Abstract
An experiment was conducted to study the effects of probiotics (Saccharomyces cerevisiae NCDC 49 and Lactobacillus acidophilus-15) on blood biochemical profile, immunity and small intestine morphology in growing finishing pigs, weaned at 28 days of age. Thirty-six cross-bred (Landrace X Desi) piglets were allocated to three treatments on the basis of the body weight in a completely randomized design. Each treatment was comprised of four replicates with three piglets in each. The three dietary treatments were: basal diet without any probiotic (Control), basal diet where 10 % of feed was replaced by feed fermented with S. cerevisiae NCDC 49 with the count of 3–5 × 106 cfu/g (SC) and basal diet with 10 % of feed fermented with L. acidophilus-15 with the count of 2–3 × 109 cfu/g (LA). The results showed that probiotic supplementation had no effect (P > 0.05) on blood biochemical profile. Antibody titre against 20 % SRBC injection was significantly higher in probiotic-supplemented groups than control. Villus height and villus height/crypt depth ratio of jejunum were increased (P < 0.01) in SC and LA groups as compared to control. It is concluded that inclusion of probiotics at 10 % level of the basal diet improved the immunity and intestinal morphology of growing finishing pigs weaned at 28 days of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The non-antibiotic approach to control the infections and the enhancement of life performance are urgently required because increases in microbial resistance to antibiotics and residues in meat products can be harmful to consumers. Probiotics have been used in swine industry not only to enhance growth performance [3, 5], but also to improve the body’s natural defences and gut health [21]. The manipulation of gut microbiota via the administration of probiotic influences the development of the immune response [10]. Probiotics have the ability to shape the immune system by their physiological action in the intestines and can improve the performance of the animals. It is suggested that the appropriate use of probiotic can reduce the use of antibiotics in pig industry. The feeding of Saccharomyces cerevisiae NCDC-49 (National Collection of Dairy Cultures; NCDC) or Lactobacillus acidophilus-15 to the piglets weaned at the age of 28 day resulted in a significant improvement (13.43 and 12.79 %) in weight gain as compared to control animals [14]. Various strains of S. cerevisiae and L. acidophilus were tested in the laboratory for their potential as probiotic [1], and the strains, S. cerevisiae NCDC 49 and L. acidophilus-15, were found to be best. Therefore, the current study was undertaken to determine the effect of dietary supplementation of these two probiotics on blood biochemical profile, immunity and small intestine morphology to find out the changes taking place in the animals along with improvement in body weight gain.
Materials and Methods
Preparation of Probiotic Products
The probiotic products were prepared as described by Agarwal et al. [1]. Microbes, S. cerevisiae (NCDC-49) and L. acidophilus (NCDC-15) procured from National Dairy Research Institute, Karnal, were maintained in laboratory as stock culture. The yeast broth containing yeast extract 3.5 g, peptone 5.0 g and glucose 10.0 g per litre of distilled water and Rogosa broth [18] were prepared and autoclaved.
From the stock culture, a loop full of S. cerevisiae and L. acidophilus culture was transferred aseptically to 100 ml of yeast broth and Rogosa broth, respectively. The broths were incubated for 24 h at 37 °C. Basal diet (1000 g), mixed with equal amount of water, was inoculated with 200 ml of 24-h old culture of S. cerevisiae, and similarly, same amount of feed was inoculated with L. acidophilus culture, separately. The S. cerevisiae-fermented feed was fed to the animals of SC group and L. acidophilus-fermented feed was fed to the animals of LA group. The same fermented feeds were used as inoculums (20 % of concentrate mixture) for preparation of next day’s fermented feed. After 15 days, fresh cultures were taken and used as described above and used consecutively for next 15 days.
Animal Diets and Management
Thirty-six cross-bred (Landrace X Desi) piglets weaned at 28 days were randomly allotted to three treatments on the basis of initial body weight in a randomized complete block design. Each treatment had 12 piglets arranged in 4 replicates of 3 piglets in each. The three dietary treatments were: basal diet without any probiotics (control), basal diet where 10 % of feed was replaced with S. cerevisiae (SC)-fermented feed (SC group) and basal diet where 10 % of feed was replaced with L. acidophilus-fermented feed (LA group). The fermented feeds had 3–5 × 106 cfu/g and 2–3 × 109 cfu/g of S. cerevisiae and L. acidophiolus. Piglets were fed basal diet (concentrate mixture) as per NRC [15] consisting of maize, soya bean, wheat bran and fish meal as major ingredients (Table 1). The animals were housed in cemented corrugated floor pens with no litter, and each pen was equipped with a feeder and tap. During experimental period, feed and water were provided ad libitum. The feeding trial was conducted for 140 days.
