Abstract
Glioblastoma multiforme is a fast-growing malignant brain tumor with poor prognosis and low survival rate. Here we investigated the potential use of anticancer drug dioxadet loaded into nanogels for glioma treatment. We used block copolymer of polyethylene glycol and polymethacrylic acid for synthesis of dioxadet carriers and two types of cross-linking agents: non-degradable ethylenediamine and biodegradable cystamine, containing disulfide bond. We analyzed physicochemical properties of nanoparticles, their loading capacity, cytotoxicity of drug-loaded nanogels, and also their internalization into glioma cells. We found the optimal conditions that promote the efficient loading of the drug. We demonstrated that dioxadet loaded nanogels have relatively high level of loading capacity (>35% w/w) and loading efficiency (>75%). We shown that nanogels with the biodegradable cross-links prone to dissociate under reducing conditions (glutathione) that allow to decrease IC50 values of the drug compared to the nanogels with ethylendiamine cross-links. This stimuli-sensitive behavior of nanogels could be beneficial for tumor treatment. Confocal analysis of glioma cells demonstrated that both types of nanogels accumulate in cells and localize in lysosomes. These results indicate that loading of dioxadet into nanoparticles can improve its performance; such formulation has a potential for further studies and practical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along with surgery and radiotherapy, chemotherapy is currently one of the basic methods of tumor treatment (Siegel et al. 2012). There are a lot of chemotherapeutic drugs with different mechanisms of action developed for cancer treatment: inter and intrastrand crosslinking of DNA (cisplatin) (Fuertes et al. 2003), induction of the microtubule dysfunction (paclitaxel) (Horwitz 1994); alkylation (carmustine) (Jones et al. 1993) or intercalation of DNA molecule (doxorubicin) (Bodley et al. 1989). Among all mentioned types of drugs, alkylating agents are one of the earliest classes of drug to treat cancer that includes different categories: triazines, nitrosoureas, nitrogen mustards, alkyl sulfonates and ethylenimines. They are most effective in therapy of cancers that grow slowly, like solid tumors (i.e., glioblastoma) and leukemia, and also used for treatment of other types of tumors.
Dioxadet (DD) is a derivative of symmetrical triazine (the group of alkylating compounds aziridines), was developed in the N. N. Petrov Research Institute of Oncology (Stukov et al. 2011). Besides, triazine family of alkylating agents includes temozolomide and procarbazine that have already widely used to treat brain tumor. Its mechanism of action is the alkylation of DNA nucleobases, which leads to the disturbances in their structure and functions, and also impairs the process of the cell division and following apoptosis (Loshadkin et al. 2002). Previously, Bespalov et al. (2013) demonstrated that DD has antitumor effect comparable with cisplatin, however, it has lower systemic toxicity. Moreover, DD showed no cardio-, pneumo-, nephro- and neurotoxicity as other chemotherapeutic drugs (nephrotoxicity of cisplatin, cardiotoxicity of doxorubicin). Generally it was shown that the main limitation of DD is inhibition of hematopoiesis in high doses, which, however, returns to normal in 2–3 weeks after drug removal. Moreover, a structure of DD has a beneficial hydrophobic–hydrophilic balance, which allows using both aqueous and oil media, for example, for chemoembolization (Stukov et al. 2011).
However, in spite of the advantages of DD mentioned above, we sought to improve its stability, therapeutic effectiveness, and to decrease its toxic effect towards non-tumor cells. One of approaches could be incorporation of the drug into delivery system to increase blood circulation time (Kabanov and Vinogradov 2009).
The choice of the delivery system mainly depends on the molecular properties of the drug: its hydrophilic and hydrophobic properties, and the presence of the charged groups. Thus, in DD molecule there is a protonated secondary amino group that allows considering the electrostatic binding with the carrier as the main mechanism of the drug loading into the selected nanocontainer. Thereby, among the different systems, nanocontainers based on the amphiphilic block copolymers (such as nanogels) seems to be the most appropriate (Nukolova et al. 2011). This type of the nanocontainers are studied by many research groups (Yokoyama et al. 1998; Bronich et al. 1999; Tian et al. 2007) because their amphiphilic properties, narrow size distribution of particles, and dispersion stability.
