicarbazones are coordinated to ruthenium, as N,S-donors in a bidentate fashion and forming four-membered chelate ring (I). Two carbonyls are also coordinated to the metal center. Ruthenium is thus having a distorted octahedral N2S2C2 coordination environment, where both nitrogen atoms are cis, both sulfur atoms are trans, and both carbon atoms are cis. The Ru–N, Ru–S and Ru–C distances are quite normal and are within the accepted values for a chelated thiosemicarbazone [24–34], and coordinated carbonyls [16–20]. The presence of an acetonitrile as solvent of crystallization per molecule of [Ru(CO)2(L-NO2)2] in the crystal lattice indicates existence of non-covalent interactions between the solvent and complex molecule, in addition to those between the complex molecules. A closer look at the packing pattern in the crystal lattice (Fig. 2) reveals that intermolecular interactions of four different types, viz. N–H···N(CH3CN), N–H···O(NO2), N–H···O(CO) and π···π interactions, are active in the lattice. Each complex molecule is thus linked with the surrounding complex molecules through such non-covalent interactions, and these extended intermolecular interactions seem to be responsible for holding the crystal together. As all the [Ru(CO)2(L-R)2] complexes were synthesized similarly and they show similar properties (vide infra), the other four [Ru(CO)2(L-R)2] complexes (with R ≠ NO2) are assumed to have similar structures as the [Ru(CO)2(L-NO2)2] complex.
3.2 Spectral Studies
Magnetic susceptibility measurements show that all the ruthenium complexes are diamagnetic, which corresponds to the +2 oxidation state of ruthenium (low-spin d6, S = 0). 1H NMR spectra of all [Ru(CO)2(L-R)2] complexes are found to be consistent with the C2 symmetry present in the molecule. The [Ru(CO)2(L-OCH3)2] and [Ru(CO)2(L-CH3)2] complexes show a sharp signal at 3.78 and 2.35 ppm respectively, for the methoxy and the methyl groups. From the coordinated thiosemicarbazone, signal for the NH2 group is observed near 5.7 ppm, signal for the azomethine proton is observed around 8.8 ppm, and the aromatic proton signals are observed in the expected region.
Infrared spectrum of each [Ru(CO)2(L-R)2] complex shows many bands of different intensities in the 400–4000 cm−1 region. Attempt has not been made to assign each band to a specific vibration. However, the N–H stretch, observed near 3290 cm−1 in the uncoordinated ligand, is found to be absent in the complexes, confirming de-protonation of the thiosemicarbazone ligand. Two weak bands have been found in all the spectra around 3443 and 3332 cm−1, which are attributable to the –NH2 fragment. Two strong bands have been observed near 2045 and 1975 cm−1 in all the [Ru(CO)2(L-R)2] complexes, indicating the presence of two coordinated carbonyl ligands. Several sharp bands (e.g. near 1602, 1583, 1509, 1416, 1369, 1325, 1306, 1287, 1180, 1096, 1052, 950, 869, 845, 808, 779, 722, 660, 583 and 508 cm−1) are also observed in the [Ru(CO)2(L-R)2] complexes, which are attributable to the coordinated thiosemicarbazone ligands. The 1H NMR and infrared spectral data are therefore found to be consistent with the composition and stereochemistry of the [Ru(CO)2(L-R)2] complexes.
The [Ru(CO)2(L-R)2] complexes are found to be readily soluble in dichloromethane, methanol, ethanol, chloroform, acetonitrile, etc., producing bright yellow solutions. Electronic spectra of the complexes were recorded in dichloromethane solution. Each [Ru(CO)2(L-R)2] complex shows two intense absorptions near 510 and 344 nm. Spectral data are presented in Table 3. The absorption in the ultraviolet region is attributable to a transition within the ligand orbitals. To have an insight into the nature of absorption in the visible region, DFT calculations were performed on the [Ru(CO)2(L-R)2] complexes [36]. Compositions of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are given in Table 4 and contour plots of these molecular orbitals for a representative complex is shown in Fig. 3. For all the five complexes, the HOMO is found to have maximum (>80 %) contribution from a coordinated thiosemicarbazone, while the LUMO is found to be delocalized mostly (>90 %) on the coordinated thiosemicarbazones.Footnote 3 The lowest energy absorption displayed by the [Ru(CO)2(L-R)2] complexes near 510 nm is therefore assignable to a transition from a filled orbital of a coordinated thiosemicarbazone to a vacant orbital spread almost equally over the coordinated thiosemicarbazones (see Footnote 3).