Blood Biochemical Analysis
On 0 and 120 days of experiment, blood was collected and serum was obtained to determine blood biochemical profile. The serum was analysed for glucose, total protein and albumin as per the methods described by Henry [8], Gornell et al. [4] and Gustaffson [6], respectively. Globulin was determined as the difference between total protein and albumin concentration in the serum. Cholesterol and triglycerides contents in the serum were estimated by the method of Wybenga et al. [26] and McGowan [13].
Immunological Studies
Immunological studies were performed during the last month of the experiment. Humoral response was studied by microhemagglutination assay as described by Wagmann and Smithies [24] against injecting 1 ml of a suspension of 20 % sheep red blood cells (SRBC) in phosphate-buffered saline solution (PBS) i/m into the ham region of pig. Blood samples were taken by venipuncture at 0, 7, 14, 21 and 28 days of post-injection. Pigs were again challenged with SRBC on day 28 to investigate the secondary immune response on day 35. Blood was centrifuged; serum was collected and analysed for antibody titre against SRBC.
Cell-mediated immune response was assessed by measuring the changes in skin-fold thickness in response to intra-dermal injection with phytohemagglutinin-P (PHA-P; Bangalore Genei, India). One mg of PHA-P was dissolved in 1 ml of sterile physiological saline solution, and resulting concentration was 100 µg/100 µl. The skin of the area to be tested (upper side of each shoulder) was shaved 24 h in advance so as to facilitate subsiding of any inflammation due to abrasion. Two hundred microlitres of intra-dermal PHA-P was injected on the upper side of each shoulder. The thickness of the skin was measured with the help of a digital vernier caliper just before injection and was represented as the basal (0 h) value. Subsequently, all piglets were administered PHA-P into the centre of a 2-cm-diameter circle marked on shaved skin. Skin thickness was monitored at 24, 48, 72 and 96 h post-inoculation and expressed as the percentage of increase in skin thickness compared with 0 h.
Small Intestine Morphology
At day 120 post-weaning, four piglets per group were slaughtered and the systemic necropsy was conducted. The entire intestinal tracts were removed, and jejunum collected from each animal was immediately fixed in 10 % neutral buffered formalin. The specimens were then dehydrated in graded alcohols, cleared with xylene and embedded in paraffin, serial microtome sections (6 μm thick) were stained with haematoxylin and eosin and examined to assess microanatomical structure, number of villi in 50 µm distance, villus height, crypt depth, width of villi (in middle), intervillous distance, number of goblet cells and infiltration in lamina propria were determined at 10 × magnification using light microscope, and villus height and crypt depth ratio were calculated.
Statistical Analysis
The experimental data generated were analysed using statistical package SPSS 17.0. Means were compared using Duncan’s multiple range test adopting standard statistical procedures [23]. Bacteria and yeast count were transformed log10 before statistical analysis.
Results and Discussion
In the present study, fermented feed was used as probiotics so that live cells of the microbes could be fed because it has been documented that probiotics are more effective when live cells are fed [20]. The fermented feeds were counted at every 15-day interval, and the counts were ranged 3–5 × 106 and 2–3 × 109 cfu/g for S. cerevisiae and L. acidophilus. The feeding of either of the probiotics significantly improved the body weight gain in the piglets.
Blood Biochemical Profile
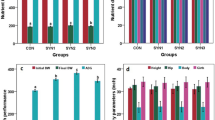
Table 2 shows the effects of probiotics supplementation on blood biochemical profile in weaned piglets. Determined serum chemistry parameters including glucose, total protein, albumin, globulin, total cholesterol and triglycerides were not affected by dietary supplementation of probiotics (P > 0.05). Chen et al. [2] also found no change in the blood indices by feeding probiotic-supplemented feed to the growing pigs. Pigs shifted to cholesterol-free diet had lower cholesterol level, but feeding of 2.5 × 1011 cells of L. acidophilus ATCC 42131 per kg feed resulted in further lowering of cholesterol in comparison with non-probiotic group [17], and the authors claimed that the probiotic has anticholesterogenic characteristics. Kumar et al. [11] also observed that the level of cholesterol was significantly reduced in yeast fed piglets as compared to control. The response of animal to the probiotic very much depends upon the cultures used and its dose. Hung et al. [9] tested two cocktails of microbial cultures as probiotic in the form of fermented soya bean meal and found that only one cocktail of cultures (FSM-A) was effective in lowering of serum cholesterol. Both the mixture of cultures contained L. acidophilus and S. cerevisise along with other cultures. In the present study, the cultures used as probiotic might not be that effective to induce the beneficial changes in the blood indices.
