Abstract
A study to determine the effect of varying soil moisture regimes on growth and carbon dioxide assimilation in soybean [Glycine max (L.) Merrill] was conducted under field conditions during 2018 and 2019 cropping seasons. The experiment was conducted in a randomized complete block design (RCBD) with a split plot treatment arrangement, replicated three times. Soil moisture regimes [100, 75, 50 and 25% of crop water requirement (CWR) and cultivars (Gazelle, Nyala, EAI 3600, DPSB 8, Hill and DPSB 19] were main and sub plot factors, respectively. Data were subjected to analysis of variance in GENSTAT release 18.1. Soil moisture stress reduced (P < 0.05) soybean shoot growth and yield of all tested soybean cultivars. Photosynthetically active radiation and sub-stomatal CO2 concentration declined (P < 0.05) due to soil moisture limitation. Soil moisture regime at 25% CWR reduced (P < 0.05) photosynthetic rate and stomata conductance by 86.45% and 36.64%, respectively, compared to CWR of 100%. Cultivar DPSB 19 had highest stomata conductance and photosynthetic rate at reduced CWR of 25% indicating moisture stress tolerance potential of the cultivar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean [Glycine max (L) Merrill] is an important legume crop as it contributes 29% of global edible oil and 70% of protein meal consumption [16]. Soybean yields in Kenya are low and range from 445 to 1200 kg ha−1 against potential yields of 3500 kg ha−1. These low yields are attributed to various biotic and abiotic factors including increased incidences soil moisture stress as a result of upward shifts in temperatures and reductions in rainfall due to global warming [35]. Soil moisture stress arising from recurring droughts has become a key limiting factor to crop production and food security [23]. In soybean, soil moisture stress may cause yield reduction of up to 45% due to suppressed biochemical and physiological processes [5, 29, 36]. Results of most previous soil moisture stress studies with soybean varied with geographical location of study areas meaning that local environmental conditions play a major role in plant response to soil moisture stress. This emphasizes the need for localized soil moisture stress studies so as to align crop production recommendations to prevailing environmental conditions. In addition, previous studies evaluated effects of short duration moisture stress which were effected either at vegetative or reproductive stages of soybean plant growth [1, 7, 10, 14, 23]. Soil moisture regimes in the current study were imposed from 30 days after seed germination up to physiological maturity of the crop thus exposing plants to soil moisture stress almost the entire growing period. This has the potential to generate better plant responses than short duration soil moisture stress.
Considering that reduction in rainfall is expected to continue due to global warming [4, 32], it is necessary to continuously understand growth, biochemical and physiological responses in plants which may act as a basis for generation of soil moisture stress mitigation measures in order to optimize crop yields. Key in soil moisture stress mitigation is the identification and use of soil moisture stress resilient crop cultivars for food self-sufficiency at national and household levels. Efforts by Government of Kenya to promote soybean as a food security crop are hampered by unavailability of information on the performance of available soybean cultivars under soil moisture stress. It was with this understanding that a study was undertaken to determine effect of varying soil moisture regimes on growth and carbon dioxide assimilation of selected soybean cultivars under field conditions in Kenya.
Materials and Methods
Site Description
The experiment was conducted under irrigation at Kenya Agricultural and Livestock Research Organization (KALRO) in Njoro (0° 20′ S; 35° 56′ E; 2120 m above sea level). Soils at the site are classified as mollic andosols in agro ecological zone III [18]. The experiment was conducted over two seasons during dry months of November to April during both seasons. Mean maximum monthly temperature and relative humidity for Njoro during 2017/2018 and 2018/2019 seasons are presented in Table 1.
Experimental Design and Treatments
The experiment was conducted as a randomized complete block design (RCBD) with a split plot treatment arrangement and replicated three times. Soil moisture regimes were evaluated at 100, 75, 50 and 25% of crop water requirement (CWR) and formed main plot treatments while soybean cultivars (Gazelle, Nyala, EAI 3600, DPSB 8, Hill and DPSB 19) were sub plot treatments. Gross plot sizes were 4.5 m long and 4 m wide (18 m2) while net plot sizes were 3 m long by 2.25 m wide (6.7 m2). Moisture regimes treatments were separated by 2 m wide path. Growth habits and phenology of soybean cultivars used in the study are shown in Table 2.
