Abstract
Lipid-based self-assembling vesicles were first described in 1961 and reported in 1964 by Dr. Alec D. Bangham (Bangham and Horne in J Mol Biol 8:660–668, 1964) at the Babraham Institute in Cambridge, UK, in which he wrote: “It is probable that at equilibrium each and every lipid bilayer forms an unbroken membrane-there being no exposed hydrocarbon/water interface-from which it follows that every aqueous compartment would be discrete and isolated from its neighbor, including a complete separation of the outermost compartment of the whole structure from the continuous phase in which it is suspended”. It is thermodynamically possible for each lipid bilayer to form a discrete membrane, separating the vesicle from the continuous aqueous phase in which it is suspended. These “unbroken membranes” were called as “liquid crystal” or “smectic mesophase”, “Bangasomes” after the name of Dr. Bangham, and finally “liposomes”. The word “liposome” derives from two Greek words of “lipo-meaning fat” and “soma-meaning body”. In the earlier days, these artificial vesicles (or liposomes) were used for the study of cell physiology such as ion (or drug) permeability, membrane fusion, membrane-bound enzyme properties or as a membrane model. More recently, much attention was brought to the uses of liposomes in medical fields as drug delivery systems and the first article of this kind was published in 1971 in FEBS Letters by Dr. George Gregoriadis and his co-workers, where amyloglucosidase and albumin were entrapped into liposome for the purpose of enzyme replacement therapy (Gregoriadis et al. in FEBS Lett 14(2):95–99, 1971). Since then, tremendous amounts of papers were published on the uses of liposomes as a drug delivery system (DDS). So, this mini-review is mainly focused on the use of liposome as DDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Liposome-overview
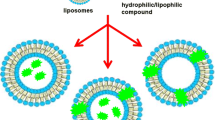
Since their discovery more than 50 years ago, liposomes are gaining more and more popularity as a drug delivery tool for better therapeutic outcomes. By definition, liposome is “a lipid vesicle or bubble in which aqueous compartments exist in the core or between the lipid bilayers and non-aqueous compartments also exist only in the lipid bilayers”. See the Fig. (1) This peculiar structure of liposomes was made possible mainly due to the hydrophobic interaction between the phospholipids as the phospholipid, which is a main component of liposomes, consists of a hydrophilic head group and two hydrophobic tails. The amphipathic properties of phospholipids can encapsulate both hydrophilic and hydrophobic drugs in aqueous compartment and lipid bilayers, respectively, in the liposomes. Moreover, liposomes can also be made in various sizes, usually 50–200 nm, in order to take advantage of the enhanced permeability and retention (EPR) effect in cancer treatment. So, liposomes can circumvent many problems associated with small molecule drugs.
Advantages versus disadvantages of liposomes
The beauty of liposomes, in the context of drug loading, lies in that both hydrophilic and lipophilic drugs can be encapsulated in aqueous core and lipid bilayer, respectively. This property of drug encapsulation ability of liposomes differentiates them from micelles in which only the lipophilic drugs are to be loaded. However, liposomes have been known to have some drawbacks of mass production, stability during storage on shelves and also in blood circulation, though most of these drawbacks have been overcome by the extensive research on new biocompatible materials, surface modification with polyethylene glycol (PEG) and size and zeta-potential optimization and more than ten liposomal drug products have entered the market after FDA’s approval in many countries.
It is highly expected that ideal formulations of drug-encapsulating liposomes with good mechanical stability, longer in vivo circulation time, improved drug-loading efficiency, enhanced EPR with exact tissue or organ targetability will emerge in the near future. This paper is designed to overview the advancement of liposome technology including stimuli-sensitive liposome, target-specific liposome, PEGylated liposome with the emphasis on commercialization possibility.
Liposomes, after administered into the blood stream, are known to be preferentially distributed to the organs or tissues with leaky vasculatures such as tumor tissue. This phenomenon, which is first named as the enhanced EPR effect of solid tumors by Dr. Maeda in 1986, has been extensively studied for cancer treatment (Matsumura and Maeda 1986). As the enhanced circulation time can also potentiate the EPR effect of liposome, long-circulating liposome or “stealth liposomes” were also invented by modulating the surface of liposome with hydrophilic polymers such as PEG which decreased the uptake of liposomes by reticuloendothelial system (RES).
