Abstract
Treatment for diabetic foot ulcer (DFU) remains as one of the biggest clinical concerns in diabetic disease. DFU often causes a prolonged treatment and eventually leads to a non-healing wound ulcer. An impaired foot ulcer is often associated with a high number of amputation cases in diabetes patients. Owing to progress in the scientific research on DFU, better understanding of the mechanisms, pathophysiology of DFU has provided an insight into the advanced treatment of DFU. This includes the use of bioactive compounds and tissue engineering approach for the regeneration of damaged cells. Despite the availability of various wound treatments and dressing products in the market, most of the current products have drawbacks and limitations in terms of the pharmaceutics perspectives. Hence, pitfalls and challenges remain to develop an effective medicinal treatment product for DFU. In this paper, we discuss the current treatments available and the promising bioactive compounds for DFU. We also review advanced treatments for DFU and their limitations from the pharmaceutical point of views.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prevalence of diabetic foot ulcer (DFU)
Diabetic foot ulcer is one of the most common complications for diabetes patients. It prolongs the treatment period and is one of the risk factors for amputation. Consequently, DFU results in a costly hospitalized expense in the patients. Approximately 15 % of the diabetes patients will develop DFU once in their life (Andrews et al. 2015). It was estimated that 12–24 % of the diabetic patients undergoes amputation and this contributes to 30 % of the mortality cases in diabetic (Gregg et al. 2004). According to a 5-year mortality statistical analysis carried out in 2011, approximately 50 % of the mortality rate for DFU patients was directly related to amputations (Armstrong et al. 2007). Moreover, the 5-year mortality rate of patients with DFU was higher than those patients with breast or prostate cancers (Armstrong et al. 2007). In addition to the high amputation rate, DFU has also been associated with the highest economic burden among different types of wounds. In the United States of America (US), chronic wound contributes to 2–4 % of the health budgets with wound care service reaching up to 50 billion annually (Fife and Carter 2012). The budget of diabetes-related amputations and diabetic wound treatment is approximately USD $3 billion and USD $9 billion per year, respectively (Gordois et al. 2003; Rice et al. 2014). The average cost to heal a single wound is about USD $20,000 (Stockl et al. 2004). The cost of DFU treatment is projected to rise approximately five times in the subsequent year after the first occurrence of ulcer (Driver et al. 2010). As the diabetic disease is expected to increase to 592 million by the year of 2035 (Guariguata et al. 2014), immediate action needs to be taken in order to tackle this problem.
Physiological aspect of wound healing
DFU is a complex and chronic wound, which is often associated with nerve tissue damage and peripheral vascular problems. It causes a series of changes in the mechanical and physiological reactions in the body. Generally, for any type of wound, the healing process requires four phases of reaction: hemostasis, inflammation, proliferation, and maturation (TNF α), Strodtbeck 2001). In brief, when a tissue is injured, hemostasis is activated to constrict the blood vessels and initiate the formation of a clot. Then the fibrin clot and platelets secrete the relevant pro-inflammatory cytokines and growth factors such as tumor necrosis factor α, platelet-derived growth factor, and others onto the wounded site (Werner and Grose 2003). These proteins stimulate epithelial cells and attract the inflammatory cells such as macrophage, neutrophils, lymphocytes to induce the initial stage of inflammation. In response to the secretion of growth factors, re-epithelization is initiated to stimulate the proliferation of keratinocytes, endothelial cells, and fibroblasts for the construction of extracellular matrix (ECM) and then induction of angiogenesis to repair the wound. For a chronic wound such as DFU, the inflammatory stage is prolonged and wounds remain trapped in the chronic inflammatory stage and without further action in the proliferation and maturation stages. This disruption prohibits the production of cytokines and growth factors for the proliferation and migration of cells. Consequently, the imbalance between the inflammatory and anti-inflammatory signals causes a stiff competition in the wound microenvironment and interferes with the normal healing process. With the reduced number of growth factors and anti-inflammatory factors in the wound environment of DFU, the interactions between proteins, cells, and ECM are disrupted and further suppressed with delayed angiogenesis response and activity of the normal keratinocytes and fibroblasts.
Current wound management and treatment for diabetic foot ulcer
To date, treatment for DFU remains a central concern for both patients and clinicians. Several factors need to be taken into consideration prior to the decision making for a suitable and effective treatment. In general, the physical condition of a patient, type of wounds, and size of a wound are the common factors to be taken into account in the selection of a treatment (International Best Practice 2013).
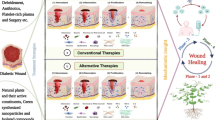
In general, the standard protocol for DFU consists of debridement, offloading, daily wound dressing, and infection control (International Best Practice 2013). However, most of the DFU patients who received this standard treatment did not show significant outcome toward the end of treatment. Hence, improved therapy such as the use of bioactive compounds has become a necessary approach in the wound management for DFU. Current and potential pharmaceutical products on the market or under development are listed in Table 1. In addition, current DFU treatment products and product development pipeline are summarized in Fig. 1.
Debridement
Debridement is the first process conducted by a clinician in the management of wound. Several debridement methods are commonly used in DFU. This includes the surgical process, hydrosurgery, ultrasonic, enzymatic reaction, and even the use of maggot (International Best Practice 2013). Debridement is an essential phase for wound management. It removes most of the cell debris and non-viable tissues and further reduces the chance of potential bacterial infection in the wound environment. Moreover, it helps to drain the secretion of pus in order to optimize the effectiveness of topical treatment. In addition, by removing the pus and exudates, the procedure prevents the prolonged inflammatory stage and stimulates the healing process by enhancing the secretion of growth factors and cytokines. Several review papers have discussed the details of availability of debridement methods and the pros-cons of these (Boateng and Catanzano 2015; International Best Practice 2013; Yazdanpanah et al. 2015).
Infection control
One of the major obstacles of foot ulcer is the bacterial infections in an open wound. Heavily infected wound is often associated with high morbidity and mortality in DFU (International Best Practice 2013). Hence, antibiotic therapy is necessary to control the infection before the wound being deteriorated. Several common topical antimicrobial agents such as silver, iodine, and poly(hexamethylene) biguanide hydrochloride (PHMB) have been widely used as standard antibacterial medicine for DFU(Moura et al. 2013). Generally, a polymicrobial infection is often observed in an open wound of DFU. A polymicrobial infection consists of various types of bacterial populations forming a biofilm on the surface of the wound. This biofilm is invisible by naked eyes and is not detected by general common bacterial culture (International Best Practice 2013). Hence, dressings with the antibacterial agent are necessary to disrupt the prevalence or spreading of bacteria onto other tissues. Although the use of the antibacterial agent has raised global concern on the prevalence of drug resistance in most of the bacterial infections, there is no reported drug resistance case with topical antibacterial treatments for wound (International Best Practice 2013). This is most likely due to the fact that topically applied antimicrobial agent does not penetrate into the skin and other deeper tissues. Furthermore, topical treatment could reduce the potential bacterial infections and protect the wound from further contamination.
Offloading
Peripheral neuropathy is a very common pathological symptom in diabetic patients, which is also known as pressure modulation. Hence, offloading the pressure in the area of foot is necessary in order to enhance the healing rate. Improper reduction in pressure often leads to the tissue damage and ulceration. The most widely used offloading technique for DFU is the total contact casts (TCC). TCC is designed to shape the foot with minimum padded and molded cast. The cast also allows the contact of the entire plantar surface of the foot and lowers leg. The previous study has shown that the use of TCC could reduce the pressure of ulceration up to 92 % (Lavery et al. 1996). Furthermore, the reduction of nonischemic plantar diabetic foot wounds reached up to 86 % using TCC (Armstrong et al. 1998; Myerson et al. 1992). Despite this high promising outcome, TCC has its own limitations. For instance, it may potentially cause skin irritation and daily inspection of the wound is rather difficult using TCC. Although there are various types of casting techniques, which are currently used for DFU, limited studies have been reported on the duration of wound healing using this method. Moreover, there is no recommended offloading pressure device for DFU in most of the countries (International Best Practice 2013).
Choice of dressing
Selection of wound dressing is very subjective. It often depends on the types, the size of the wound, exudates, risk of infection, and others (International Best Practice 2013). Most dressings are designed to create a moist wound environment for healing. Due to the complexity of the diabetic disease, there is no a single dressing which can be used for different complexity and type of wound in DFU. In response to the huge market demands of advancement in wound management, there is an extensive growth in the production of wound dressing products in the market in the last decade. A number of different dressings are available in the market, such as hydrogel or hydrocolloid, foam, and film. Most of the dressing products in DFU are incorporated with antibacterial agents. The combinations of different polymers incorporated with the antibacterial agent have long been in clinical use as a dressing for DFU. Additionally, topically applied antibacterial agents are also available in the form of solution, cream, or ointment.