Planting and Crop Management
Soybean seed was inoculated with BIOFIX (Bradyrhizobium japonicum) inoculant strain USD 110 from Mea Limited–Kenya at the rate of 10 g kg−1 of seed prior to planting. Triple Super Phosphate and Muriate of Potash fertilizers were applied at the rates of 30 kg P2O5 ha−1 and 30 kg K2O ha−1, respectively, as basal dressing fertilizers. Planting was done at inter and intra row spacing of 45 cm and 10 cm, respectively. Planting of first season experiment was done on 8 November 2017, while second season experiment was planted on 6 November 2018.
Determination of Crop Water Requirement
Crop water requirement (ETc) on daily basis was determined according to Savva and Frenken [34] as follows:
where \({\text{ET}}_{{\text{c}}}\) Crop water requirement/crop evapotranspiration (mm/day), \(K_{{\text{c}}}\) crop factor, \({\text{ET}}_{{\text{o}}}\) reference evapotranspiration (mm/day). \(K_{c}\) coefficient for each irrigation treatment in the experiment.
Reference crop evapotranspiration was determined using pan evaporation method [34] as indicated below.
where \({\text{ET}}_{{\text{o}}}\) reference crop evapotranspiration (mm/day), \(E_{{{\text{pan}}}}\) evaporation pan reading (mm/day), \(K_{{{\text{pan}}}}\) pan coefficient (0.70 for class A pan).
Crop water requirement on daily basis was translated into volume of water per unit area using the following equation [6].
Respective soil moisture regimes treatments were initiated 30 days after planting and after depletion of soil moisture to 50% of field capacity [8]. Irrigation frequency was determined using the following equation by Savva and Frenken [34].
where \({\text{IF}}\) irrigation frequency (days), \({\text{SM}}_{{{\text{ta}}}}\) total available soil moisture [= field capacity − permanent wilting point] mm/m, \(P\) allowable depletion (0.5 for soybean), \({\text{RZD}}\) effective root zone depth (m), \({\text{ET}}_{{\text{c}}}\) crop water requirement (mm/day).
Data Collection
Morphological Parameters
Plant height was measured using a measuring tape from the soil surface to the last node of soybean plant. The number of branches per plant was determined by making individual counts of branches arising from the primary stem of soybean plant. Leaf area was measured using a manual method developed by Norman and Campbell [27]. It involved determination of individual leaf length (l) and width (w) and multiplied the product by a coefficient (k) which is 0.67 for legumes.
Intercepted Photosynthetically Active Radiation
Intercepted photosynthetically active radiation (IPAR) was measured at 50% flowering stage using an AccuPar Ceptometer (LP-80 PAR/LAI Decagon Devices). Measurements were done above and below canopy of soybean plants during clear sunny days. Intercepted photosynthetically radiation (IPAR) was determined as a percentage of incoming photosynthetically active radiation (PAR) using following modified formula by Purcell [30].
where \({\text{IPAR}}\) intercepted photosynthetically active radiation (\({\text{PAR}}\)); \({\text{PAR}}_{{\text{a}}}\) is \({\text{PAR}}\) (µmol m−2 s−1) measured above soybean canopy and \({\text{PAR}}_{{\text{b}}}\) is \({\text{PAR}}\) (µmol m−2 s−1) measured below soybean canopy.
Leaf Relative Water Content
Leaf relative water content (LRWC) was measured on a third trifoliate leaf from top of the plant at 50% flowering stage. Leaf samples were collected at midday and cut leaves were put in pre-weighed 150 millilitre tubes and sealed to avoid moisture loss. Equal amounts (150 millilitres) of distilled water were then added to tubes and samples placed in a refrigerator at 4 °C for 24 h for leaves to reach full turgor. After 24 h, leaf samples were removed from plastic containers, blotted dry with paper towel and weighed to get turgid weights. Leaf samples were then oven dried at 65 °C for 24 h after which dry weights were measured [33]. Leaf relative water content was determined using the following formula:
where \(\mathrm{LRWC}\) is leaf relative water content.