Even with many advantages, liposomes still need to be improved with their biodistribution, pharmacokinetic and tissue-targeting properties. So, more advanced technologies are to be developed including as follows.
Preparation of liposomes
Since the finding of liposomes by Dr. Bangham, numerous methods of liposome preparation have been developed including lipid hydration, ethanol injection, freeze-thawing and reverse phase evaporation, etc. Here, I describe two major types of liposome preparation: (1) small unilamellar vesicles (SUVs) prepared by “bath sonication” method, (2) large unilamellar vesicles (LUVs) prepared by “reverse-phase evaporation” method.
Bath sonication method for SUVs
This method is usually used for simple encapsulation of drug or fluorescent dye in the liposome. Briefly, phospholipids and cholesterol are dissolved in chloroform, followed by evaporation of chloroform, resulting in dry lipid film. Then, drug-dissolved buffer is added to the lipid film (hydration), sonicated in a bath-type sonicator and mixed vigorously. The resulting suspension is centrifuged in a swinging bucket rotor to remove any large vesicle and the supernatant is used as a SUVs.
Reverse-phase evaporation method for LUVs
The pro of LUVs by reverse-phase evaporation method is a large capture volume; however, the con is the use of diethyl (or isopropyl) ether, which precludes large-scale preparation. Overall preparation method is similar to the bath sonication method for SUVs except that ether is added to the lipid film before the addition of one-third amount of drug-dissolved buffer. Then, the ether is evaporated and a gel is formed. A short (5–10 s) vigorous mixing is applied to break up this gel and evaporation is resumed. This step is repeated a couple times until an aqueous opalescent suspension is formed. Then, the residual two-thirds of drug-dissolved buffer is added with thorough evaporation of ether. The resulting LUVs can be passed through polycarbonate membranes of 0.2 μm or other pore sizes to achieve a uniform size distribution.
Various kinds of liposomes
Temperature-sensitive liposome (TSL)
Different membrane compositions of liposomes can alter the way in which the liposomal contents are released. The compositions can be formulated such a way that the release is in response to the environmental stimuli including temperature or pH.
Temperature-sensitive lipids undergo phase transition from gel (tightly ordered, solid) to liquid–crystal (high freedom of movement) state at/above the elevated temperature. If liposomes are made out of temperature-sensitive lipids, the drug contents can be released at/above the phase transition temperature of lipids used. In 1978, Prof. M. Yatvin and Dr. J. Weinstein at the University of Wisconsin and National Cancer Institute, respectively, and their colleagues published an article in Science, where they first suggested possible applications of the TSL in the treatment of tumors or local injections (Yatvin et al. 1978). In this article, they reported that liposomes can be designed to release the entrapped drug preferentially at temperatures attainable by mild local hyperthermia. In a test system in vitro, protein synthesis by E. coli is inhibited and killing of the cells is enhanced by heating neomycin-containing liposomes to their phase transition temperature to maximize drug release. They also reported the enhanced (4-times more) delivery of methotrexate-loaded temperature-sensitive liposomes to Lewis Lung tumors which was heated to 42 °C compared to unheated control tumors (Weinstein et al. 1979). Various combinations of phospholipids can generate different phase-transition temperatures of lipids. For example, dimyristoyl phosphatidylcholine (DMPC) has phase transition temperature of 23 °C, dipalmitoyl phosphatidylcholine 41 °C, and distearoyl phosphatidylcholine (DSPC) 58 °C. So, combination of appropriate amounts of DMPC and DSPC might result in a phase transition temperature of 39 °C (this could be determined by a differential scanning calorimetry).