In this study we used nanogels based on block copolymer of polyethylene glycol and polymethacrylic acid (PEG-b-PMAA) as a delivery system for DD. Several advantages of this type of nanocontainers compared to other delivery systems were previously shown: high loading capacity (Kabanov and Vinogradov 2009), sustained and controlled drug release (Bontha et al. 2006), and also its sensitivity to external stimuli (pH, ionic strength, etc.) (Bae et al. 2005). Moreover, the use of biodegradable cross-linking agents (cystamine, methacrylic acid alginate, etc.) provides selective release of the drug in tumor cells due to the difference of glutathione concentrations between blood plasma and cells (Kim et al. 2010). The other feature of these nanocontainers is their core–shell structure, where shell is constructed of PEG. This coating allows to prolongate the circulation time of nanoparticles in the blood (Kataoka et al. 2000), and, hence, improves its therapeutic effect due to more pronounced accumulation of the drug in tumor [the enhanced permeability and retention (EPR) effect, Maeda 2001].
In this study we synthesized nanogels for delivery of DD using two types of cross-linking agents: non-degradable ethylenediamine and biodegradable cystamine, containing disulfide bond. We analyzed physicochemical properties of loaded nanoparticles, capacity and effectiveness of loading, cytotoxicity of drug-loaded nanogels, and also their internalization into glioma cells.
Materials and methods
Materials
Diblock copolymer of polyethylene glycol (PEG) and polymethacrylic acid (PMAA) with terminal methoxy group in PEG block was purchased from Polymer Source Inc., Canada. The block lengths were 170 and 180 repeating units for PEG and PMAA, respectively (PEG170-b-PMAA180, Mn = 23,000, Mw/Mn = 1.45). Dioxadet was provided by our colleagues from N. N. Petrov Research Institute of Oncology (Saint-Petersburg, Russia). Ethylenediamine (ED), cystamine (CYS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), ethylenediaminetetraacetic acid (EDTA), glutathione, calcium chloride, 6-aminofluorescein were bought from Sigma-Aldrich, USA. Centrifugal filters Amicon™Ultra (MWCO 10, 30, 50 kDa) were provided by Milipore, USA. Desalting NAP10 and PD10 columns were bought from GE Healthcare Life Sciences (USA), CellTiter 96® AQueous MTS Reagent Powder was bought from Promega (USA).
Synthesis of nanocontainers
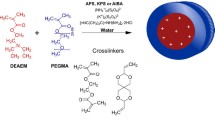
Synthesis of nanogels was performed as previously described (Kim et al. 2009). Briefly, block ionomer complexes of polyethylene glycol and polymethacrylic acid (PEG-b-PMAA) and calcium ions were formed in water with molar ratio [Ca2+]/[COO−] = 1.1–1.3. Carboxyl groups of obtained particles were activated using EDC and then were conjugated with ED or CYS in molar ratio [COOH]:[EDC]:[ED] = 1:5:10 for 12 h, room temperature (r.t.). After that calcium ions were removed by chelating with EDTA and following dialysis (0.5% NH4OH for 4 h, H2O for 24 h). Then the solution was concentrated on the centrifugal filters (30 kD, 2000 rpm for 15 min), filtered (0.22 μM, Fisherbrand, USA) and stored at +4 °С until further use.
To analyze internalization with confocal microscopy fluorescein-labeled nanogels were synthesized. For that purpose, PEG-b-PMAA [C(COO−) = 210 mM] was mixed with EDC (5 min, r.t.) at molar ratio [COO−]/[EDC] = 1, and then with 6-aminofluorescein (6-AF) at molar ratio [COO−]/[6-AF] = 10. The mixture was incubated for 12 h at r.t., dialyzed against water to remove unbound fluorescein and concentrated (Amicon filters, MWCO 30 kDa), then stored at +4 °С. After that, nanocontainers were synthesized from the fluorescein-labeled polymer as described earlier.