Measurement of Conductance
Stomata conductance was determined on three plants per plot at 50% flowering stage of soybean growth on abaxial side of a middle leaflet of a third trifoliate leaf from top of the plant. It was measured between 12.00 and 14.00 h on sunny days using a steady state leaf porometer (SC1, Decagon Devices, USA).
Determination of Sub-stomatal Carbon Dioxide Concentration, Photosynthetic and Transpiration Rates
Sub-stomatal CO2 concentration, photosynthetic and transpiration rates were determined on two plants per plot at vegetative (V5) and 50% flowering stages of soybean on a middle leaflet of a third trifoliate leaf from top of the plant. Measurements were done between 12.00 and 14.00 h during sunny days using a TPS-2 portable photosynthesis system (V2.02-PP systems Inc., USA).
Grain Yield
Grain yield was obtained by harvesting all plants in a net plot when all pods had dried. Harvested pods were then threshed and grains separated. Obtained grains were sun dried to a constant weight and then adjusted to a storage moisture content of 12%.
Statistical Analysis
Data obtained were checked for fulfilment of analysis of variance (ANOVA) assumption of normality by using Shapiro–Wilk normality test in Genstat release 18. Data that did not meet the aforesaid ANOVA assumption were subjected to square root transformation before analysis. Data were then subjected to ANOVA using the linear mixed model for RCBD with split plot arrangement in Genstat (Restricted Maximum Likelihood-REML.
Results
Plant Height
Plant height was significantly affected by the interaction effects of soil moisture regimes and seasons (P < 0.05) and of cultivars and seasons (P < 0.01). Tallest soybean plants were obtained at 100% crop water requirement (CWR) during 2018 season, while shortest plants were obtained at 25% CWR during 2019 season (Fig. 1). Overall, there was 31.54% reduction in plant height at the lowest soil moisture regime of 25% CWR compared to plants grown at 100% CWR.
Number of Branches
The number of branches borne from primary stem was significantly (P < 0.05) responsive to interaction effects of soil moisture regimes, cultivars and seasons (Table 3). Cultivars Hill and Gazelle had highest number of branches at 100% CWR during 2018 and 2019 seasons, respectively. Cultivar DPSB 19 had highest mean number of branches at the lowest soil moisture regime of 25% CWR. Overall, increase in soil moisture stress negated formation of branches regardless of season and cultivar.
Leaf Area
Leaf area per plant changed with interaction of soil moisture regimes and seasons (P < 0.01. Largest leaf area per plant was attained at 100% CWR during 2018 season (Fig. 2). Soil moisture limitation reduced leaf area regardless of seasons. Overall, there was 40.15% reduction in leaf area at 25% CWR compared to leaf area obtained at 100% CWR.
Leaf Area Index
Leaf area index (LAI) was significantly (P < 0.01) responsive to interaction effects of soil moisture regimes and seasons and of cultivars and seasons. Highest LAI was attained at 100% CWR during both seasons which was, nonetheless, at par with LAI attained at 75% CWR (Fig. 3). Cultivar and season interaction led to cultivars DPSB 8 (4.09), EAI 3600 (4.13) and Hill (3.25) having highest LAI during 2018 season. The same cultivars also registered highest LAI during 2019 season which were 4.09, 3.13 and 2.69, respectively.
Photosynthetically Active Radiation
Interception of photosynthetically active radiation (IPAR) was significantly dependent on interactions of soil moisture regimes and cultivars (P < 0.01). Soybean cultivars had highest IPAR at 100% CWR after which IPAR decreased with increased soil moisture limitation (Fig. 4). Overall, variety DPSB 8 had highest mean IPAR of 73.67%, while DPSB 19 had lowest mean IPAR of 63.99%.
Leaf Relative Water Content
Leaf relative water content (LRWC) was significantly dependent on interactions of soil moisture regimes and cultivars (P < 0.05) and of cultivars and seasons (P < 0.001). All soybean cultivars registered higher LRWC at the highest soil moisture regime of 100% CWR. Cultivar EAI 3600 had lowest per cent reduction (2.9%) in LRWC between the highest and lowest soil moisture regimes. Soybean cultivars had relatively higher LRWC during 2018 season than during 2019 season (Table 4).