In addition to the common anti-bacterial wound dressing products, incorporation of the bioactive agent into the dressing product has become the new direction in the advanced treatment. There is a significant increase in efforts to incorporate bioactive compound as a wound medicinal treatment. These bioactive substances have been reported to enhance the wound healing process. Application of antibacterial agents and bioactive compounds in dressing is potentially a better and more effective medicinal treatment for DFU.
Active compound incorporated in the dressing can be released via the hydrolysate activity of enzyme, which can be found in the exudates (DuBose et al. 2005). In addition, hydrates, swelling and diffusion are the common methods to release the bioactive compound from dressing to the wound areas (Higuchi 1961; Korsmeyer et al. 1983; Lee and Kim 1991). However, due to the rapid absorption of compounds by exudates, the bioactive agent in the dressing are found to be less effective (Futrega et al. 2014). Hence, an ideal dressing should be a platform to deliver the active compounds to the wound area while protecting the compound from being absorbed and degraded by the exudates in the wound. In addition, the continuous release of bioactive compounds from the dressing at the wound site is another important feature of dressing product in the near future.
Growth factors
With better understanding of wound healing process, studies have been concentrated on the use of biologically active molecules to enhance wound healing (Barrientos et al. 2008). Exogenously applied biologically active compounds such as growth factors were found to interact with the cells and ECM and further stimulate the proliferation and angiogenesis process in the wound area (Günter and Machens 2012; Gainza et al. 2015; Okabe et al. 2013). Subsequently, a huge number of in vitro and in vivo studies have been performed to investigate the use of growth factors as the therapeutic agent in an impaired wound (Futrega et al. 2014; Moura et al. 2013; Yazdanpanah et al. 2015).
Growth factors belong to biologically active polypeptides, which are crucial in wound healing process with specific roles in cell growth, proliferation, migration, and signal transduction of the healing process of the skin wound. Growth factors modulate the inflammatory response, angiogenesis, ECM formation, and re-epithelization in wounded skin. It was reported that for chronic wound such as DFU, the levels of most of the growth factors were reduced whereas the inflammatory factors levels were increased (Barrientos et al. 2014). Such adverse in the proteins levels disrupts the interaction and signaling transduction of the physiological reaction in the body and further attributes to the impaired wound in DFU. Therefore, controlling the level of growth factors is the key factors in wound healing.
Epidermal growth factor (EGF)
EGF was first described by Cohen et al. in 1962 (Cohen 1965). It was a fascinating finding, which was discovered unexpectedly upon separation of the neuronal growth factors from the murine submandibular gland (Cohen 1965). EGF is secreted by platelets, activated macrophages, and fibroblasts. It can be used to stimulate the proliferation, growth, and migration of various types of cells such as fibroblast and keratinocytes. In addition, EGF also promotes angiogenesis in a wounded skin. Several studies have successfully demonstrated that EGF is vital in wound healing process. In a chronically impaired wound, the levels of EGF were found to be reduced and the exogenously supplied EGF can be used to enhance the migration and proliferation of cells and further accelerate the wound healing process (Hong et al. 2006; Tsang et al. 2003). However, it was also reported that exogenous growth factors encountered substantial degradation by metalloproteinases or other proteinase present in the wound areas (Futrega et al. 2014). As EGF is susceptible to the proteolytic environment of the wound, high dosage of EGF is often required to increase the chance of active EGF in the wound. Currently, there are three growth factor-based medications for DFU in the market. These include HEBERPROT-P® (Heber, Biotech, Havana, Cuba), EASYEF® (Daewoong Pharmaceutical, Seoul, Korea), and REGEN-D™ 150 (Bharat Biotech International Limited, Hyderabad, India).
Heberprot-P® was previously known as Citoprot-P during the preclinical development. It is a registered intralesional administration recombinant human EGF (rhEGF) in Cuba since 2006 (Berlanga et al. 2013). Heberprot-P® has been made available in the market as a medication for DFU since 2007 and has been registered in more than 15 countries with more clinical phase III studies planned in the European continent and China (Berlanga et al. 2013).
Heberprot-P® is available as an injectable rhEGF in the market. Each vial of the Heberprot-P contains either 25 μg or 75 μg of freeze-dried EGF purified from Saccharomyces cerevisiae (Fernández-Montequín et al. 2007). It is administrated intralesionally three times per week. Heberprot-P® is reconstituted in 5 ml of water prior to infiltration using a disposable syringe with a 26 G × 1/2 and approximate 0.5–1 ml is administrated by injection (Heberprot 2013). The injection is performed at lesion contours and deep into the wound bottom and then covered with the dressing containing saline to keep moist and clean wound environment. Preclinical data and current clinical data have demonstrated the efficacy of using Heberprot-P as the treatment for DFU. Clinical studies showed that more than 85 % of full recovery and complete response to wound closure was observed after 5–6 weeks of treatment (Ertugrul et al. 2015; Fernández-Montequín et al. 2007). To date, over 15,000 Cuban patients were treated with Heberprot-P with 4.4-fold reduction of amputations and quick recovery time (Mola 2012). As for the stability of the protein, each vial of Heberprot-P® can be stored at 2–8 °C and can be maintained up to 24 months. Once it is opened, it should be used within 24 h to prevent the loss of activity (Heberprot 2013).
Another EGF product for DFU is Regen-D™, which is a topical gel used at a dose of 150 μg/g and applied twice per day until complete healing of a wound (Mohan 2007). Regen-D™ was a genetically engineered EGF and expressed in Escherichia coli prior to being purified. The phase III clinical study carried out in India showed quick healing rate of about 9 weeks and with 86 % of successful cases were reported (Mohan 2007). The healing duration for DFU was about 4 weeks during the post-marketing surveillance. However, there is limited clinical data from other regions of the world using this product.
Unlike topically applied gel or injectable EGF products, Easyef® is a dermal solution spray designed for DFU (50 mg/100 mL, 60,000,000 IU EGF). Easyef® is the first rhEGF which obtained a generic name of ‘nepidermin’ from WHO in 2008 in accordance to its active pharmaceutical ingredient. Easyef® is recommended to administrate twice per day until full wound closure. In a clinical study carried out using Easyef® in South Korea and Vietnam, more than 50 % of them showed complete healing (Hong et al. 2006; Tuyet et al. 2009). The distinctive feature of Easyef® is the product stability during storage at 2–8 °C for 24 months compared to other products available in the market.
Platelet-derived growth factor (PDGF)
PDGF is an important protein factor in wound healing process. It is secreted by platelets in response to the injured skin and attracts the inflammatory cells to the wound site and further stimulates the production of insulin growth factors (IGF) and thrombospondin-I, for the subsequent migration of keratinocytes and protection from the degradation of protein, respectively. In addition, PDGF also enhances the proliferation, re-epithelization, revascularization of cells, and the building of ECM of skin.
To date, the first and only PDGF product approved by US Food and Drug Association (US FDA) is Regranex® (Smith and Nephew, Inc, London, UK), which contains the recombinant human PDGF (rhPDGF-BB) (generic name: Becaplermin). Becaplermin is genetically engineered PDGF expressed in Yeast, S. cerevisiae. It was designed for topical usage and with wound area less than 5 cm2 at a dose of 10 μg/cm2 once a day. Upon application to the ulcer, the area is then covered with a saline moistened dressing. The initial clinical study showed that rhPDGF-BB had improved wound closure in DFU and reduced the chances of amputations (Krupski et al. 1991). In another study carried out in 118 patients, 45 % of these patients showed statistically significant response to the rhPDGF-BB in comparison to the control group in term of wound closure and reduction of wound size (Rees et al. 1999). Based on the data from several research groups, US FDA has subsequently approved the use of Regranex® as a medicinal treatment for wound (Embil et al. 2000; Rees et al. 1999; Smiell et al. 1999; Steed 2006; Weiman et al. 1998). Later, a safety concern has been raised regarding the risk of cancer development. An analysis found that patients who used more than three tubes of becaplermin gel had the mortality rate of 3.9 in 1000 persons (Becaplermin Regranex Gel 2012). In light with the increased of cancer development on the use becaplermin gel in patients, US FDA announced a ‘black box warning’ with the use of more than three tubes of becaplermin gel in 2008 (Becaplermin Regranex Gel 2012; Embil et al. 2000). Despite this warning, subsequent follow-up studies showed that the risk of cancer development decreased and found to be not statistically significant (Papanas and Maltezos 2010; Ziyadeh et al. 2011). In terms of the stability of Regranex® as a medicinal product, it is recommended to be stored at 2–8 °C. The duration of storage depends on the manufacturing date.