Dioxadet loading into nanocontainers
The obtained particles were loaded with the drugs by mixing of water solutions of nanocontainers and DD at different pH (7–9) and concentration (drug/nanocontainer) ratio (0.25–2) for 12 h, r.t. Unbound drug was removed using gel-filtration chromatography (Sephadex G-25). As a result, we have obtained the samples of DD-loaded nanocontainers with ethylenediamine (NG-ED-DD) and cystamine as cross-linking agents (NG-CYS-DD).
The amount of incorporated drug was evaluated using absorption spectrophotometry on 221 nm (Hitachi UV-2800, Japan). Loading efficiency was calculated as the ratio of loaded drug to the total weight of the drug added in the process of loading. Loading capacity was estimated as the ratio of the loaded drug to the total weight of the DD-nanogel.
Size and ζ-potential of nanoparticles
Dynamic light scattering
Size and ζ-potential of synthesized nanocontainers with or without DD were evaluated using dynamic light scattering analysis with Zetasizer Nano ZS (Malvern Instrument, UK). For this purpose sample was diluted to the concentration of 10 μg/ml and then filtered with 0.22 μM filter (Corning, Germany). Mean values of hydrodynamic diameter and ζ-potential for all samples were calculated as a result of at least three measurements. Data are shown as average value ± SD.
Atomic force microscopy
To perform measurements using atomic force microscopy, 5 μl of nanogel water suspension (1 mg/ml) was placed on micaceous plate and dried, then the measurements were performed followed by drying. The measurement was made using NT-MDT NEXT device on air in a tapping mode with 5 μm/s scanning rate and NT-MDT HA_HR cantilever with force constant 34 N/m. Obtained pictures were then processed with Gwyddion software (Czech Metrology Institute, Czech Republic).
Evaluation of nanocontainers colloidal stability
The stability of nanogels stored at 4 °C was studied for 12 days. The analysis was based on the data on hydrodynamic diameter of the particles.
Cell lines
Cell cultures of rat glioma (C6), human glioblastoma (U87) and Chinese hamster ovarian cells (CHO-K1) were incubated in DMEM supplemented with 1 mM of sodium pyruvate, 2 mM of l-glutamine, 1% of antibiotic–antimycotic, and 10% of fetal bovine serum. Human glioblastoma U-87 MG line (ATCC HTB-14) was cultured in complete DMEM with low concentration of d-glucose (1 g/l). Cells were cultured in the plastic flasks (Corning, USA) in humidified atmosphere with 5% CO2 at 37 °С until monolayer confluency value 80–100%. Cell dissociation was performed using 0.125% trypsin–EDTA. All growth media and supplements were bought from Thermo Fisher Scientific (Invitrogen™, USA).
Cytotoxicity of DD and DD-nanogels
Firstly, cytotoxicity of the free drug was analyzed with MTS-assay (Mosmann 1983) on several cell cultures: C6, U87 and CHO-K1. Besides, dependence of a cytotoxicity on pH of the drug solution was analyzed on the CHO-K1 cell line. Second, cytotoxicity of DD-loaded nanocontainers with different cross-linking agents (NG-CYS-DD and NG-ED-DD) was conducted on rat glioma (C6) and human glioblastoma cell lines (U87). For all experiments standard protocol was followed. Briefly, the studied samples were added in the concentrations from 2 μg/ml to 5 mg/ml and incubated for 24 h (37 °С, 5% СО2). Next day, the cells were washed, replaced with a new medium and incubated for additional 24 h. Then cells were incubated with MTS-reagent for 4 h and measure the optical density was measured using Victor X3 (PerkinElmer, USA). Dose–response curve was plotted to find the concentration corresponding to 50% viability level (IC50) using Origin 8.1 software (USA).
Confocal microscopy
The analysis of internalization of fluorescein-labeled nanocontainers was studied on C6 glioma cells. Сo-localization of the nanocontainers with lysosomes were studied using LysoTracker® Red DND-99 (Molecular Probes®, USA). Obtained samples (6-AF-NG-ED and 6-AF-NG-CYS) were incubated with C6 glioma cell culture for 20 min or 1.5 h. After that, cells were washed, and fluorescence of fluorescein-labeled nanogels and lysosomes were analyzed using confocal microscopy with 488 and 561 nm laser emission (Nikon A1R MP+, Japan).