Conductance
Stomata conductance at vegetative stage significantly varied with interaction of soil moisture regimes and cultivars (P < 0.05) and of cultivars and seasons (P < 0.001), while at 50% flowering stage, stomata conductance was significantly (P < 0.01) responsive to interaction of soil moisture regimes and cultivars (Table 5). Regardless of growth stage, increased rates of stomata conductance were attained at the highest soil moisture regime of 100% CWR where cultivars DPSB 19 and Gazelle had the highest stomata conductance rates at vegetative and flowering stages, respectively (Table 5). Stomata conductance was generally higher during 2019 season than during 2018 season (Table 5).
Sub-stomatal Carbon Dioxide Concentration
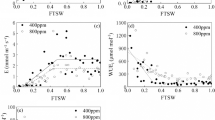
Sub-stomatal CO2 concentration was significantly responsive to main effects of soil moisture regimes (P < 0.05) and seasons (P < 0.001) at both vegetative and 50% flowering stages. Highest sub-stomatal CO2 concentration was attained at 100% CWR which corresponded to 56.88 and 47.99% increase over the lowest soil moisture regime of 25% CWR at vegetative and 50% flowering stages, respectively (Fig. 5). Highest sub-stomatal CO2 concentration of 104.65 µmol CO2 mol−1 was attained during 2019 season which was 8.75% more than sub-stomatal CO2 concentration registered during 2018 season.
Effect of soil moisture regimes on sub-stomata CO2 concentration at vegetative and 50% flowering stages during 2018 and 2019 seasons. Error bars represent ± standard error. Values significantly different at P < 0.05 and P < 0.01 at vegetative and flowering stages, respectively. CWR crop water requirement
Photosynthetic Rate
Photosynthetic rate significantly varied with main effects of soil moisture regimes (P < 0.05), cultivars (P < 0.01) and seasons (P < 0.001) at vegetative stage. At 50% flowering stage, photosynthetic rate varied with soil moisture regimes. Soil moisture limitation reduced photosynthetic rate of soybean plants (Fig. 6) and there was 76.53 and 77.29% reduction in photosynthetic rate at the lowest soil moisture regime of 25% CWR relative to highest soil moisture regime of 100% CWR at vegetative and 50% flowering stages, respectively. Cultivars DPSB 19 and EAI 3600 had highest and lowest photosynthetic rates of 12.89 and 6.05 µmol CO2 m−2 s−1, respectively. Photosynthetic rate was higher during 2019 season (11.09 µmol CO2 m−2 s−1) which corresponded to 35.84% increase over photosynthetic rate registered during 2018 season.
Transpiration Rate
Transpiration rate at vegetative stage was significantly (P < 0.01) responsive to interaction of soil moisture regimes, cultivars and seasons. All soybean cultivars had higher transpiration rates at 100% CWR (Fig. 7). Cultivars Gazelle and DPSB 19 had relatively lower transpiration rates than other cultivars at the lowest soil moisture regime of 25% CWR during 2019 season, while cultivar responses were not apparent during 2018 season. Seasonal variations led to higher transpiration rates during 2019 season than during 2018 season.
Correlations of Photosynthetic Rate with Stomata Conductance and Sub-stomatal Carbon Dioxide Concentration
There was a significant positive association between photosynthetic rate and stomatal conductance (0.2534 at P < 0.01) and between photosynthetic rate and sub-stomatal carbon dioxide concentration (0.2513 at P < 0.01) in soybean plants. Coefficients of determination (r2) show that 99.32 and 93.42% variations in photosynthetic rates amongst soybean plants grown at different soil moisture levels may be due to differences in the degree of stomata conductance and sub-stomatal carbon dioxide concentration, respectively.
Days to Flowering and Pod Maturity
Number of days of 50% flowering by soybean plants significantly (P < 0.001) varied with main effects of soil moisture regimes and cultivars. Soybean plants grown at soil moisture regime of 25% CWR flowered 82 days after planting which was 5 days earlier than plants grown at 100% CWR. Cultivar DPSB 19 took 71.46 days to flower which was the earliest compared to other test cultivars. Number of days to 50% pod maturity significantly varied with main effects of soil moisture regimes, (P < 0.05) and cultivars (P < 0.001). Soil moisture stress hastened pod maturity with soybean plants grown at 25% CWR maturing after 132.72 days after planting which was 7.54 days earlier than plants grown at higher soil moisture levels. Cultivar DPSB 19 was the earliest to mature at 115.6 days after planting, while cultivar DPSB 8 matured late at 146.3 days after planting.