Basic fibroblast growth factor (bFGF)
Among all the FGF family members, bFGF has been found to be expressed in a variety of tissues promoting the cell division, and angiogenesis. bFGF was found to promote the proliferation and migration of mesenchymal cells, such as fibroblasts, epidermal cells, endothelial cells, and vascular smooth muscle cells (Okumura et al. 1996). Furthermore, bGFG induces neovascularisation through vessel cell proliferation and new capillary tube formation (Akasaka et al. 2007). Clinical studies reported the effectiveness of the use of bFGF in wound healing (Robson et al. 1992). However, later finding showed that the exogenously supplied bFGF as the treatment in the DFU failed to support the use of bFGF in wound closure (Richard et al. 1995). It was reported that bFGF failed to stay in the wound areas and had been washed away by other molecules in the wound areas (Richard et al. 1995). Clinical study reported the use of Fiblast® Spray, a commercially available product of recombinant human basic fibroblast growth factor (rhbFGF), which binds to ECM of various tissues showed improved wound healing process (Uchi et al. 2009).
Fiblast® Spray is a lyophilized powder of rhFGF (generic name: trafermin) and supplied with a solution to reconstitute the powder into solution (Uchi et al. 2009). This is a spray-like formulation, which requires reconstitution prior to being used. The product is administrated by spraying from approximate 5 cm away from the ulcer site, 5 times with a total of approximately 30 μg of trafermin once a day with approximate 5 cm away from ulcer site. A clinical study showed that bFGF with the use of Fiblast® showed 75 % or greater reduction in the area of the wound (Uchi et al. 2009). The spray-Fiblast® can be stored at 15 °C for up to 36 months. Upon reconstitution, spray-Fiblast® is recommended to be used within 2 weeks and stored at the temperature below 10 °C (Okabe et al. 2013).
Vascular endothelial growth factor (VEGF)
VEGF has direct effect on inducing the angiogenesis of a wound. It is secreted by platelets, macrophages, and keratinocytes during the wound healing process. VEGF is often considered as an important factor in the vascular generation. Several pre-clinical data have shown that VEGF was effective in stimulating the growth of vascular vessels (Galiano et al. 2004; Saaristo et al. 2006). In DFU, recombinant human rhVEGF (generic name: telbermin) showed convincing results for wound closure (Hanft et al. 2008; Kusumanto et al. 2006). Topically administrated telbermin (72 μg/cm2) for up to 6 weeks showed a positive outcome for wound closure (Hanft et al. 2008). However, there is no further clinical study report for phase III using VEGF.
ECM components in wound healing
ECM is a collection of non-cellular components, which can be found in the tissues and organs. The building block of ECM is made up of collagen, fibronectin, glycosaminoglycans, proteoglycans, thrombospondins, tenascin, and vitronectin (Midwood et al. 2004; Raghow 1994). ECM consists of the largest portion of the skin components and plays an important role in the wound healing process (Raghow 1994). ECM can act as a scaffold, which undergoes dynamic interaction with cells and participates in the signal transduction of a reaction (Schultz and Wysocki 2009). In a normal healing process, the components of ECM interact with cells and growth factors to regulate the wound closure (Schultz and Wysocki 2009). On the other hand, for a chronic wound skin, the ECM is destructed and subsequently the action of growth factors and the reaction with cells are interrupted and thus affecting the normal wound repair and prolonged the healing process.
In light of the importance of ECM in wound healing, a number of research has been conducted on the development of scaffold product to mimic the structure and function of a native ECM. The scaffold materials can provide a temporary support for cells to migrate, proliferate, regenerate, and further interacts with the growth factors to initiate the wound closure via cellular migration and revascularization (Schultz and Wysocki 2009). ECM scaffolds can be developed from the use of polymers and decellularised products from human and animal. For instance, the use of porcine small intestinal submucosa and human placenta-derived ECM products have been conducted in the preclinical and clinical studies (Choi et al. 2012; Mostow et al. 2005). Several ECM products are also available in the market these days. This includes Oasis® wound matrix (Smith and Nephew Inc, London UK), MatrixStem® wound matrix (Acell, Inc), Alloderm select ™ regenerative tissue matrix (Acelity, TX, US), PriMatrix® (TEI Bioscience, Boston, MA, US), and Graftjacket® regenerative tissue matrix (KCI, TX, US).
A preclinical study has demonstrated that human placenta-derived ECM is a potential candidate for wound healing (Choi et al. 2012). This type of ECM is rich in bioactive molecules such as growth factors, collagen, elastin, which are useful in wound healing process and the maintenance of blood vessel (Choi et al. 2012; Futrega et al. 2014). Most of the bioactive compounds are transferred to the fetus by the mother during pregnancy through the placenta and they carry several unique properties such as anti-inflammatory, antibacterials, low immunogenic, anti-scarring, and wound protection (Koob et al. 2013, 2014; Tseng et al. 2004). Several examples of devitalized placenta tissue products include EpiFix® (MiMedx, GA, US), AmnioExcel® (Derma Sciences, NJ, US), AmnioGraft® (BioTissue, FL,US) and XWRAP® ECM (Applied Biologics, AZ, US). In addition to decellularised products, skin substitutes that are derived from growing cells of autologous or allogenic source seeded onto natural polymer such as collagen of polylactic acid can be another choice of biomimicking ECM. Cellular ECM containing the live cells was also found to enhance the wound closure. Apligraft® (Organogenesis, Inc. La Jolla, CA, US) and Dermagraft® (Organogenesis, Inc. La Jolla, CA, US) are the commercially available acellular ECM product in the market. Apligraft® is the first cell products approved by FDA as a DFU therapy. It is a bovine-derived collagen gel seeded with human neonatal keratinocytes and fibroblasts (Zaulyanov and Kirsner 2007). Apligraft® is known to secrete interferons alpha, beta PDGF, interleukin 1, and interleukin 6 (Eaglstein and Falanga 1997). Dermagraft® is a bovine collagen gel seeded with human neonatal fibroblast on synthetic polyglactin mesh, which obtained the approval from US FDA in 2001 (Organogenesis 2013). Dermagraft® carries the ability to secrete dermal collagen, matrix proteins, and cytokines in enhancing wound repair. Clinical studies for Apligraft® and Dermagraft® have been conducted and showed increased in healing rate of more than 50 % in DFU (Edmonds 2009; Marston et al. 2003). Apligraft® can be stored at room temperature for up to 2 years shelf life whereas Dermagraft® is ought to be stored at −75 ± 10 °C with a shelf life of 6 months. The use of cryopreserved vitalized placenta membrane as a therapeutic agent in wound healing will be discussed further in “Amnion/chorion membrane” section.
Application of short peptides and nucleic acids as potential therapeutic agents for DFU
In addition to growth factors, short peptides, and nucleic acids were also found to have therapeutic potential in the wound healing process. Several types of short peptides have been studied as potential drugs for DFU and convincing results have been reported in the preclinical studies. To date, clinical trials are on-going for these potential peptides.
One of the potential therapeutic peptide agents for DFU is NorLeu3-angiotensin (NorLeu3-A (1–7)), an analog of the naturally occurring peptide, angiotensin. NorLeu3-A (1–7) was reported to accelerate the wound healing and increase the proportion of wound closure in DFU by induction of progenitor cells proliferation, re-epithelization of damaged cells, vascularization, and collagen deposition (Rodgers et al. 2015). Several preclinical studies have demonstrated the use of active peptides such as angiotensin II and angiotensin 1-7 A (1-7) to accelerate the healing of injuries (Rodgers et al. 1997, 2005). The use of topical gel of hydroxyethyl cellulose (DSC127) containing NorLeu3-A (1–7) has shown to accelerate the wound healing process (Rodgers et al. 2015). Previously, clinical study has been carried out using NorLeu3-A (1–7) as a treatment for DFU (Balingit et al. 2012). The reduction of wound areas was observed at week 4 and full wound closure was observed at week 12 (Balingit et al. 2012). Currently, clinical phase III study in larger populations has been initiated by Derma Sciences and expected to finish by the end of 2016 (Rodgers et al. 2015).