pH-sensitive liposome (PSL)
Lower pH values of ~pH 6.5 can destabilize the lipid bilayers of liposomes and the drug contents are released at tumor, infection and inflammation. Dr. Yatvin and his colleagues also published an article in Science in 1980 in which, drugs could be specifically released from liposomes by a change of pH in the ambient serum when pH-sensitive molecules, such as palmitoyl homocysteine, are incorporated into liposomes (Yatvin et al. 1980). Such liposomes are known to be useful clinically if they can be targeted to areas of the body (tumors, inflammation, or infection site) where pH is less than physiological range. At the intracellular level, pH of endosome or lysosomes is also lower than that of cellular membrane of other organelles due to the ATP-driven H+ pumps in the endosomal (or lysosomal) membrane that pump H+ from the cytosol into the lumen of endosome (or lysosome). Considering that the conventional liposomes are entering into the cells by endocytosis, the pH-sensitive liposomes can also be used to deliver liposome-loaded drugs to the cytosol after internalization and acidification of endosomal pH. In addition to palmitoyl homocysteine, cholesteryl hemisuccininate, dipalmitoyl succinyl glycerol, dioleoyl succinyl glycerol and oleic acid can also be used in this system (Madni et al. 2014).
One thing I’d like to point out is that both temperature-sensitive liposome and pH-sensitive liposome are best beneficial when these systems are delivered to the targeted sites of body with optimum stimuli, either temperature or pH, respectively. That’s one of the reasons why studies on temperature- or pH-sensitive immunoliposomes, where targeting antibody is also conjugated to the liposomes, were followed.
Polymerized liposomes
One of the major drawbacks of conventional liposomes is the leakage of drug contents before they reach the target sites. This problem can be solved by polymerization of lipids by thermal polymerization or irradiation method in the presence of free radical donor such as azobis-isobutyronitde or UV, respectively. Polymerized liposomes can be made in nano-sizes, and used for parenteral, oral and mucosal delivery of drugs with possible surface modification.
Ligand-conjugated liposomes
Drs. Gregoriadis and Neerunjun first suggested the possibility of homing of liposome to target cells in 1975 (Gregoriadis and Neerunjun 1975). Basic idea is very simple: ligand molecules on the liposome are to recognize the complementary molecules on the surface of the target cell. This is known to be the most popular and direct method for targeting of drug molecules with liposomes.
Several kinds of ligands have been conjugated to liposome surfaces including sugars, lectins, peptide hormones, small haptens, antibodies, and other proteins. Conjugation of ligands is achieved either before or after liposome preparation and either covalently or non-covalently. Typical conjugation of ligands to the surface of liposomes is performed using thioether (–S–) bonds through the reaction of thiols (usually from ligands) and maleimide group (usually from lipid-derivatives).
Antibody-conjugated immunoliposome
The most fascinating advantage of liposomes as a DDS is their possible use for targeting of drug molecules to the desired site of the body, thereby avoiding off-target toxicity. To this end, many scientists have been attracted by the possibility of conjugating target-specific antibodies to the liposome with the therapeutic drug encapsulated in the liposomes. On popular review of this concept, terms like “magic bullet”, “molecular zip-codes” or “guided missiles” became familiar to the general public (Ehrlich 1906).
Due to their high specificity, immunoliposome (liposomes with antibody attached to their surface) have emerged as one of the most promising tools for drug targeting in medical fields. Historically, Prof. V. Torchilin et al. reported the first paper of this kind in 1979, in which the immunoliposomes were successfully localized in acute myocardial infarction (Torchilin et al. 1979). Dr. Leserman and co-workers also showed a specific receptor-mediated delivery of the carboxyfluorescein (as a fluorescent marker) and methotrexate (as a pharmacologic agent) to murine tumor cells by using hapten-conjugated (Leserman et al. 1980) or antibody-conjugated liposomes (Leserman et al. 1981). Three major steps need to be elucidated in ligand-mediated delivery of liposomal contents to the cells for therapeutic benefit: (1) binding (or interaction) of ligand to the cell surface receptors, (2) endocytosis of ligand-receptor complex, and (3) release of liposome-encapsulated drugs into the cytosol or nucleus, though each step also contains numerous other hurdles to overcome.
Folate-conjugated liposomes
Folate receptor (FR) has two glycosyl phosphatidylinositol-anchored isoforms, α and β. FR-α is frequently overexpressed in many epithelial cancers, whereas FR-β is found in myeloid leukemia and other inflammatory diseases-related macrophages. As the conjugates of folic acid (FA) or anti-FR antibodies (FR mAb) can be taken up by numerous cancer cells via receptor (FR)-mediated endocytosis, conjugation of FA or FR mAb to liposomes can be used for targeted delivery of therapeutic drugs to FR-overexpressed cancer cells. FR-targeted liposomes have shown very promising efficacy in preclinical models and significant potential for future clinical application in a wide range of diseases including cancer.