Results
Physicochemical characteristics of nanocontainers
In this study we synthesized nanogels with different types of cross-linking agents (cystamine and ethylenediamine) and loaded them with antitumor drug DD. The dependence between the size and ζ-potential of the particles in different pH was analyzed (Fig. 1). Thus, the hydrodynamic diameter (Fig. 1a) and ζ-potential (Fig. 1b) of nanogels were increased along with the increase of pH from 7 to 9: 119 to 138 nm and from − 7 to − 36 mV, respectively. It also can be seen that nanoparticles loaded with drug had smaller size and ζ-potential value within the studied pH range. Similar results were obtained for empty and DD-loaded nanogels with ethylenediamine as cross-linking agent. The size of the empty nanogels was also analyzed using atomic force microscopy (AFM) (Fig. 1d). The average height of the particles was 45 nm and average lateral width was 145 nm, which corresponds to the particles with almost twofold smaller volume than it was estimated by dynamic light scattering analysis (DLS). This difference can be explained by swelling of nanoparticles in solution due to their hydrogel-like structure, while in AFM the dry particles were measured.
Physicochemical characteristics of nanogels. a Hydrodynamic diameter and b ζ-potential of cystamine cross-linked empty and DD-loaded nanogels as a function of pH; c hydrodynamic diameter of ethylendiamine and cystamine cross-linked nanogels and DD loaded nanogels as a function of time; d semi-contact mode AFM image of cystamine cross-linked nanogels
Degradation of disulfide bond was shown for nanogels with cystamine in the presence of reducing agents (here we used glutathione). It was shown that polydispersion index significantly increases from 0.09 to 0.60 for the sample of nanocontainer incubated with 10 mM glutathione solution for 24 h, while mean hydrodynamic diameter (found from volume distribution) decreased from 131 to 15 nm, which attests to destruction of the particles (Table 1).
We studied dispersion stability of empty and loaded nanocontainers with different cross-linkers using DLS (Fig. 1c). There were no significant changes in the average size and PDI of the particles during 12 days, which may attest to its stability. Thus, it was shown that loading of the drug into nanogels has an effect on their size and ζ-potential, but does not affect their colloid stability.
Loading capacity and efficiency of nanogels
Drug was loaded into nanocontainers under different pH conditions (pH 6–9) and different R = 0.25–2 (where R is the ratio of the drug concentration to the concentration of the carboxyl groups of nanogels that were estimated by titration). Based on the drug concentration and the mass of the loaded particles, we calculated loading capacity (Fig. 2a) and efficiency (Fig. 2b) of nanogels with ethylenediamine or cystamine cross-links. For both types of the nanocontainers we have obtained similar results: maximal loading capacity (>35%) and efficiency (>75%) was reached at pH 7. While the concentration of the drug was raising, loading capacity also tended to grow at any pH value, but loading efficiency had an opposite trend. Taking these data into account, we have chosen samples that were loaded at R = 1, because at this value, the ratio between loading efficiency and capacity was optimal.
Since DD could not be detected in the presence of reducing agents due to their interference (absorbance at 221 nm), the release kinetics of the particular drug was analyzed only in the phosphate buffer. We found that during the first 2 h, more than half of the drug is released. Knowing that nanogels with cystamine links degrade under reducing conditions (10 mM glutathione), we can anticipate that the release kinetics of the drug depends on the particle degradation.
Cytotoxicity of DD and DD-nanogels
Cytotoxicity of free DD and DD loaded nanogels was evaluated using MTS assay on different cell cultures: C6, U87 and CHO-K1. In order to evaluate effectiveness of the drug in different conditions, the cytotoxicity of free DD was analyzed on the CHO-K1 cell line at different pHs. The least toxicity was observed at pH 6, IC50 was 117 μg/ml, whereas at the other investigated pH values IC50 was near 50 μg/ml (Fig. 3). Therefore, in following experiments we used DD solution with pH 7. Moreover, we found that DD exhibits much greater toxicity on U87 (IC50 = 34 μg/ml) and CHO-K1 (IC50 = 53 μg/ml) cells compared to the C6 cells (IC50 = 214 μg/ml).