Besides, gap-junctional protein connexin 43(Cx43) is another potential option of the therapeutic agent for DFU. It was reported that Cx43 increased the rate of wound healing and accelerated wound reepithelization in the diabetic skin (Wang et al. 2007). In the in vitro assessment carried out using the human diabetic keratinocytes and fibroblasts, the abnormal elevation in Cx43 expression and gap junction communication has been observed consistently at the cellular levels (Abdullah et al. 1999). In a diabetic mouse model, the synthetic peptide ACT-1, which contains the binding domain for Cx43, was found to accelerate the wound closure significantly (Moore et al. 2014). In addition to wound closure, reduced inflammatory neutrophil infiltration and granulation tissue deposition were also being observed (Moore et al. 2014). A clinical trial has also been performed to assess the therapeutic effects of ACT-1 in augmenting the reepithelialization of chronic DFU (Grek et al. 2015). The outcomes showed that wound closure was significantly improved by 79 % at week 12 compared to the traditional bandage therapy.
Erythropoietin (EPO), a glycosylated protein hormone, which participates in all stages of wound healing has gained attention as a therapeutic agent for wound healing. Preclinical and clinical studies have successfully demonstrated the potential of using EPO in wound healing (Galeano et al. 2004; Hamed et al. 2014). A short case report showed a promising result of wound healing for DFU patients treated with topical recombinant human erythropoietin (rhEPO) hydrogel (Christina I Guenter et al. 2015). A clinical trial has also been planned for rhEPO in burn and scalding injury (Günter et al. 2013). Despite the initial findings from rhEPO, there is no information regarding the use of rhEPO in the clinical trials for DFU. However, rhEPO could still be an attractive therapeutic agent for DFU due to the beneficial effects of EPO at all phases of wound healing.
In addition to the short peptide, nucleic acids are another macromolecules worth to be investigated. A previous study has successfully demonstrated the potential of using polydeoxyribonucleotides (PDRN) as a potential therapeutic agent for DFU (Altavilla et al. 2009). PDRN is a short molecular weight of DNA ranging between 50–2000 bp (Sini et al. 1999). It was suggested that PDRN is cleaved by enzymes from the active cell membrane. Upon cleavage, it becomes the source rich in purine, pyrimidine, deoxyribonucleosides, and deoxyribonucleotides, which can be used for the proliferation and activities of the cells (Squadrito et al. 2014). It was also reported that PDRN increased the proliferation of primary cell cultures of human fibroblast cells and osteoblasts (Sini et al. 1999). PDRN also enhanced the angiogenesis and neovessel formation in a model of peripheral artery occlusive disease (Altavilla et al. 2009). Studies also demonstrated that at the hypoxia condition of wounded ulcer, the increased expression of adenosine A2A receptor by PDRN enhanced the angiogenesis and vasculogenesis (Altavilla et al. 2009). For the application in DFU, PDRN has shown to improve wound healing in a genetically diabetic mouse by increasing the reepithelialization surface of DFU (Galeano et al. 2008). The clinical trial has also been performed using PDRN and showed the promising outcome of PDRN in wound healing (Squadrito et al. 2014). Hence, it was then proposed that PDRN could be an alternative therapeutic approach to improve the wound healing in a DFU model.
Anti-inflammatory cytokines
It was hypothesized that pro-inflammatory cytokines S100A8 and IL-8 proteins could cause persistent inflammation in chronic wounds like DFU and may contribute to impaired wound healing in type II diabetes patients (Singh et al. 2016). Proinflammatory factors such as interleukin-1 (IL-1), interleukin- 6 (IL-6), interleukin-8 (IL-8), and TNF-α are the common factors found to be increased upon activation of inflammatory phase in the wound healing process. TNF-α suppresses the synthesis of ECM proteins, and metallopeptidase inhibitors whereas increases the synthesis of matrix metalloproteinases (MMPs), collagenases, and gelatinases. The increased levels of MMPs prohibit the wound repair by breaking down the ECM, inhibit the cell migration, collagen deposition, and further break down the growth factors and their target cell receptors. To counteract the effect of inflammatory effects, anti-inflammatory factors are necessary to balance the effect of upregulation of inflammatory factors.
Consequently, an alternative therapeutic target for wound healing could be the use of anti-inflammatory cytokine therapy such as factors such as IL-10, TGF-β, and IL-4 in wound healing (Barrientos et al. 2014; Barrientos et al. 2008). Cytokine therapy has long been used as a therapeutic agent for cancer, and chronic disease such as osteoarthritis (Barrientos et al. 2014, 2008). In addition, several preclinical studies reported the application of honey and nano silver agent (nAg) in decreasing the inflammation via downregulation of TNF- α (Shin et al. 2007; Tian et al. 2007). To date, there is no study conducted on the use of anti-inflammatory cytokines cocktail therapy, the use of TGF-α in wound healing was previously reported in the in vivo animal study (Bitar and Labbad 1996; Brown et al. 1994). The clinical study for TGF- β was terminated due to the unmet requirement of the drug company. Despite the failure of TGF- β, usage of cocktail based anti-inflammatory therapy is a potential approach to stimulating the wound repair in DFU.
Stem cell therapy
Stem cells such as mesenchymal stem cells (MSCs) have long been characterized and documented with the capability of self-renewal, differentiation into other cell types and stimulation of angiogenesis. In the preclinical studies, stem cells such as MSCs, hematopoietic stem cells (HPCs) have been shown to differentiate into the fibroblast, epithelial cells, and vascular endothelial cells, the essential cells for wound healing (Chan et al. 2007; Chen et al. 2008; Sasaki et al. 2008). In addition, MSCs were found to carry immunomodulatory effects by attenuating the prolonged inflammatory response in wounded skin. Studies have found that MSCs have the ability to secrete pro-inflammatory cytokines and anti-inflammatory factors such as TNF-α, interferon-γ and IL 10 (Chen et al. 2008; Tark et al. 2010). MSCs also release paracrine factors to stimulate the vasculogenesis and angiogenesis factors such as IGF-1, PDGF-BB, VEGF, angiopoietin-1, MMPs, and bFGF (Chen et al. 2008).
The use of stem cell has been suggested as an alternative and effective therapy for tissue regeneration such as in DFU since early 2000. Wound healing is a complicated process, which requires many cells and proteins to interact to reach the wound closure. Stem cell- based therapy could possibly introduce new cells into the wounded site in order to regenerate cells activities. Preclinical studies have successfully demonstrated that the secretion of cytokines, growth factors from stem cells stimulates the regeneration of cells and ECM for subsequent wound healing. Living cells secrete appropriate amount of growth factors to enhance cell regeneration at the physiology relevance level (Futrega et al. 2014). Topically delivered exogenous growth factors may not be physiologically similar to the natural secretion of proteins from cells in the body (Futrega et al. 2014). Furthermore, the interaction of cells with other proteins available in the skin helps to synthesize ECM of the skin and transduction of signal at the cellular levels. As a result, current studies focused on the use of stem cells as a potential treatment for DFU as the secretion of growth factors are of physiological relevance.
A case report of delivery of MSCs to subdermal tissue successfully enhanced the tissue repair (Badiavas and Falanga 2003). Different types of stem cells have actively been used in DFU, in particular, autologous stem cells which include the bone marrow-derived mesenchymal stem cells (BM-MSCs), MSC derived from adipose tissues (AD-MSCs), bone-marrow derived endothelial progenitors cells, HPC, and others (Heublein et al. 2015). Studies carried out on a diabetic mouse model has successfully shown that the use of BM-MSCs is a potential agent to carry anti-inflammatory effects and stimulate the healing of the wound by secreting the EGF, PDGF, VEGF, and TGF (Kato et al. 2014; Kwon et al. 2008). In addition to MSCs, human umbilical cord blood (UCB) is another potential source of MSCs for wound healing. Preliminary studies using UCB showed that the wound repair has been improved by increasing the secretion of growth factors from cells (Luo et al. 2010; Tark et al. 2010). Currently, phase II clinical trial is on-going using AD-MSCs as a therapy in DFU.
Conditioned medium (CM)
With the abundant unique properties of stem cells, the CM from BM-MSCs, AD-MSCs, and amniotic fluid-derived MSCs (AF-MSCs) were found to enhance the migration and proliferation of epithelial and fibroblasts cells in an in vitro study (Li et al. 2015; Walter et al. 2010; Yew et al. 2011). In vivo studies using animal model also showed improved wound healing using the CM-from stem cells (Chen et al. 2014; Yew et al. 2011). In addition to the reconstruction of skin tissues, the CM derived from MSC (CM-MSCs) has also been found to carry the ability to promote the regeneration of skin tissues (Chen et al. 2014; Shen et al. 2015). CM-MSCs accelerated wound closure by enhancing the epithelial and endothelial cell migration (Chen et al. 2014). It also increased re-epithelialization, cell infiltration, granulation formation, and angiogenesis (Shen et al. 2015). In the CM, there are substantial amounts of paracrine factors secreted by cells (Yew et al. 2011). These factors such as cytokines, growth factors, chemokines are accumulated in the CM and regulate the paracrine effect in accordance to the microenvironment (Yew et al. 2011).