3.3 Electrochemical Properties
Electrochemical properties of the [Ru(CO)2(L-R)2] complexes have been studied by cyclic voltammetry in acetonitrile solution (0.1 M TBHP). Voltammetric data are given in Table 3 and a selected voltammogram is shown in Fig. 4. Each complex shows two oxidative responses on the positive side of SCE and a reductive response on the negative side. All the responses are found to be irreversible in nature. In view of composition of the HOMO in all these complexes, the first oxidative response near 0.87 V versus SCE is assigned to oxidation of a coordinated thiosemicarbazone ligand. Similarly, based on the composition of the LUMO, the reduction near −1.24 V versus SCE is assigned to reduction of the second coordinated thiosemicarbazone ligand. The second oxidative response near 1.28 V versus SCE is tentatively assigned to ruthenium(II)–ruthenium(III) oxidation. Potential of the redox responses does not show any systematic variation with the nature of the substituent R in the thiosemicarbazone ligand.
4 Conclusion
The present study shows that the 4-R-benzaldehyde thiosemicarbazones (HL-R) can readily interact with [Ru(CO)2Cl2]n to afford a group of ruthenium carbonyl complexes of type [Ru(CO)2(L-R)2], where the thiosemicarbazones display N,S-binding mode (I) forming a four-membered chelate ring. Presence of two mutually cis carbonyl ligands, together with the two anionic N,S-coordinated thiosemicarbazones, has made these complexes good candidates for application in catalysis, which is currently under exploration.
5 Supporting Information
Crystallographic data have been deposited with the Cambridge Crystallographic Data Center, CCDC 1486586.
Notes
The mmol calculation was done based on the mass of the repeating Ru(CO)2Cl2 fragment.
Chemical shifts are given in ppm and multiplicity of the signals along with the associated coupling constants (J in Hz) are given in parentheses. Overlapping signals are marked with an asterisk.
For [Ru(CO)2(L-NO2)2] the LUMO is spread over mostly (~90%) on one of the two coordinated thiosemicarbazones.
References
Tamizh MM, Mereiter K, Kirchner K, Karvembu R (2012) Ruthenium(II) carbonyl complexes containing ‘pincer like’ ONS donor Schiff base and triphenylphosphine as catalyst for selective oxidation of alcohols at room temperature. J Organomet Chem 700:194–201
Kalaivani P, Prabhakaran R, Poornima P, Dallemer F, Vijayalakshmi K, Padma VV, Natarajan K (2012) Versatile coordination behavior of salicylaldehyde thiosemicarbazone in ruthenium(II) carbonyl complexes: synthesis, spectral, X-ray, electrochemistry, DNA binding, cytotoxicity, and cellular uptake studies. Organometallics 31:8323–8332
Prabhu RN, Ramesh R (2012) Synthesis, structural characterization, electrochemistry and catalytic transfer hydrogenation of ruthenium(II) carbonyl complexes containing tridentate benzoylhydrazone ligands. J Organomet Chem 718:43–51
Fagundes FD, da Silva JP, Veber CL, Barison A, Pinheiro CB, Back DF, de Sousa JR, de Araujo MP (2012) Ruthenium-carbonyl complexes with P/O or P/N donor ligands: effect of the chelate ring size and donor atom. Polyhedron 42:207–215
Naziruddin AR, Huang ZJ, Lai WC, Lin WJ, Hwang WS (2013) Ruthenium(II) carbonyl complexes bearing CCC-pincer bis-(carbene) ligands: synthesis, structures and activities toward recycle transfer hydrogenation reactions. Dalton Trans 42:13161–13171
Cavarzan DA, Fagundes FD, Fuganti O, da Silva CWP, Pinheiro CB, Back DF, Barison A, Bogado AL, de Araujo MP (2013) Mixed phosphine/diimines and/or amines ruthenium carbonyl complexes: synthesis, characterization and transfer-hydrogenation. Polyhedron 62:75–82
Vijayan P, Viswanathamurthi P, Silambarasan V, Velmurugan D, Velmurugan K, Nandhakumar R, Butcher RJ, Silambarasan T, Dhandapani R (2014) Dissymmetric thiosemicarbazone ligands containing substituted aldehyde arm and their ruthenium(II) carbonyl complexes with PPh3/AsPh3 as ancillary ligands: synthesis, structural characterization, DNA/BSA interaction and in vitro anticancer activity. J Organomet Chem 768:163–177