Grain Yield
Grain yield significantly varied with interactions of soil moisture regimes and seasons (P < 0.01) and of cultivars and seasons (P < 0.001). The highest and the lowest grain yields were attained at 100 and 25% CWR, respectively, during both seasons (Fig. 8). Soybean yield was higher during 2018 season than 2019 season at each soil moisture regime but only significantly different at 50% and 25% CWR.
Discussion
Plant growth is anchored by photosynthesis through production of photoassimilates for cell division, multiplication and elongation [16]. Findings of this study have shown that soil moisture stress reduced conductance, sub-stomatal CO2 concentration which led to reduced photosynthetic rate. Plants grown at lower than optimal soil moisture levels had limited cell division and elongation leading to reductions in plant height, branching and leaf production [31]. Leaf relative water content was lower at higher soil moisture limitation which reduced plant cellular and metabolic activities translating into retarded soybean shoot growth. Under soil moisture stress, there is limited uptake of mineral nutrients to support plant growth. Limited mineral nutrient uptake may arise from suppressed root growth, limited root penetration in the soil profile and limited uptake of less mobile nutrients like phosphorous due to absence of water as solvent and medium through which nutrients are acquired [11]. Reduced leaf area at lower soil moisture levels could have been the result of suppressed leaf production in addition to increased leaf senescence [25]. Interception of PAR was higher in 2018 than 2019 season which contributed to increased synthesis of photoassimilates during 2018 season that supported faster plant growth leading to taller plants during 2018 than during 2019 season.
Results show that soil moisture stress reduced IPAR, LRWC, conductance, sub-stomatal CO2 concentration, transpiration and photosynthetic rates. Reductions in IPAR, LRWC, stomata conductance, sub-stomatal CO2 concentration, transpiration and photosynthetic rate due to soil moisture stress effects were reported by Kirnak et al. [21], Krivodska and Filova [22] and Jha et al. [20] which is in agreement with findings of this study. Results, however, contradict findings of Zhang et al. [38] who reported increased sub-stomatal CO2 concentrations at reduced soil moisture levels. Plants close stomata at limited soil moisture levels to prevent excessive water loss to the environment which leads to reduction in stomata conductance [38]. Furthermore, stomata closure at lower soil moisture levels could have emanated from reduction in leaf water potential as evidenced from lower LRWC [37]. Stomata closure may also have been caused by secondary effect of increased production of endogenous abscisic acid which triggers closure of stomata to avoid desiccation of plants through tissue water loss by transpiration [28]. Stomata closure under soil moisture stress reduced transpiration rate, diffusion of CO2 into intercellular spaces of plant leaves [9, 26]. Soil moisture stress reduces activity of photosynthetic enzymes such as Rubisco which may also have led to a reduction in photosynthesis [39]. Soil moisture stress may also lead to overproduction of reactive oxygen species (ROS) which have deleterious effects on photosynthesis [2, 24]. Increased IPAR at higher soil moisture regimes may be attributed to larger leaf area and LAI which was registered at optimum soil moisture regimes.
Day time temperatures were 2.4% higher and relative humidity 14.0% lower during 2019 season compared to 2018 season which could have triggered increased stomata resistance to water loss. Higher photosynthetic rate during 2019 season could have been due to increased sub-stomatal CO2 concentration in soybean leaves relative to 2018 season. Even under drier conditions, plants will open stomata to allow more CO2 to enter for photosynthesis to take place [3]. Lower LRWC during a hotter and drier season of 2019 concurs with findings of Jeon et al. [17, 19] who indicated that plants will generally have reduced LRWC when temperatures are higher. Stomata conductance and photosynthetic rate were generally higher for soybean cultivars with indeterminate growth habit (DPSB 19 and DPSB 8) compared to cultivars with determinate growth habit. Soybean cultivars with indeterminate growth habit exhibit greater stomata density, higher number of epidermal cells per unit area and are able to recover from soil moisture stress much faster than determinate cultivars [39]. This explains why indeterminate soybean cultivars had relatively higher carbon dioxide concentration and photosynthetic rate compared to determinate cultivars.