Also MTS test revealed that DD decreased its cytotoxicity upon loading into nanogels (Table 2). Thus, in the case of the nanogels with ethylenediamine cross-linkers, IC50 values increased 2.6 times for C6, and 11.3 times for U87 cells compared to free drug. It should be emphasized that DD loaded into the nanogels with cystamine cross-linkers was more toxic compared to ethylenediamine ones: the IC50 values decreased by 23% for C6, and by 47% for U87 cells.
Internalization of fluorescein-labeled nanocontainers
Internalization analysis of fluorescein-labeled nanogels with ethylenediamine (Fig. 4) and cystamine (Fig. 5) linkers was performed by confocal microscopy. Nanogels conjugated with 6-aminofluorescein were incubated with C6 glioma cells for 20 min or for 1.5 h. To analyze the co-localization of the nanocontainers with lysosomes we used LysoTracker® Red DND-99. We found that after 20 min of incubation both types of nanogels accumulate in the tumor cells. It should be emphasized that signal intensity of cystamine-containing nanogel in lysosomes was two times higher than the one of ethylenediamine-containing nanogel (Pearson correlation coefficient for 6-aminofluorescein and LysoTracker® Red was 0.62 and 0.34, respectively). Although, there was no significant difference in the signal intensities of ED-nanogels and CYS-nanogels after the increase of incubation time up to 1.5 h. The co-localization analysis revealed that both types of nanocontainers internalize and localize in lysosomes (Pearson coefficients for biodegradable and non biodegradable nanogels were 0.84 and 0.88, respectively).
Internalization analysis of ethylendiamine cross-linked nanogels in glioma C6 cells. Line 1 20 min incubation; line 2 90 min incubation; column a merged image; column b aminoflourescein-6 (green); column c LysoTracker® Red DND-99 (red); column d colocalization analyzis of nanogels (red channel, X axis) and lysosomes (green channel, Y axis). Bar −10 μm. (Color figure online)
Internalization analysis of cystamine cross-linked nanogels in glioma C6 cells. Line 1 20 min incubation; line 2 90 min incubation; column a merged image; column b aminoflourescein-6 (green); column c LysoTracker® Red DND-99 (red); column d colocalization analyzis of nanogels (red channel, X axis) and lysosomes (green channel, Y axis). Bar −10 μm. (Color figure online)
Discussion
A lot of research groups are focused on the development of the delivery systems for different anticancer drugs (Kabanov and Vinogradov 2009). These systems allow to (1) increase the solubility of hydrophobic drugs (Kato et al. 2007), (2) prolong their circulation time in the body (Kataoka et al. 2000), and (3) initiate the selective release of drug in the tumor due to sensitivity to the specific stimuli (Kim et al. 2010).
One of the most important parameters of drug loaded formulation is its loading capacity and encapsulation efficacy. Here we used nanoparticles based on block ionomer complexes that are known for a quite high loading capacity of anticancer drugs (Kim et al. 2013). Thus, the studied nanocontainers based on PEG-b-PMAA had the loading capacity (>35 wt%) that is much higher compared to other drug delivery systems. For example, the loading capacity of N3-O-toluyl-fluorouracil-loaded liposomes was 9% (Sun et al. 2008), dexamethasone-loaded carbon nanotubes −20% (Murakami et al. 2004), doxorubicin-loaded hybrid superparamagnetic particles −19% (Yang et al. 2009). Previously, nanogels was efficiently loaded with cisplatin (Yokoyama et al. 1996) and doxorubicin (Kim et al. 2009), indicating that this type of carriers is suitable for a variety of drugs. As mentioned before, the maximum loading capacity of nanogels was achieved at pH 7. Similar results were obtained by Kim et al. (2009) for doxorubicin-loaded micelles. This trend might be associated with the mechanism of drug loading into nanocontainers, and, particularly, with the fact that the optimal balance between deprotonated carboxyl groups in the particle and protonated amino groups in the drug can be reached at pH 7. At higher pH, the number of protonated amino groups decreases, and, hence, the loading capacity becomes lower. Loading capacity at pH 6 is comparable with that in the neutral medium, but further decrease of pH might reduce the loading capacity due to the decrease in the number of deprotonated carboxyl groups.