A recent study has shown that high level of IL6 was found in the CM-MSCs (Chen et al. 2014). The preclinical study demonstrated that exogenously supplied IL6 enhanced the cell migration and would improve healing in a mice model (Luckett and Gallucci 2007). IL6 also stimulated angiogenesis of circulating blood-derived endothelial progenitors cells in vitro (Yew et al. 2011). Nevertheless, genetically modified with IL 6 knockout in mice showed deteriorated wound healing with impaired granulation tissue formation and decreased in function of fibroblast in wound healing (Luckett and Gallucci 2007). In addition to IL-6, chemokine CXCL 1 was also found to stimulate wound healing and cell migration and wound repair in a knockout mouse (KO) model (Luckett and Gallucci 2007). CM derived from BM-MSCs were found to secrete paracrine factors such as VEGF-α, insulin-like growth factor (IGF), EGF, KGE, and others (Shen et al. 2015). Despite a number of preclinical research studies had reported the advantages of using CM in wound healing, there is no clinical study on the use of stem cell-derived conditioned medium as a therapeutic agent for DFU.
Amnion/chorion membrane
The amniotic membrane of the placenta consists of a thick layer membrane known as amnion and chorion membrane. These are the membranes used in most of the studies as a potential therapeutic agent for DFU. It was previously reported that amnion and chorion membranes contain a high number of growth factors, cytokines, chemokines, and metalloproteinases inhibitors, which are the useful components for wound healing (Koob et al. 2013, Koob et al. 2014; Massee et al. 2015).
Placenta products are rich with properties such as growth factors, anti-inflammatory, antibacterials, angiogenetic activities, and anti-scarring, which favor the wound healing process (Brantley and Verla 2015). Nevertheless, placenta membrane is also rich with human MSCs, collagen matrix, growth factors to support the tissue regeneration and repair (Koob et al. 2013; Parolini et al. 2008). In addition to that, the moist environment of a placenta is an ideal choice of dressing for wound healing. Hence, reservation of placenta membranes with those properties is of great interest. Most of these placenta products are produced in decellularized or devitalized form by removing all the cells from the placenta in order to prevent the short life span. The decellularized placenta may potentially carry host-pathogen and suffer from product inconsistency. However, no case report has been published regarding this issue.
Grafix® (Osiris Therapeutics, Inc, Columbia, USA) is a placenta membrane products, which contains viable endogenous cells such as the MSC, epithelial cells, and fibroblasts (Gibbons 2015). Grafix® is produced via cryopreservation technology. Currently, there are two placenta membrane products manufactured based on the cryopreserved placenta tissues in the market. Both are manufactured by Osiris Therapeutics. The first uses the amnion (Grafix Prime) whereas the other one uses chorionic (Grafix Core) (Brantley and Verla 2015; Gibbons 2015). Clinical data using Grafix® showed that wound closure was enhanced compared to the standard wound treatment and the adverse effects and complication of DFU were reduced (Lavery et al. 2014). In order to prevent the degradation of bioactive molecules reserved via cryopreservation, Grafix® should be stored at −80 °C before use and has at least 2-year shelf life.
Drug delivery systems (DDS)
Drug delivery systems are one of the vital factors in the development of a therapeutic product. DDS can be used to carry the pharmaceutical compounds such as growth factors and peptides into the wound healing areas. As there are limitations in DFU treatment, in terms of the degradation and instability of proteins, DDS for wound healing have been used in order to maintain the stability of the proteins and achieve the desired therapeutic effects. There are several types of DDS, which can be used to carry proteins using polymeric DDS such as microspheres, nanospheres, nanofiber, hydrogel, film, sponge, liposome, and others. A number of review papers have discussed the DDS for wound healing in details (Boateng and Catanzano 2015; Gainza et al. 2015; Moura et al. 2013). The combinations of various types of polymers are actively used to develop a sustainable, controlled, and reliable system in delivering the therapeutic agent. Details on the advanced delivery methods for growth factors such as EGF, FGF, PDGF, and others were also previously discussed (Boateng et al. 2008; Gainza et al. 2015). Although, some of these advanced DDS have been proven for their effectiveness in the in vivo study using an animal model, none of them has been applied in the clinical study or available in the market. Recently, protein delivery has been reported using methods such as crystallization, co-crystallization, and coacervation, which can potentially improve the stability of proteins. The advanced delivery systems aim to provide a direct and easy way to maintain the stability of protein prior to achieving a significant positive effect.
Development of protein pharmaceuticals products faces a number of challenges as reported previously in several research papers (Kim et al. 2014; Park et al. 2014). Adequate delivery systems for protein should address the integrity of the protein, ease of administration, storage stability, and optimal drug release rate. Early research studies focused on the kinetics of protein release from DDS whereas current field emphasizes on maintaining protein integrity and other factors associated with the shelf life of a protein. Proteins delivered via skin often have a short half-life in vivo. By using DDS technologies such as nanoparticles and stimuli-responsive nanomaterials, proteins can be released in a controlled and sustained manner. In addition, the use of DDS conjugates with proteins can protect them from the physical and chemical damage upon exposure to the change of temperature and moisture environment. Protein formulation strategy with excipients or modification of proteins can reduce the chance of degradative reactions in the proteins. Additionally, the presence of a ‘smart’ delivery technology can control the delivery rate and elucidate the protein stability in a controllable system. With those developmental works, the optimized therapeutic proteins with effective DDS can be useful in clinical settings.
Limitations of current treatment for DFU
One of the major obstacles in DFU treatment is the heterogeneity among individuals in terms of physical health, history of diabetes, and others. Treatment selection is also subjected to the types of ulcer and microenvironment of the wound (Gottrup and Apelqvist 2012). Hence, clinical outcomes vary among individuals even though the same treatment was used. Clinicians often encounter problem to find a suitable dressing, which perfectly matches the size of the wound in DFU. As the toes and the plantar surface of foot vary among individuals, these have narrowed the choice of dressing. Foam dressing is considered as a suitable dressing for DFU. However, pressure ulcer often occurs on the plantar surface of toes even with the use of foam dressing. Hence, the selected dressing for the plantar wound is crucial in order to reduce the pressure for the foot.
The delivery method of therapeutic agents into wound area is another major focus in DFU treatment. There are many ways to deliver the active compounds to the wound site. One of the most common methods is the topical application of active agent into the wounded skin. However, it was reported that topically applied formulation might not be able to reach deeper tissue of the wound (Berlanga et al. 2013). Moreover, the diffusion of proteins such as growth factors might be restricted by the necrotic tissues, sepsis, inflammation, and the presence of proteases (Gainza et al. 2015). It was then suggested that intralesional injection of the growth factor can potentially deliver to the desired areas without being degraded by other proteases (Fernández-Montequín et al. 2009). Despite the advantage of avoiding degradation by proteases, intralesional injection is potentially invasive and may attribute to the transmission of bacterial infection and development of sepsis (Fernández-Montequín et al. 2009). Consequently, the combination of topical and subcutaneous application could be a potential administrative route of a therapeutic agent. Currently, only limited information can be obtained regarding the delivery route for bioactive compounds and there is no study conducted on the comparison between the different routes of administration of bioactive compounds in DFU.
In addition, the delivery of protein into wound areas is often reported with limitations, especially the stability and activity of protein in the wound areas in the presence of MMPs (Barrientos et al. 2014). In many chronic wounds, high levels of inflammatory cells lead to the elevated levels of proteases that appear to degrade ECM components, growth factors, and other protein. Hence, the degradation of topical and injectable growth factors in the wound has become a major concern in treatment for DFU (Futrega et al. 2014). As the application of growth factors such as EGF and PDGF have proved to enhance the wound healing, some contradictory data between the in vitro research and clinical settings suggest the dissociation between growth factors and wound healing was due to the protein degradation in the wound in clinical settings (Barrientos et al. 2014). Hence, the next step appears to be to identify the genuine type of proteases in the wound bed, which degrade the growth factors specifically, and then to develop an agent to counteract the degradation molecules.