Immunological Study
The antibody titre was significantly higher in both the probiotic-fed groups as compared to control representing improvement in humoral immune response of the pigs (Table 3). After one week of SRBC injection, the antibody increased and continued to increase up to 21 days post-injection and thereafter they reduced at 28 days. At every week, the increase in the antibody titre was more in both probiotic-fed groups. Similarly, the cell-mediated immune response as indicated by significantly more skin thickness by injecting PHA-P was improved in both the probiotic-fed groups as compared to control (Table 4). The skin thickness was at 6 h post-injection and continued to increase up to 24 h and thereafter it reduced. Again the increase in skin thickness was more in probiotic-fed groups at every stage of analysis. The results revealed that both S. cerevisiae and L. acidophilus when used as probiotic were able to make the animals healthier than the control animals. Hung et al. [9] also demonstrated significantly higher antibody titre against CSF vaccine in probiotic-fed groups revealing improved humoral immune response. In porcine production, it is very important to improve immunity in order to prevent infectious diseases. Similarly, Kumar et al. [12] concluded that S. cerevisiae feeding improved the immune response of the early weaned cross-bred piglets.
Small Intestine Morphology
Histometry analysis showed that villus height and villus height to crypt depth increased (P < 0.01) in piglets fed probiotic-supplemented diets (SC and LA) compared to the piglets fed control diet (Table 5). Villus height is a direct indication of the maturity and functional capacity of enterocytes [7]. It is assumed that an increased villus height is paralleled by an increased digestive and absorptive function of intestine due to increased surface area, expression of brush border enzymes and nutrient transport systems [16]. In present study, supplementation of S. cerevisiae and L. acidophilus to piglet’s diet resulted in increase villus height in jejunum and this might be due to healthier intestinal environment because of increased humoral immune response. Shen et al. [21] observed that supplementation of 5 g yeast culture/kg diet improved the villus height and villus/crypt ratio in the jejunum as compared to control piglets, but was comparable to the animal received antibiotic growth promoters and suggested that yeast can be an effective replacer of antibiotics growth promoters. Likewise, Shirkey et al. [22] have reported that villus height was the longest in jejunum of pigs supplemented with Lactobacillus fermentum as probiotic. The crypt depth in the present study did not show any change by probiotic supplementation. Scharek et al. [19] also reported no significant change in the crypt depth in proximal jejunum of pigs supplemented with Enterococcus faecium 68. However, Willing and Van Kessel [25] observed increased crypt depth in piglets inoculated with Lactobacillus fermentum as compared to control animals.
Conclusions
The results obtained in present study indicated that the feeding of feed fermented with either Saccharomyces cerevisiae or Lactobacillus acidophilus as probiotic at 10 % level of basal diet improved intestinal morphology through modulating gut immune response in the growing finishing pigs. But the discussion revealed discrepancy in the results of various experiments which might be due to the reason that response of animal to probiotic feeding is regulated by number of factors such as strain of probiotic, dose of probiotic, diet composition and mode of feeding indicating that a probiotic is highly specific, and therefore, each probiotic should be defined very specifically.