Here we also analyzed the change of the particle size of loaded and free nanogels at different pH. Swelling of nanogels is determined by two factors: electrostatic repulsion of carboxyl groups in the polymer and osmotic pressure in the particle. When pH increases, the number of the deprotonated carboxyl groups and, consequently, the total charge of the nanocontainer rises. This, in turn, enhances repulsion between PMAA chains and increases the osmotic pressure inside the nanocontainer, which leads to its swelling. At the same time, drug loaded particles have lower hydrodynamic diameter and ζ-potential compared to free nanogels. This attests to electrostatic interaction of the drug with carboxyl groups of nanocontainers leading to a decrease in the number of free carboxyl groups and, consequently, to weaker repulsion of these groups. Similar results were obtained for analogous polymer nanocontainers loaded with cisplatin (Oberoi et al. 2011) and doxorubicin (Kim et al. 2009).
Moreover, DLS analysis demonstrated degradation of nanogels with cystamine cross-linking agent in the presence of the reducing agents (glutathione). Similar to the work of Kim et al. (2010), nanogels with ethylenediamine linkers were stable at 10 mM concentration of glutathione, whereas nanogels with cystamine linkers degraded during 4 h. This feature might be used for selective release of the drug only after its accumulation in tumor cells, because intracellular concentrations of the glutathione (1–10 mM) is sufficient for nanocontainer destruction, while in blood they would be stable due to low glutatione concentration in plasma (1–10 μM) that also was demonstrated by Kim et al. (2010).
We performed the MTS assay on glioma (C6, U87) and normal (CHO-K1) cell lines to compare cytotoxicity of free and encapsulated form of DD. First, using CHO-K1 cells, we found that IC50 values increase with the decreasing of pH solution of free DD. This might be associated with the loss of drug activity in the acidic medium due to the opening of ethyleneimine cycles, which are responsible for the alkylating mechanism of DD.
Second, the cytotoxicity analysis demonstrated lower toxicity of DD loaded into the nanocontainer in comparison with its free form. This can be explained by slow release of the drug from the container during incubation, and, consequently, the lower dose of the drug interacting with cells. Similar decrease of cytotoxicity was reported by Yokoyama et al. for doxorubicin (1990) and cisplatin (1996). Generally, IC50 for all forms of DD, including unbound drug, in C6 cell culture remained high compared to doxorubicin (Bennis et al. 1994) or cisplatin (Tokunaga et al. 1997). This effect can be associated with the resistance of C6 glioma to DD or triazines at all. For example, another alkylating agent from triazine family, temozolomide, also possessed much higher IC50 for C6 cell line and at the same time it demonstrated much higher toxicity on human U87 glioma cells (data not shown). However, it should be noted that toxicity of free DD on U87 glioblastoma cells was similar to that of doxorubicin (Lu et al. 2012) and cisplatin (Khiati et al. 2011), while it was less toxic for non-tumor CHO-K1 cells. In addition to the fact that this drug has low systemic toxicity and effective against human glioblastoma cells compared to non-tumor cells, allows us to consider that DD is a promising chemotherapeutic drug for the treatment of brain tumor.
Finally, it was also demonstrated that drug-loaded biodegradable nanocontainers are more efficient than nanocontainers with ethylenediamine cross-linking agent. Their higher toxicity can be explained by cleavage of cystamine linkers followed by degradation of the particle due to intracellular glutathione concentration ~1–10 mM. This process, in turn, leads to drug release, which explains higher toxicity of this nanocontainer form. Similar results have been shown for doxorubicin-loaded biodegradable formulations (Kim et al. 2010), but the difference between linkers was more significant. It could be associated with sensitivity of cell line used (ovarian cancer A2780 cell line). Also the loading capacity of doxorubicin-loaded particles was higher, what in turn might lead to higher concentration of available drug upon degradation of nanoparticles. The difference in loading capacity between this two drugs could be related to the difference in the functional groups of doxorubicin and DD: the first one interacts with carboxyl groups of polymer via primary amine group while the second one interacts via secondary amine group, what can cause some steric hindrance and as a result lower loading capacity.