Another major limitation of the treatment approach for DFU is the instability of bioactive agent and the shelf life of the product. Bioactive compounds such as proteins are highly organized and carry a complex structure. Maintaining the integrity of the protein as its native form is vital in the pharmaceutical industry processes, which include the formulation, processing, storage, before being delivered to the patients. In terms of storage, the products are usually required to be stored at 2–8 °C or −20 °C to maintain the bioactivity of the products. Additionally, safety is another important issue for pharmaceutical products. Some of the products may carry the potential risk of cancer development and disease transmission.
Incorporation of growth factors, stem cells, peptides in the dressing products, is the current manufacturing direction of commercial products. Most of the growth factor-based products for wound healing are available in the form of solution, cream, or freeze-dried. Some use gel as a delivery platform whereas spray-drying of protein can also be found. None of the products for DFU in the market utilized the conjugation of proteins with smart DDS to control the release of protein upon reacting with stimuli in a desired timely manner. Hence, it may suggest that the DDS for proteins are still under development stage and not ready for clinical trials.
Emergence of DFU and increasing number of wound treatment products in the markets have raised issues concerning the choice of treatment for DFU. Despite the strict regulations for clinical trial applications and marketing authorization, most of the products have yet to show more convincing evidence for efficacy. Some clinical studies lack strong validity due to the low number of participants in the clinical trials (Brantley and Verla 2015; Maderal et al. 2012). Scientific findings often did not convincingly support the clinical outcomes and lack sufficient evidence for wound management. Case reports with a small number of patients were mostly reported by the manufacturer company of the pharmaceutical products (Brantley and Verla 2015; Maderal et al. 2012). For instance, the biological properties of placental membranes showed benefits for the treatment of chronic DFUs, but scientific and clinical data for commercially available placental products are insufficient and limited.
In addition, most of the studies were conducted at a single center instead of multicenter at different geographical areas (Brantley and Verla 2015). Some clinical studies performed were not randomized double blind placebo control trials. For instance, studies carried out by Epifix® were performed at the same location with the limited number of participants (Brantley and Verla 2015). Some of the clinical studies were performed with variations in the routine dressing protocol between the control participants and patients (Brantley and Verla 2015). This may potentially create bias in analyzing the outcome of the study. Another major pitfall, which should be taken into account is the fact that many of the studies were sponsored by a company and with the same investigator group (Brantley and Verla 2015; Frykberg and Banks 2015). It may be necessary to consider the distribution of works into different research groups to prevent the possibility of favoritism. Moreover, there is no longitudinal study conducted on the use of these DFU treatments. As it was previously reported that the rate of recurrence of foot ulceration is very high, study on the effects of pharmaceutical products on the recurrence of diabetic foot ulcer is essential to provide substantial supportive information on the products in a long term (Dubský et al. 2013).
There is limited number of information or report on the post-marketing or continuous monitoring safety and tolerability of pharmaceutical products, after completion of phase III clinical trial. Hence, there is no data to justify the safety toward the end of the clinical trial study. In addition to post-marketing monitoring, currently, FDA only accepts complete wound healing as an efficacy outcome of a chronic wound (Maderal et al. 2012. Definition of a complete wound is the re-epithelialization of skin without a drainage at two consecutive weeks (Maderal et al. 2012). Problem was raised with the justification of completely healed wound due to the fact that participants were not healed over the course of the two consecutive weeks (Maderal et al. 2012). Hence, it becomes hard to show the difference between the control and patient groups. Consequently, some of the clinical trials use alternative end points measurements such as the wound measurements, the rate of wound reduction, and patient measure in terms of the health condition. Furthermore, there is another concern regarding the characterization of the wound, and original size of the wound. Therefore, the multi-endpoint measurements and criteria of patient selection should be taken into account prior to the commencement of the study.
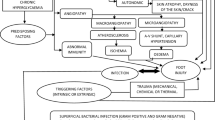
There are a few issues to be solved in stem cell and CM therapy for DFU. This includes the secretome factors, safety, choice of the cells and delivery methods of cells (Jayaraman et al. 2013). Current stem cells therapy is cumbersome, time-consuming, expensive, and may not sufficiently address the underlying mechanisms that contribute to the chronic nature of the ulcers. Stem cells derived from various tissues carry a different but specific lineage and may result in the variation in secretome factors, secretion cytokines and growth factors (Jayaraman et al. 2013). As a result, the stem cells of choice need to be studied prior to being used in the wound healing treatment. In order to prevent the introduction of animal derived cytokines and growth factors in medium, complete defined serum-free media is a more desirable condition although it may be slightly costly. There is also a chance to introduce dead cells and cells debris while using the conditioned medium. Hence, investigation of the product safety of the CM product is mandatory before being used in clinical settings. Limitations of DFU treatments is summarized in Fig. 2.
Research direction for pharmaceutical products in DFU
Current growth factor treatment uses single growth factor therapy for DFU. Even though it is well documented that growth factors are necessary to enhance the wound healing process, the single growth factor may not be sufficient to create physiological interactions between growth factors and cells for the wound repair process (Borena et al. 2015). In the physiological context of the body, growth factors usually work synergistically in their natural context. Hence, exogenously supplied growth factors should create an optimum active environment for wound healing in DFU. Perhaps, more than one type of growth factors is indeed needed for optimal healing of a wound. Delivery of multiple growth factors seems to be a potential solution for wound healing. Although, use of high concentration growth factors has been suggested to compete for the enzymatic reaction and reduce the degradation of growth factors (Andree et al. 1994), the use of a high amount of growth factors may not be an ideal solution in DFU. For example, in the case of EGF, when the exogenously supplied EGF concentration is much higher than the physiological levels, it may contribute to the adverse effects such as the development of malignant neoplasia (Andree et al. 1994). Even though combination of more than one growth factors would increase the cost of active ingredients, it may eventually lower the cost of treatment by enhancing the activity of synergistic growth factors. Moreover, each growth factor in combination product would be at lower level than single component products, which would have less chance of overdosing and adverse effects. In addition, DDS with suitable compartmentalization may be desirable to achieve a specific profile of each component. An effective DDS is necessary for controlling the release of proteins and maintaining the stability, functional activity of a protein over a period of time. In spite of the advancement of DDS technologies, the main obstacle of DDS is the inconsistency outcomes of the in vitro and in vivo studies, which is very likely due to the physiological differences in the cells culture and animal model (Gainza et al. 2015). Therefore, more studies should be carried out to determine the suitable model for basic research to provide evidences in subsequent clinical settings.
In addition to the cost efficiency, in vivo fate of topically delivered growth factors is another great concern in wound treatment. As described earlier, wound exudate can physically displace the topically applied growth factors and high concentration of MMPs can inactivate and further degrade the growth factors (Barrientos et al. 2008, 2014). In order to overcome such challenge, several studies have reported the strategies to minimize the degradation of the protein. For instance, the use of recombinant strategies to produce an engineered ECM binding motif for growth factors and removal of MMPs cleavage site to stabilize the tertiary structure of growth factors (Sun et al. 2007; Yan et al. 2010). Alternatively, polyethelene glycol can also be attached to the amino acid side chains of growth factors to protect them from MMP cleavage (Szlachcic et al. 2011). In addition to engineered ECM, genetically modified growth factors with a lipid layer could be another potential approach to maintain the integrity of protein in the wound area. In fact, in the actual repair, growth factors often interact with non-protein soluble mediators such as phospholipids membrane and lipid. The lipid acts synergistically with growth factors to enhance the wound healing (Demidova-Rice et al. 2012). Hence, modification of growth factors to pre-interact with lipids may increase wound repair and prevent degradation of protein in the wound.
Several types of biological molecules such as growth factors, cytokines, peptides, ECM components have been studied as pharmaceutically active compounds for impaired DFU. Most of these compounds were previously proven effective in stimulating the cell proliferation and migration in the basic preclinical scientific research. Although abundant potentially bioactive compounds for DFU have been reported, general concerns remain in terms of efficiency, cost-effectiveness, and side effects of the product. These compounds have yet to fulfill the demand of DFU from the pharmaceutical perspectives. There are several issues concerning the manufacturing of the pharmaceutical products. The key aspects of the pharmaceutical products are the integrity, stability, and release efficiency. For the benefit of patients and clinicians, studies should be conducted at clinical trials in a larger population at geographically distinct locations simultaneously to understand the mechanisms and actions of the pharmaceutics agent in the wound repair process. In addition, it is also important to determine the criteria for selection of patients in the treatment and to understand the duration of healing for each treatment. Hence, randomized and controlled clinical trials for the commercially available product are desirable. It is also necessary to conduct a study comparing the commercially available product in their efficacy in the wound healing rate. For instance, comparison of the outcome of products from different companies would help clinicians to identify the selection criteria of patient required for each product. A summary of future research direction for DFU treatments is shown in Fig. 2.