Ramachandran R, Prakash G, Nirmala M, Periasamy V, Malecki JG (2015) Ruthenium(II) carbonyl complexes designed with arsine and PNO/PNS ligands as catalysts for N-alkylation of amines via hydrogen autotransfer process. J Organomet Chem 791:130–140
Graux LV, Giorgi M, Buono G, Clavier H (2015) Ruthenium carbonyl complexes bearing secondary phosphine oxides and phosphinous acids: synthesis, characterization, and application in catalysis. Organometallics 34:1864–1871
Pranckevicius C, Fan L, Stephan DW (2015) Cyclic bent allene hydrido-carbonyl complexes of ruthenium: highly active catalysts for hydrogenation of olefins. J Am Chem Soc 137:5582–5589
Cheng CH, Guo RY, Cui Q, Song HB, Tang LF (2015) Synthesis and catalytic activity of N-heterocyclic carbene metal carbonyl complexes based on 1-[2-(pyrazol-1-yl)phenyl]imidazole. Trans Met Chem 40:297–304
Manikandan R, Anitha P, Prakash G, Vijayan P, Viswanathamurthi P, Butcher RJ, Malecki JG (2015) Ruthenium(II) carbonyl complexes containing pyridoxal thiosemicarbazone and trans-bis(triphenylphosphine/arsine): synthesis, structure and their recyclable catalysis of nitriles to amides and synthesis of imidazolines. J Mol Catal A Chem 398:312–324
Suganthy PK, Prabhu RN, Sridevi VS (2015) Synthesis, structural characterization and catalytic transfer hydrogenation of ruthenium(II) carbonyl complexes bearing N,N,O pincer type benzoylhydrazone ligands. Polyhedron 88:57–62
Kuhn PS, Meier SM, Jovanović KK, Sandler I, Freitag L, Novitchi G, González L, Radulović S, Arion VB (2016) Ruthenium carbonyl complexes with azole heterocycles: synthesis, X-ray diffraction structures, DFT calculations, solution behavior, and antiproliferative activity. Eur J Inorg Chem 1566–1576
Selvamurugan S, Ramachandran R, Prakash G, Viswanathamurthi P, Malecki JG, Endo A (2016) Ruthenium(II) carbonyl complexes containing bidentate 2-oxo-1,2-dihydroquinoline-3-carbaldehyde hydrazone ligands as efficient catalysts for catalytic amidation reaction. J Organomet Chem 803:119–127
Saha Chowdhury N, Seth DK, Drew MGB, Bhattacharya S (2011) Ruthenium mediated C–H activation of benzaldehyde thiosemicarbazones. Synthesis, structure and spectral and electrochemical properties of the resulting complexes. Inorg Chim Acta 372:183–190
Datta S, Seth DK, Halder S, Sheldrick WS, Mayer-Figge H, Drew MGB, Bhattacharya S (2012) Mononuclear palladium and heterodinuclear palladium–ruthenium complexes of semicarbazone ligands. Synthesis, characterization, and application in C–C cross-coupling reactions. RSC Adv 2:5254–5264
Saha Chowdhury N, GuhaRoy C, Butcher RJ, Bhattacharya S (2013) Mixed-ligand 1,3-diaryltriazenide complexes of ruthenium: synthesis, structure and catalytic properties. Inorg Chim Acta 406:20–26
Dey BK, Dutta J, Drew MGB, Bhattacharya S (2014) Chloro–ruthenium complexes with carbonyl and N-(aryl)pyridine-2-aldimines as ancillary ligands. Synthesis, characterization and catalytic application in C–C cross-coupling of arylaldehydes with arylboronic acids. J Organomet Chem 750:176–184
Dutta J, Richmond MG, Bhattacharya S (2014) Cycloruthenation of N-(naphthyl)salicylaldimine and related ligands: utilization of Ru–C bond in catalytic transfer hydrogenation. Eur J Inorg Chem 4600–4610
Garoufis A, Hadjikakou SK, Hadjiliadis N (2009) Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord Chem Rev 253:1384–1397
Lobana TS, Sharma R, Bawa G, Khanna S (2009) Bonding and structure trends of thiosemicarbazone derivatives of metals—an overview. Coord Chem Rev 253:977–1055
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Advances in copper complexes as anticancer agents. Chem Rev 114:815–862
Basu S, Acharyya R, Basuli F, Peng SM, Lee GH, Mostafa G, Bhattacharya S (2010) Iridium assisted S–H and C–H activation of benzaldehyde thiosemicarbazones. Synthesis, structure and electrochemical properties of the resulting complexes. Inorg Chim Acta 363:2848–2856