Photosynthesis is a source of photoassimilates for pod and seed development [12]. Results of this study have however shown that soil moisture stress reduced photosynthetic rate which could have limited supply of photosynthates to reproductive sinks of soybean plants under soil moisture stress leading to lower yields [15]. Effective nodulation and biological nitrogen fixation are key to realization of optimal soybean grain yield. It is however reported that under soil moisture stress, rhizobia undergo morphological changes that cause reduction in infection and nodulation of soybean roots leading to low grain yield [8, 13].
Conclusions
Soil moisture limitation reduces stomata conductance and sub-stomata carbon dioxide concentration which leads to reduced rates of photosynthesis. Cultivar DPSB 19 was the earliest to mature and had relatively higher mean stomata conductance, photosynthetic rate and lower transpiration rate at reduced soil moisture level which indicates drought tolerance potential of the cultivar. Soil moisture level at 50% CWR should be a cut-off point for optimal soybean production beyond which supplementary irrigation be employed to optimize soybean grain yield.
References
Adeboye OB, Schultz B, Adekalu KO, Prasad K (2015) Crop water productivity and economic evaluation of drip irrigated soybean (Glycine max L. Merr.). Agric Food Secur 4:1–13
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol 23:731–744
Araújo WL, Fernie AR, Nunes-Nesi A (2011) Control of stomatal aperture: a renaissance of the old guard. Plant Signal Behav 6:1305–1311
Arnell NW, Lowe JA, Hughes BL, Osborn TJ (2018) The impacts avoided with a 1.5 °C climate target: a global and regional assessment. Clim Chang 147:61–76
Barnabas B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Brouwer C, Heibloem M (1985) Irrigation water management: irrigation water needs. FAO training manual No. 5. Food and Agricultural Organisation of the United Nations, Rome
Candoğan BN, Yazgan S (2016) Yield and quality response of soybean to full and deficit irrigation at different growth stages under sub-humid climatic conditions. Tar Bil Der 22:129–144
Chafi AA, Gohari AA (2013) The effect of various irrigation regimes and moisture variations and water infiltration in soil on yield and water use efficiency in soybean (Glycine max). Intl J Farm Alli Sci 2:27–31
Dalal VK, Tripathy B (2012) Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ 35:1685–1703
Demirtas Ç, Yazgan S, Candogan BN, Sincik M, Büyükcangaz H, Göksoy AT (2015) Quality and yield response of soybean (Glycine max L. Merrill) to drought stress in sub–humid environment. Afr J Biotechno 9:6873–6881
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Farooq M, Hussain M, Siddique KH (2014) Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci 33:331–349
Farooq M, Gogoi N, Barthakur S, Baroowa B, Bharadwaj N, Alghamdi SS, Siddique M (2017) Drought stress in grain legumes during reproduction and grain filling. J Agron Crop Sci 203:81–102
Giménez L, Paredes P, Pereira LS (2017) Water use and yield of soybean under various irrigation regimes and severe water stress. Application of Aqua Crop and SIMDualKc models. Water 9:1–18
Gusmao M, Siddique KHM, Flower K, Nesbitt H, Veneklaas EJ (2012) Water deficit during the reproductive period of grass pea (Lathyrus sativus L.) reduced grain yield but maintained seed size. J Agron Crop Sci 198:430–441
Hartman GL, West EO, Herman TK (2011) Crops that feed the world 2. Soybean worldwide production, use and constraints caused by pathogens and pests. Food Secur 3:5–17
Hossain M, Liu X, Qi X, Lam H, Zhang J (2014) Differences between soybean genotypes in physiological response to sequential soil drying and rewetting. Crop J 2:366–380
Jaetzold R, Schmidt H, Hometz B, Shisanya C (2010) Farm management handbook of Kenya vol. 2. Natural conditions and farm management information, 2nd edn. Southern Rift Valley Province
Jeon MW, Ali MB, Hahn EJ, Paek KY (2006) Photosynthetic pigments, morphology and leaf gas exchange during ex vitro acclimatization of micropropagated CAM Doritaenopsis plantlets under relative humidity and air temperature. Environ Exp Bot 55:183–194