In conclusion, there are still unmet medical needs for DFU and a number of challenges ahead to develop optimal treatment options. From pharmaceutical perspectives, DDS based on combination products appear to be one of the future directions. Combination of biomaterials which could be various growth factors, scaffolds, or dressings. The release profile of each active component of the combination product might have to be controlled to achieve desired clinical efficacy. In vivo degradation of active growth factors in wound area needs to be minimized to enhance the overall delivery efficiency. Critical factors for successful product development, apart from the clinical efficacy, are as follows; product stability during storage and shipping and upon application, the cost of goods, feasibility for industry scale-up, and patient compliance.
References
Abdullah KM, Luthra G, Bilski JJ et al (1999) Cell-to-cell communication and expression of gap junctional proteins in human diabetic and nondiabetic skin fibroblasts. Endocrine 10(1):35–41
Akasaka Y, Ono I, Tominaga A et al (2007) Basic fibroblast growth factor in an artificial dermis promotes apoptosis and inhibits expression of α-smooth muscle actin, leading to reduction of wound contraction. Wound Repair Regen 15(3):378–389
Altavilla D, Bitto A, Polito F et al (2009) Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovascular & Hematological Agents in Medicinal Chemistry 7(4):313–321
Andree C, Swain WF, Page CP et al (1994) In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci 91(25):12188–12192
Andrews KL, Houdek MT, Kiemele LJ (2015) Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int 39(1):29–39
Armstrong DG, Lavery LA, Bushman TR (1998) Peak foot pressures influence the healing time of diabetic foot ulcers treated with total contact casts. J Rehabil Res Dev 35(1):1
Armstrong DG, Wrobel J, Robbins JM (2007) Guest editorial: are diabetes-related wounds and amputations worse than cancer. Int Wound J 4(4):286–287
Badiavas EV, Falanga V (2003) Treatment of chronic wounds with bone marrow–derived cells. Arch Dermatol 139(4):510–516
Balingit PP, Armstrong DG, Reyzelman AM et al (2012) NorLeu3-A (1–7) stimulation of diabetic foot ulcer healing: results of a randomized, parallel-group, double-blind, placebo-controlled phase 2 clinical trial. Wound Repair and Regeneration 20(4):482–490
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601
Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M (2014) Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 22(5):569–578
Becaplermin Regranex Gel (2012) Smith and Nephew Inc (www.regranex.com)
Berlanga J, Fernández JI, López E et al (2013) Heberprot-P: a novel product for treating advanced diabetic foot ulcer. MEDICC Rev 15(1):11–15
Bitar MS, Labbad ZN (1996) Transforming growth factor-β and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 61(1):113–119
Boateng J, Catanzano O (2015) Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci 104(11):3653–3680
Boateng JS, Matthews KH, Stevens HN, Eccleston GM (2008) Wound healing dressings and drug delivery systems: a review. J Pharm Sci 97(8):2892–2923
Borena BM, Martens A, Broeckx SY et al (2015) Regenerative skin wound healing in mammals: state-of-the-art on growth factor and stem cell based treatments. Cell Physiol Biochem 36(1):1–23
Brantley JN, Verla TD (2015) Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv Wound Care 4(9):545–559
Brown RL, Breeden MP, Greenhalgh DG (1994) PDGF and TGF-α act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res 56(6):562–570
Chan RK, Garfein E, Gigante PR et al (2007) Side population hematopoietic stem cells promote wound healing in diabetic mice. Plast Reconstr Surg 120(2):407–411
Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4):e1886
Chen L, Xu Y, Zhao J et al (2014) Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One 9(4):e96161
Choi JS, Kim JD, Yoon HS, Cho YW (2012) Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Eng Part A 19(3–4):329–339
Christina I Guenter LK, Shibashish Giri, Hans-Günther Machens, and Augustinus Bader (2015) First results on the three patients treated with topical recombinant human erythropoietin (rhEPO) to improve wound healing in diabetic foot ulcers. J Transplant Stem Cells Biol 2(1):4
Cohen S (1965) The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol 12(3):394–407
Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 25(7):304
Driver VR, Fabbi M, Lavery LA, Gibbons G (2010) The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 52(3):17S–22S
DuBose JW, Cutshall C, Metters AT (2005) Controlled release of tethered molecules via engineered hydrogel degradation: model development and validation. J Biomed Mater Res Part A 74(1):104–116
Dubský M, Jirkovská A, Bem R et al (2013) Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J 10(5):555–561
Eaglstein WH, Falanga V (1997) Tissue engineering and the development of Apligraf®, a human skin equivalent. Clin Ther 19(5):894–905
Edmonds M (2009) Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds 8(1):11–18
Embil JM, Papp K, Sibbald G et al (2000) Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacy. Wound Repair Regen 8(3):162–168
Ertugrul BM, Buke C, Ersoy OS, Ay B, Demirez DS, Savk O (2015) Intralesional epidermal growth factor for diabetic foot wounds: the first cases in Turkey. Diabetic Foot Ankle. doi:10.3402/dfa.v6.28419
Fernández-Montequín JI, Infante-Cristiá E, Valenzuela-Silva C et al (2007) Intralesional injections of Citoprot-P®(recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 4(4):333–343
Fernández-Montequín JI, Betancourt BY, Leyva-Gonzalez G et al (2009) Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure. Int Wound J 6(1):67–72
Fife CE, Carter MJ (2012) Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds 24(1):10–17
Frykberg RG, Banks J (2015) Challenges in the treatment of chronic wounds. Adv Wound Care 4(9):560–582
Futrega K, King M, Lott WB, Doran MR (2014) Treating the whole not the hole: necessary coupling of technologies for diabetic foot ulcer treatment. Trends Mol Med 20(3):137–142
Gainza G, Villullas S, Pedraz JL, Hernandez RM, Igartua M (2015) Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed Nanotechnol Biol Med 11(6):1551–1573
Galeano M, Altavilla D, Cucinotta D et al (2004) Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes 53(9):2509–2517
Galeano M, Bitto A, Altavilla D et al (2008) Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen 16(2):208–217
Galiano RD, Tepper OM, Pelo CR et al (2004) Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 164(6):1935–1947
Gibbons GW (2015) Grafix®, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv Wound Care 4(9):534–544
Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA (2003) The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 26(6):1790–1795
Gottrup F, Apelqvist J (2012) Present and new techniques and devices in the treatment of DFU: a critical review of evidence. Diabetes Metabolism Res Rev 28(S1):64–71
Gregg EW, Sorlie P, Paulose-Ram R et al (2004) Prevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes 1999–2000 National Health and Nutrition Examination Survey. Diabetes Care 27(7):1591–1597
Grek CL, Prasad G, Viswanathan V, Armstrong DG, Gourdie RG, Ghatnekar GS (2015) Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: a multicenter, randomized trial. Wound Repair Regen 23(2):203–212
Guariguata L, Whiting D, Hambleton I, Beagley J, Linnenkamp U, Shaw J (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2):137–149
Günter C, Machens H-G (2012) New strategies in clinical care of skin wound healing. Eur Surg Res 49(1):16–23
Günter CI, Bader A, Dornseifer U et al (2013) A multi-center study on the regenerative effects of erythropoietin in burn and scalding injuries: study protocol for a randomized controlled trial. Trials 14(1):124
Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmouliere A (2014) Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen 22(1):23–33
Hanft J, Pollak R, Barbul A et al (2008) Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 17(1):34–37
Heberprot (2013) (http://heberprot-p.cigb.edu.cu/)
Heublein H, Bader A, Giri S (2015) Preclinical and clinical evidence for stem cell therapies as treatment for diabetic wounds. Drug Discov Today 20(6):703–717
Higuchi T (1961) Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci 50(10):874–875
Hong JP, Jung HD, Kim YW (2006) Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg 56(4):394–398
International Best Practice GWM, in Diabetic Foot Ulcers. (2013) Wounds Int
Jayaraman P, Nathan P, Vasanthan P, Musa S, Govindasamy V (2013) Stem cells conditioned medium: a new approach to skin wound healing management. Cell Biol Int 37(10):1122–1128
Kato J, Kamiya H, Himeno T et al (2014) Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complicat 28(5):588–595
Kim NA, Lim DG, Lim JY et al (2014) Evaluation of protein formulation and its viscosity with DSC, DLS, and microviscometer. J Pharmaceut Investig 44(4):309–316