Prof. R. Lee and P. Low reported the use of a low molecular weight ligand, folic acid, as a targeting agent for liposomal drug delivery. As the receptors for folic acid are highly expressed on certain cancer cells, it was studied that conjugation of folic acid might allow targeting of liposomes to tumor tissues (Lee and Low 1994).
Sterically-stabilized (long-circulating or stealth) liposomes
Conventional liposomal drug delivery has been hampered by short circulation time in blood due to the preferential accumulation of liposomes in organs of RES. In an effort to address this problem, various surface modifying molecules were investigated including synthetic or natural polymers and oligosaccharides, etc. Among these molecules, PEG (PEG, av. MW 2000–5000 Da) is known to be the most popular one because PEG is hydrophilic, conformationally flexible and highly mobile, resulting in decreased interactions of PEG-modified liposomes with various plasma proteins. Consequently, PEG modification have extended the half-life of conventional liposomes and reduced the liver uptake. These so-called PEGylated liposomes were later named as Stealth™ Liposome for their ability to avoid the RES uptake (The word Stealth™came from a military term for the fighter jet which can avoid the radar detection).
Prof. D. Papahadjopoulos and his co-workers published an article in PNAS in 1991 in which they showed that a liposome formulations incorporating a synthetic PEG-derivatized phospholipid exerted a pronounced effect on the biodistribution of liposome and produced a large increase in the pharmacological efficacy of encapsulated antitumor drugs.
Papahadjopoulos et al. (1991) liposome formulation with PEG–DSPE/HSPC/Chol showed a significant increase in the therapeutic index of antitumor drugs in mice against both a lymphoma and a colon carcinoma, presumably due to the prolonged circulation time in blood and diminished uptake by the liver and spleen. So, the term “stealth liposomes” has been named after the fighter aircraft as they can evade from the detection and clearance by RES in the blood. Most of the currently available liposomal drug products, such as Doxil, are this kind of PEGylated liposomes.
Cationic liposomes for gene delivery
Gene therapy is defined as “the therapeutic delivery of nucleic acids into a patient’s cells as a drug to treat disease”. Once successful “delivery” is achieved, one can expect the “addition”, “correction” and “replacement” of the genes to remove the cause of the disease. One of the major hurdles in the success of gene therapy is inefficiency of gene delivery. Even though many viral vectors have been used for gene delivery due to their high gene delivery efficiency, several drawbacks and limitations, such as toxicities and potential replication of competent viruses, still need to be thoroughly investigated before wide uses. Non-vial vectors, on the other hand, are drawing much attention due to their non-immunogenicity, low toxicity and large production capability, though low efficiency issue still needs to be addressed.
Liposomes are also studied as carriers of antisense oligodeoxynucleotide (AS-ODN), DNA and small interfering RNA (siRNA) for gene silencing or gene delivery. This was made possible especially with cationic liposomes as the DNA or siRNA is negatively charged at physiological pH so that the liposome (cationic)–DNA (anionic) complexes, called lipoplexes, are easily formed and condensed for delivery into the cells. Fusogenic lipids, such as dioleoyl phosphatidylethanolamine (DOPE), are often added to cationic lipid formulation for better transfection efficiency. These genetic materials were also encapsulated in the neutral or negatively-charged liposomes, though the encapsulation efficiency was not high enough to be used widely.
Prof. L. Huang’s group reported an efficient delivery and controlled expression of a foreign gene (chloramphenicol acetyltransferase gene) in mouse using a pH-sensitive immunoliposome (Wang and Huang 1987). Even though other groups reported earlier the successful delivery of exogenous gene and its expression in the cell, it has not yet been used widely mainly due to the poor transfection efficiency and safety issue.