Paul P, Datta S, Halder S, Acharyya R, Basuli F, Butcher RJ, Peng SM, Lee GH, Castineiras A, Drew MGB, Bhattacharya S (2011) Syntheses, structures and efficient catalysis for C–C coupling of some benzaldehyde thiosemicarbazone complexes of palladium. J Mol Catal A Chem 344:62–74
Datta S, Seth DK, Butcher RJ, Bhattacharya S (2011) Mixed-ligand thiosemicarbazone complexes of nickel. Synthesis, structure and catalytic activity. Inorg Chim Acta 377:120–128
Seth DK, Bhattacharya S (2011) Reactivity of the sulfur center in rhodium-bound benzaldehyde thiosemicarbazones towards molecular oxygen. A theoretical investigation. J Organomet Chem 696:3779–3784
Halder S, Paul P, Peng SM, Lee GH, Mukherjee A, Dutta S, Sanyal U, Bhattacharya S (2012) Benzaldehyde thiosemicarbazone complexes of platinum: syntheses, structures and cytotoxic properties. Polyhedron 45:177–184
Datta S, Seth DK, Butcher RJ, Gangopadhyay S, Karmakar P, Bhattacharya S (2012) Nickel complexes of some thiosemicarbazones: synthesis, structure, catalytic properties and cytotoxicity studies. Inorg Chim Acta 392:118–130
Dutta J, Datta S, Seth DK, Bhattacharya S (2012) Mixed-ligand benzaldehyde thiosemicarbazone complexes of palladium containing N,O-donor ancillary ligands. Syntheses, structures and catalytic application in C–C and C–N coupling reactions. RSC Adv 2:11751–11763
Paul P, Sengupta P, Bhattacharya S (2013) Palladium mediated C–H bond activation of thiosemicarbazones: catalytic application of organopalladium complexes in C–C and C–N coupling reactions. J Organomet Chem 724:281–288
Dutta J, Bhattacharya S (2013) Controlled interaction of benzaldehyde thiosemicarbazones with palladium: formation of bis-complexes with cis-geometry and organopalladium complexes, and their catalytic application in C–C and C–N coupling. RSC Adv 3:10707–10721
Paul P, Seth DK, Richmond MG, Bhattacharya S (2014) Unusual chemical transformations of acetone thiosemicarbazone mediated by ruthenium: C–H bond activation, thiolation, and C–N bond cleavage. RSC Adv 4:1432–1440
Paul P, Butcher RJ, Bhattacharya S (2015) Palladium complexes of 2-formylpyridine thiosemicarbazone and two related ligands: synthesis, structure and spectral and catalytic properties. Inorg Chim Acta 725:67–75
Anderson PA, Deacon GB, Haarmann KH, Keene FR, Meyer TJ, Reitsma DA, Skelton BW, Strouse GF, Thomas NC, Treadway JA, White AH (1995) Designed synthesis of mononuclear tris(heteroleptic) ruthenium complexes containing bidentate polypyridyl ligands. Inorg Chem 34:6145–6157
Frisch MJ, Tracks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR Jr, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalamani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa I, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskroz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision D01. Gaussian Inc, Pittsburgh
Sheldrick GM (1997) SHELXS-97 and SHELXL-97, Fortran programs for crystal structure solution and refinement. University of Gottingen, Gottingen
Acknowledgments
Financial assistance received from the Department of Science and Technology, Government of West Bengal, Kolkata [Sanction No. 746(Sanc.)/ST/P/S&T/2G-4/2013)], University Grants Commission, New Delhi [Sanction No. F.19-122/2014(BSR)] and Council of Scientific and Industrial research, New Delhi [Grant No. 01(2788)/14/EMR-II] is gratefully acknowledged. The authors thank Dr. Saurabh Das (Department of Chemistry, Jadavpur University) for his help in recording the electronic spectra of the complexes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deb, R., Paul, P. & Bhattacharya, S. Ruthenium Carbonyl Complexes with 4-R-Benzaldehyde Thiosemicarbazone as an Ancillary Ligand: Synthesis and, Structural, Spectral and Electrochemical Properties. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 86, 551–559 (2016). https://doi.org/10.1007/s40010-016-0303-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-016-0303-z