Jha PK, Kumar SN, Ines AV (2018) Responses of soybean to water stress and supplemental irrigation in upper Indo-Gangetic plain: field experiment and modelling approach. Field Crops Res 219:76–86
Kirnak H, Dogan E, Türkoğlu H (2010) Effect of drip irrigation intensity on soybean seed yield and quality in the semi-arid Harran plain, Turkey. Span J Agric Res 8:1208–1217
Krivosudska E, Filova A (2013) Evaluation of selected soybean genotypes (Glycine max L.) by physiological responses during water deficit. J Cent Eur Agric 14:213–228
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529:84–87
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Mangena P (2018) Water stress: Morphological and anatomical changes in soybean (Glycine max L.) plants. In: Plant, abiotic stress and responses to climate change. InTechOpen, p 72889
Monteiro MV, BlanušaT VA, Hadley P, Cameron RWF (2016) Relative importance of transpiration rate and leaf morphological traits for regulation of leaf temperature. Aust J Bot 64:32–44
Norman JM, Campbell GS (1989) Canopy structure. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology: field methods and instrumentation. Chapman and Hall, New York, pp 301–325
Osakabe Y, Osakabe K, Shinozaki K, Tran LSP (2014) Response of plants to water stress. Front Plant Sci 5:1–8
Pedersen P, Lauer JG (2004) Response of soybean yield components to management system and planting date. Agron J 96:1372–1381
Purcell LC (2000) Soybean canopy coverage and light interception measurements using digital imagery. Crop Sci 40:834–837
Rehem BC, Bertolde FZ, de Almeida AAF (2012) Regulation of gene expression in response to abiotic stress in plants. In: Cell metabolism-cell homeostasis and stress response. InTechOpen, p 26769
Rosenzweig C, Colls J (2005) Global warming and agriculture. In: Sylvester-Bradley R, Wiseman J (eds) Yield of farmed species: constraints and Opportunities. University of Nottingham, Nottingham, pp 143–165
Sade N, Galkin E, Moshellon M (2015) Measuring Arabidopsi, tomato and barley leaf relative water content. Bio Protoc 5:1–4
Savva AP, Frenken K (2002) Crop water requirement and irrigation scheduling: Food and Agricultural Organization of the United Nations Irrigation Manual No. 4. East and Southern Africa FAO sub-regional office, Harare, Zimbabwe
Sentelhas PC, Battisti R, Câmara GMS, Farias JRB, Hampf AC, Nendel C (2015) The soybean yield gap in Brazil–magnitude, causes and possible solutions for sustainable production. J Agric Sci 153:1394–1411
Wijewardana C, Reddy KR, Alsajri FA, Irby JT, Krutz J, Golden B (2018) Quantifying soil moisture deficit effects on soybean yield and yield component distribution patterns. Irrig Sci 36:241–255
Yan W, Zhong Y, Shangguan Z (2016) A meta-analysis of leaf gas exchange and water status responses to drought. Sci Rep 6:1–9
Zhang J, Liu J, Yang C, Du S, Yang W (2016) Photosynthetic performance of soybean plants to water deficit under high and low light intensity. S Afr J Bot 105:279–287
Zlatev Z, Lidon FC (2012) An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir J Food and Agric 24:57–72
Acknowledgements
The study was conducted with financial support from Government of Malawi through Agricultural Productivity Programme for Southern Africa (APPSA) under the Department of Agricultural Research Services (DARS). We thank Egerton University for all the logistical support towards conducting of the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Louis Hortensius Mwamlima. The first draft of the manuscript was written by Louis Hortensius Mwamlima and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists regarding funding of the study and writing of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mwamlima, L.H., Cheruiyot, E.K. & Ouma, J.P. Soil Moisture Modulates Carbon Dioxide Assimilation in Soybean (Glycine max). Agric Res 11, 652–662 (2022). https://doi.org/10.1007/s40003-021-00601-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-021-00601-8