Clinical use of liposome
During the last 50 years, liposome researches had focused on many life-threatening diseases such as cancer and fungal infection as shown in Table 1. More recently, liposomes are gaining much attention as gene delivery vectors, more favorably than the corresponding viral vectors for safety issue. Human clinical data of cationic liposomes for gene therapy are reported in many areas including cystic fibrosis (CF). Vical, Inc., a San Diego based bio-company, and University of Alabama team reported a result of a phase I clinical trial of safety and efficacy of cationic liposome mediated CFTR gene transfer in the nasal airway (Sorscher et al. 1994). The UK Cystic Fibrosis Gene Therapy Consortium reported a positive clinical result of a Phase I/II safety and efficacy study of nebulized liposome-mediated gene therapy for cystic fibrosis (Alton et al. 2015).
Many conventional chemical drugs with unfavorable properties, such as water insolubility, poor pharmacokinetic parameters and organ toxicity, are considered for liposomal encapsulation. Moreover, studies on the synthesis of new phospholipids are also important for the development of new liposomal drug products.
Summary
Liposomes have emerged as one of the most investigated drug delivery system during the last three decades, leading to the 13 liposomal drug products approved by US FDA as of 2014 and many others in clinical trials all over the world. Cancer chemotherapy, antimicrobial treatments, vaccines and gene therapy are major medial areas which received the most benefits from the liposome researches. With the advancement of technology for industrial scale-up, more liposomal drug products are expected to get marketing approval to benefit the patients in the near future.
References
Alton EW, Boyd AC, Porteous DJ, Davies G, Davies JC, Griesenbach U, Higgins TE, Gill DR, Hyde SC, Innes JA, UK Cystic Fibrosis Gene Therapy Consortium (2015) A phase I/IIa safety and efficacy study of nebulized liposome-mediated gene therapy for cystic fibrosis supports a multidose trial. Am J Respir Crit Care Med 192(11):1389–1392
Bangham AD, Horne RW (1964) Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol 8:660–668
Ehrlich P (1906) Collected studies on immunity. Wiley, New York
Gregoriadis G, Neerunjun ED (1975) Homing of liposomes to target cells. Biochem Biophys Res Commun 65(2):537–544
Gregoriadis G, Leathwood PD, Ryman BE (1971) Enzyme entrapment in liposomes. FEBS Lett 14(2):95–99
Lee RJ, Low PS (1994) Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem 269(5):3198–3204
Leserman LD, Weinstein JN, Blumenthal R, Terry WD (1980) Receptor-mediated endocytosis of antibody-opsonized liposomes by tumor cells. Proc Natl Acad Sci USA 77(7):4089–4093
Leserman LD, Machy P, Barbet J (1981) Cell-specific drug transfer from liposomes bearing monoclonal antibodies. Nature 293(5829):226–228
Madni A, Sarfraz M, Rehman M, Ahmad M, Akhtar N, Ahmad S, Tahir N, Ijaz S, Al-Kassas R, Löbenberg R (2014) Liposomal drug delivery: a versatile platform for challenging clinical applications. J Pharm Pharm Sci 17(3):401–426
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res 46:6387–6392
Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C et al (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 88(24):11460–11464
Sorscher EJ, Logan JJ, Frizzell RA, Lyrene RK, Bebok Z, Dong JY, Duvall MD, Felgner PL, Matalon S, Walker L et al (1994) Gene therapy for cystic fibrosis using cationic liposome mediated gene transfer: a phase I trial of safety and efficacy in the nasal airway. Hum Gene Ther 5(10):1259–1277
Torchilin VP, Khaw BA, Smirnov VN, Haber E (1979) Preservation of antibody activity after covalent coupling to liposomes. Biochem Biophys Res Commun 89(4):1114–1119
Wang CY, Huang L (1987) pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc Natl Acad Sci (USA) 84(22):7851–7855
Weinstein JN, Magin RL, Yatvin MB, Zaharko DS (1979) Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science 204(4389):188–191
Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R (1978) Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202(4374):1290–1293
Yatvin MB, Kreutz W, Horwitz BA, Shinitzky M (1980) pH-sensitive liposomes: possible clinical implications. Science 210(4475):1253–1255
Acknowledgments
Jin-Seok Kim declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, JS. Liposomal drug delivery system. Journal of Pharmaceutical Investigation 46, 387–392 (2016). https://doi.org/10.1007/s40005-016-0260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0260-1