Koob TJ, Rennert R, Zabek N et al (2013) Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 10(5):493–500
Koob TJ, Lim JJ, Massee M, Zabek N, Denoziere G (2014) Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 102(6):1353–1362
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15(1):25–35
Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH (1991) A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg 14(4):526–536
Kusumanto Y, Van Weel V, Mulder N et al (2006) Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther 17(6):683–691
Kwon DS, Gao X, Liu YB et al (2008) Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 5(3):453–463
Lavery LA, Vela SA, Lavery DC, Quebedeaux TL (1996) Reducing dynamic foot pressures in high-risk diabetic subjects with foot ulcerations: a comparison of treatments. Diabetes Care 19(8):818–821
Lavery LA, Fulmer J, Shebetka KA et al (2014) The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 11(5):554–560
Lee PI, Kim C-J (1991) Probing the mechanisms of drug release from hydrogels. J Controll Release 16(1–2):229–236
Li M, Zhao Y, Hao H et al (2015) Mesenchymal stem cell-conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds 14(1):73–86. doi:10.1177/1534734615569053
Luckett L, Gallucci R (2007) Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol 156(6):1163–1171
Luo G, Cheng W, He W et al (2010) Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound repair and regeneration 18(5):506–513
Maderal AD, Vivas AC, Eaglstein WH, Kirsner RS (2012) The FDA and designing clinical trials for chronic cutaneous ulcers. In: Seminars in cell & developmental biology, vol 23. Elsevier, p 993–999
Marston WA, Hanft J, Norwood P, Pollak R (2003) The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers results of a prospective randomized trial. Diabetes Care 26(6):1701–1705
Massee M, Chinn K, Lei J, Lim JJ, Young CS, Koob TJ (2015) Dehydrated human amnion/chorion membrane regulates stem cell activity in vitro. J Biomed Mater Res B
Midwood KS, Williams LV, Schwarzbauer JE (2004) Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36(6):1031–1037
Mohan VK (2007) Recombinant human epidermal growth factor (REGEN-D™ 150): effect on healing of diabetic foot ulcers. Diabetes Res Clin Pract 78(3):405–411
Mola EL (2012) Heberprot-P®: an idea turned into a product. Biotecnología Aplicada 29(4):262–265
Moore K, Ghatnekar G, Gourdie RG, Potts JD (2014) Impact of the controlled release of a connexin 43 peptide on corneal wound closure in an STZ model of type I diabetes. PLoS One 9(1):e86570
Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D, Group OVUS (2005) Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 41(5):837–843
Moura LI, Dias AM, Carvalho E, de Sousa HC (2013) Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater 9(7):7093–7114
Myerson M, Papa J, Eaton K, Wilson K (1992) The total-contact cast for management of neuropathic plantar ulceration of the foot. J Bone Joint Surg Am 74(2):261–269
Okabe K, Hayashi R, Aramaki-Hattori N, Sakamoto Y, Kishi K (2013) Wound treatment using growth factors
Okumura M, Okuda T, Okamoto T, Nakamura T, Yajima M (1996) Enhanced angiogenesis and granulation tissue formation by basic fibroblast growth factor in healing-impaired animals. Arzneimittelforschung 46(10):1021–1026
Organogenesis (2013) DERMAGRAFT Directions for use (www.organogenesis.com)
Papanas D, Maltezos E (2010) Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf 33(6):455–461
Park M-H, Baek J-S, Lee C-A, Kim D-C, Cho C-W (2014) The effect of Eudragit type on BSA-loaded PLGA nanoparticles. J Pharmaceut Investig 44(5):339–349
Parolini O, Alviano F, Bagnara GP et al (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first International workshop on placenta derived stem cells. Stem Cells 26(2):300–311
Raghow R (1994) The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J 8(11):823–831
Rees RS, Robson MC, Smiell JM, Perry BH (1999) Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen 7(3):141–147
Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB (2014) Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 37(3):651–658
Richard J-L, Parer-Richard C, Daures J-P et al (1995) Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot: a pilot, randomized, double-blind, placebo-controlled study. Diabetes Care 18(1):64–69
Robson MC, Phillips LG, Lawrence WT et al (1992) The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 216(4):401
Rodgers K, Abiko M, Girgis W, St Amand K, Campeau J, Dizerega G (1997) Acceleration of dermal tissue repair by angiotensin II. Wound Repair Regen 5(2):175–183
Rodgers K, Ellefson D, Espinoza T et al (2005) Fragments of Nle3-angiotensin (1–7) accelerate healing in dermal models. J Peptide Res 66(s1):41–47
Rodgers KE, Bolton LL, Verco S, diZerega GS (2015) NorLeu3-Angiotensin (1–7)[DSC127] as a Therapy for the Healing of Diabetic Foot Ulcers. Adv Wound Care 4(6):339–345
Saaristo A, Tammela T, Fārkkilā A et al (2006) Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol 169(3):1080–1087
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180(4):2581–2587
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17(2):153–162
Shen C, Lie P, Miao T et al (2015) Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol Med Rep 12(1):20–30
Shin S-H, Ye M-K, Kim H-S, Kang H-S (2007) The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int Immunopharmacol 7(13):1813–1818
Singh K, Agrawal NK, Gupta SK, Sinha P, Singh K (2016) Increased expression of TLR9 associated with pro-inflammatory S100A8 and IL-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (T2DM) cases with impaired wound healing. J Diabetes Complications 30(1):99–108
Sini P, Denti A, Cattarini G, Daglio M, Tira M, Balduini C (1999) Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem Funct 17(2):107–114
Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH (1999) Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 7(5):335–346
Squadrito F, Bitto A, Altavilla D et al (2014) The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial. J Clin Endocrinol Metab 99(5):E746–E753
Steed DL (2006) Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg 117(7S):143S–149S
Stockl K, Vanderplas A, Tafesse E, Chang E (2004) Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care 27(9):2129–2134
Strodtbeck F (2001) Physiology of wound healing. Newborn Infant Nurs Rev 1(1):43–52
Sun W, Sun W, Lin H et al (2007) Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors 25(5):309–318
Szlachcic A, Zakrzewska M, Otlewski J (2011) Longer action means better drug: tuning up protein therapeutics. Biotechnol Adv 29(4):436–441
Tark K-C, Hong J-W, Kim Y-S, Hahn S-B, Lee W-J, Lew D-H (2010) Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 65(6):565–572
Tian J, Wong KK, Ho CM et al (2007) Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2(1):129–136
Tsang MW, Wong WKR, Hung CS et al (2003) Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 26(6):1856–1861
Tseng SC, Espana EM, Kawakita T et al (2004) How does amniotic membrane work? The Ocular Surface 2(3):177–187
Tuyet HL, Quynh N, Tran T et al (2009) The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results. Int Wound J 6(2):159–166
Uchi H, Igarashi A, Urabe K et al (2009) Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 19(5):461–468
Walter M, Wright KT, Fuller H, MacNeil S, Johnson WEB (2010) Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 316(7):1271–1281
Wang CM, Lincoln J, Cook JE, Becker DL (2007) Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes 56(11):2809–2817
Weiman T, Smiell J, Yachin S (1998) Efficacy and safety of a topical gel formulation of rh-PDGF-BB/becaplermin in patients with chronic neuropathic diabetic ulcers: a phase III randomized placebo-controlled double-blind study. Diabetes Care 21:822–827
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870
Yan X, Chen B, Lin Y et al (2010) Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract 90(1):66–72
Yazdanpanah L, Nasiri M, Adarvishi S (2015) Literature review on the management of diabetic foot ulcer. World J diabetes 6(1):37
Yew T-L, Hung Y-T, Li H-Y et al (2011) Enhancement of wound healing by human multipotent stromal cell conditioned medium: the paracrine factors and p38 MAPK activation. Cell Transplant 20(5):693–706
Zaulyanov L, Kirsner RS (2007) A review of a bi-layered living cell treatment (Apligraf®) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2(1):93
Ziyadeh N, Fife D, Walker AM, Wilkinson GS, Seeger JD (2011) A matched cohort study of the risk of cancer in users of becaplermin. Adv Skin Wound Care 24(1):31–39
Acknowledgments
This study was supported by the Ministry of Trade, Industry and Energy (10047890) in Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors (Aeri Kim, H.-C. Lau) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lau, HC., Kim, A. Pharmaceutical perspectives of impaired wound healing in diabetic foot ulcer. Journal of Pharmaceutical Investigation 46, 403–423 (2016). https://doi.org/10.1007/s40005-016-0268-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0268-6