We performed confocal microscopy to compare internalization of ethylendiamine cross-linked and cystamine cross-linked nanogles. Confocal analysis of glioma C6 cells, incubated with nanogels for 20 min, revealed the enhancement of signal intensity from 6-AF-nanogels with cystamine linkers in lysosomes in comparison with non-biodegradable nanocontainers. This difference could be explained by increased rate of degradation of nanogels with cystamine after internalization. If cells were incubated for 1.5 h, the difference between studied nanogels was diminished and both types of nanocontainers were accumulated in lysosomes at the same level that explains by saturation of cell by nanogels. Hence, the uptake of nanogels by cells was observed throughout the incubation period irrespective of the cross-linking agent.
Summarizing, we have shown that nanogels based on PEG-b-PMAA polymers could be used as a system for efficient loading of anticancer drug DD. The drug-loaded nanogels are characterized by the high loading capacity and efficiency and dispersion stability. In the case of cystamine cross-links, we detected selective degradation of particles under reducing conditions, which could be beneficial for a drug release in the tumor cells. Taking into account the low systemic toxicity of DD, specifically the absence of cardio-, pneumo-, nephro- and neurotoxicity, which are often side effects of many other antitumor drugs, the nanoparticle form of this drug might further improve its performance and deserves further detailed study.
References
Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K (2005) Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem 16:122–130. doi:10.1021/bc0498166
Bennis S, Chapey C, Couvreur P, Robert J (1994) Enhanced cytotoxicity of doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres against multidrug-resistant tumour cells in culture. Eur J Cancer 30:89–93. doi:10.1016/S0959-8049(05)80025-5
Bespalov VG, Zhabin AA, Stukov AN, Beljaeva OA, Murazov JaG, Semenov AL, Kon’kov SA, Krylova IM (2013) Synergism of antitumor action of dioxadet and cisplatin in model of ascitic ovarian tumor. Sibirskij Onkol J 55:42–46 (in Russian)
Bodley AL, Liu LF, Israel M, Ramakrishnan S, Yoshihiro K, Giuliani FC, Kirschenbaum S, Silber R, Potmesil M (1989) DNA topoisomerase II-mediated interaction of doxorubicin and daunorubicin congeners with DNA. Cancer Res 49:5969–5978
Bontha S, Kabanov AV, Bronich TK (2006) Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Control Release 114:163–174. doi:10.1016/j.jconrel.2006.06.015
Bronich TK, Nehls A, Eisenberg A, Kabanov VA, Kabanov AV (1999) Novel drug delivery systems based on the complexes of block ionomers and surfactants of opposite charge. Coll Surf B Biointerfaces 16:243–251. doi:10.1016/S0927-7765(99)00075-2
Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV (2005) Polymer micelle with cross-linked ionic core. J Am Chem Soc 12:8236–8237. doi:10.1021/ja043042m
Fuertes MA, Castilla J, Alonso C, Perez JM (2003) Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 10:257–266. doi:10.2174/0929867033368484
Horwitz SB (1994) Taxol (paclitaxel): mechanisms of action. Ann Oncol 5(Suppl 6):S3–S6
Jones RB, Matthes S, Shpall EJ et al (1993) Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine. J Natl Cancer Inst 85:640–647. doi:10.1093/jnci/85.8.640
Kabanov AV, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed 48:5418–5429. doi:10.1002/anie.200900441
Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS (2000) Doxorubicin-loaded poly(ethylene glycol)–poly(b-benzyl-l-aspartate copolymer micelles: their pharmaceutical characteristics and biological significance. J Control Release 64:143–153. doi:10.1016/S0168-3659(99)00133-9
Kato N, Hasegawa U, Morimoto N, Saita Y, Nakashima K, Ezura Y, Kurosawa H, Akiyoshi K, Noda M (2007) Nanogel-based delivery system enhances PGE2 effects on bone formation. J Cell Biochem 101:1063–1070. doi:10.1002/jcb.21160
Khiati S, Luvino D, Oumzil K, Chauffert B, Camplo M, Barthelemy P (2011) Nucleoside lipid-based nanoparticles for cisplatin delivery. ACS Nano 11:8649–8655. doi:10.1021/nn202291k