References
Agarwal N, Kamra DN, Chaudhary LC, Agarwal I, Sahoo A, Pathak NN (2002) Microbial status and rumen enzyme profile of crossbred calves fed on different microbial feed additives. Lett Appl Microbiol 34:329–336
Chen YJ, Son KS, Min BJ, Cho JH, Kwon OS, Kim IH (2005) Effects of dietary probiotic on growth performance, nutrients digestibility, blood characteristics and fecal noxious gas content in growing pigs. Asian-Australas J Anim Sci 18:1464–1468
Giang HH, Viet TQ, Ogle B, Lindberg JE (2011) Effects of supplementation of probiotics on the performance, nutrient digestibility and fecal microflora in growing- finishing pigs. Asian Australas J Anim Sci 24:655–661
Gornell AG, Bardawill CJ, David MM (1949) Biuret method of protein determination. J Biol Chem 177:751
Guerra NP, Bernardez PF, Mendez J, Cachaldora P, Pastrana Castro L (2007) Production of four potentially probiotic lactic acid bacteria and their evaluation as feed additives for weaned piglets. Anim Feed Sci Technol 134:89–107
Gustaffson EJ (1978) Improved specificity of serum albumin determination and estimation of acute phase reactant by use of the bromocresol green reduction. Clin Chem 22:616–622
Hampson DJ (1986) Alterations in piglets small intestinal structure at weaning. Res Vet Sci 40:32–40
Henry RJ (1963) Standard methods of clinical chemistry (Ed. D. Seligson). Academic Press, New York
Hung ATY, Su TM, Liao CW, Lu JJ (2008) Effect of probiotic combination fermented soybean meal on growth performance, lipid metabolism and immunological response of growing-finishing pigs. Asian J Anim Vet Adv 3:431–436
Isolauri E, Sutas Y, Kankaanpaan P, Arvilommin H, Salminen S (2001) Probiotics: effects on immunity. Am J Clin Nutr 73:444S–450S
Kumar S, Verma AK, Mondal SK, Gupta M, Patil AK, Jangir BL (2012) Effect of live Saccharomyces cerevisiae feeding on serum biochemistry in early weaned cross bred piglets. Vet World 5:663–666
Kumar S, Verma AK, Singh P (2012) Effect of live Saccharomyces cerevisiae on immune response in early weaned crossbred piglets. Indian J Anim Nutr 29:393–396
McGowan MW (1983) A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem 29:538–542
Mishra DK, Verma AK, Agarwal N, Mondal SK, Singh P (2014) Effect of dietary supplementation of probiotics on growth performance, nutrients digestibility and faecal microbiology in weaned piglets. Anim Nutr Feed Technol 14:283–290
NRC (1998) Nutrient Requirements of swine, 10th edn. National Academy Press, Washington
Pluske JR, Williams IH, Aherne FX (1996) Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim Sci 62:131–144
Rodas BZD, Gilliland SE, Maxwell CV (1996) Hypercholesterolemic action of Lactobacillus acidophilus ATCC 43121 and calcium in swine with hypercholesterolemia induced by diet. J Dairy Sci 79:2121–2128
Rogosa M, Mitchell JA, Wiseman RF (1951) A selective medium for isolation and enumeration of oral and faecal lactobacilli. J Bacteriol 62:136
Scharek L, Guth J, Reiter K, Weyrauch KD, Taras D, Schwerk P, Schierack P, Schmidt MF, Wieler LH, Tedin K (2005) Influence of a probiotic Enterococcus faecium strain on development of the immune system of sows and piglets. Vet Immunol Immunopathol 105:151–161
Scholten RHJ, van der Peet-Schwering CMC, Verstegen MWA, den-Hartog LA, Schrama JW, Vesseur PC (1999) Fermented co-products and fermented compound diets for pigs: a review. Anim Feed Sci Technol 82:1–19
ShenYB Piao XS, Kim SW, Wang L, Liu P, Yoon I, Zhen YG (2009) Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci 87:2614–2624
Shirkey TW, Siggers RH, Goldade BG, Marshall JK, Drew MD, Laarveld B, Van Kessel AG (2006) Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med (Maywood) 231:1333–1345
Snedecor GW, Cochran WG (1994) Statistical methods, 8th edn. Oxford and IBH Publishing Company, New Delhi
Wagmann NTG, Smithies O (1966) A simple hemagglutination system requiring small amounts of red cells and antibodies. Transfusion 6:67
Willing BP, Van Kessel AG (2007) Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J Anim Sci 85:3256–3266
Wybenga DR, Pileggi VJ, Dirstine PI, Giorgio D (1970) Direct manual determination of serum total cholesterol with a single stable reagent. Clin Chem 16:980–984
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, D.K., Verma, A.K., Agarwal, N. et al. Effect of Probiotics on Blood Biochemical Profile, Immunity and Small Intestine Morphology in Growing Finishing Pigs. Agric Res 5, 407–412 (2016). https://doi.org/10.1007/s40003-016-0231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-016-0231-9