Kim JO, Kabanov AV, Bronich TK (2009) Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J Control Release 138:197–204. doi:10.1016/j.jconrel.2009.04.019
Kim JO, Sahay G, Kabanov AV, Bronich TK (2010) Polymeric micelles with ionic cores containing biodegradable cross-links for delivery of chemotherapeutic agents. Biomacromolecules 11:919–926. doi:10.1021/bm9013364
Kim JO, Ramasamy T, Yong CS, Nukolovа NV, Bronich TK, Kabanov AV (2013) Cross-linked polymeric micelles based on block ionomer complexes. Mendeleev Commun 23:179–186. doi:10.1016/j.mencom.2013.07.001
Loshadkin NA, Kurlyandskiy BA, Daryina LV (2002) Toksikologiya alkiliruyushchikh soedineniy. In: Kurlyandskiy BA, Philov VA (ed) Obshchaya toksikologiya. Meditsina, Moscow, pp 236–257 (in Russian)
Lu YJ, Wei KC, Ma CCM, Yang SY, Chen JP (2012) Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Coll Surf B 89:1–9. doi:10.1016/j.colsurfb.2011.08.001
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul 41:189–207. doi:10.1016/S0065-2571(00)00013-3
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi:10.1016/0022-1759(83)90303-4
Murakami T, Ajima K, Miyawaki J, Yudasaka M, Iijima S, Shiba K (2004) Drug-loaded carbon nanohorns: adsorption and release of dexamethasone in vitro. Mol Pharm 1:399–405. doi:10.1021/mp049928e
Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK (2011) Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 32:5417–5426. doi:10.1016/j.biomaterials.2011.04.006
Oberoi HS, Laquer FC, Marky LA, Kabanov AV, Bronich TK (2011) Core cross-linked block ionomer micelles as pH-responsive carriers for cis-diamminedichloroplatinum(II). J Control Release 153:64–72. doi:10.1016/j.jconrel.2011.03.028
Siegel R, Desantis R, Virgo C et al (2012) Cancer treatment and survivorship statistics. CA Cancer J Clin 0:1–22. doi:10.3322/caac.21149
Stukov AN, Gershanovich ML, Blank MA et al (2011) Antitumor agents. NIKA, St. Petersburg (in Russian)
Sun WT, Zhang N, Li AG, Zou WW, Xu WF (2008) Preparation and evaluation of N3-O-toluyl-fluorouracil-loaded liposomes. Int J Pharm 353:243–250. doi:10.1016/j.ijpharm.2007.11.017
Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC (2007) Complexation and release of doxorubicin from its complexes with Pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release 121:137–145. doi:10.1016/j.jconrel.2007.05.010
Tokunaga Y, Nakashima M, Shibata S, et al (1997) Antitumor effects of 4-pyridoxate diamine hydroxy platinum, a novel cisplatin derivative, against malignant gliomas in vitro and in vivo: a comparison with cisplatin. Pharm Sci 3:353–356.
Yang XY, Zhang XY, Ma YF, Huang Y, Wang YS, Chen YS (2009) Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem 19:2710–2714. doi:10.1039/b821416f
Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, Inoue S (1990) Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adryamicin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res 50:1700–1703. doi:10.1016/j.ijpharm.2008.08.011
Yokoyama M, Okano T, Sakurai Y, Suwa D, Kataoka K (1996) Introduction of cisplatin into polymeric micelle. J Control Release 39:351–356. doi:10.1016/0168-3659(95)00165-4
Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, Okano T (1998) Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Release 50:79–92. doi:10.1016/S0168-3659(97)00115-6
Acknowledgements
We thank Anna Gabashvili for the help with cells for confocal microscopy. All authors declare that they have no conflict of interest. This article does not contain any studies with human and animal subjects performed by any of the authors. The study was supported by grant from Russian Scientific Foundation 14-15-00698.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voeikov, R., Abakumova, T., Grinenko, N. et al. Dioxadet-loaded nanogels as a potential formulation for glioblastoma treatment. Journal of Pharmaceutical Investigation 47, 75–83 (2017). https://doi.org/10.1007/s40005-016-0294-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0294-4