Abstract
Purpose
The prevalence of obesity is an escalating concern in modern populations, predominantly attributed to the widespread adoption of sedentary lifestyles observed globally. Extensive research has established a significant association between obesity and Helicobacter pylori (H. pylori). Nonetheless, a comprehensive assessment of the global prevalence of H. pylori among individuals with obesity remains undetermined.
Methods
A systematic search strategy was applied to PubMed, Scopus, and Web of Science. The resulting records were screened using the Rayyan online tool for the management of systematic reviews. Freeman–Tukey double arcsine transformation was used. Subgroup analyses (continent, regional classifications, developmental status, religion, global hemisphere, income, access to international waters, and H. pylori eradication) and multivariate meta-regression (latitude, longitude, male-to-all ratio, mean age, and body mass index) were done to estimate the effects of the moderators. Risk of bias assessment was done using JBI checklist for prevalence studies.
Results
A total of 472,511 individuals with obesity from 208 studies were included. The global estimation of H. pylori prevalence among individuals with obesity was 32.3% (95% CI 26.9%, 38.0%). South America had the highest prevalence. Based on the different classifications of countries, resource-rich, low-/middle-income, developing, and Islamic countries had the highest prevalence. Lower pooled prevalence was observed in the studies with adequate sample sizes (n ≥ 270).
Conclusion
The findings have the potential to influence future health policies for preventing and treating H. pylori infection. However, there is variability among the included studies, indicating the need for more population-based research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Given the potential for transmission and the significant complications that can arise, particularly in individuals with chronic infections, the prevalence of Helicobacter pylori (H. pylori) infection and its association with host and environmental factors have become a subject of scientific interest. In this regard, obesity represents one such host factor that is prevalent worldwide, and understanding its association with H. pylori infection poses a complex challenge. Mostly due to sedentary lifestyles and dietary changes, the global prevalence of obesity has multiplied in recent decades and become a growing public health concern throughout the world [1]. In this regard, the association between obesity and several gastrointestinal (GI) complications has been quantified [2]. However, the mutual relationship between obesity and H. pylori infection is complex. Extensive research efforts have been dedicated to examining the prevalence of H. pylori infection among the general population, both on a global and regional scale [3,4,5]. However, an important knowledge gap still exists regarding the estimation of H. pylori prevalence among individuals with obesity.
A recent meta-analysis has provided conclusive evidence that individuals infected with H. pylori are more prone to obesity and, conversely, individuals who are obese present an elevated risk of acquiring H. pylori infection [6]. The mutual relationship between H. pylori and obesity is intricate and the simultaneous coexistence of H. pylori infection and obesity can lead to a heightened susceptibility to complications. For instance, individuals may experience elevated levels of glycosylated hemoglobin in diabetes as well as an increased risk of developing gastric cancer [7, 8]. Moreover, due to the similarities in GI pathologies and subsequent clinical presentations, it is feasible that individuals with obesity experiencing heartburn and indigestion be erroneously diagnosed with H. pylori-associated symptoms and consequently receive excessive treatment.
An estimation of H. pylori infection prevalence among individuals with obesity can provide valuable guidance to health policymakers and clinicians in devising efficient diagnostic and eradication strategies for H. pylori infection among individuals with obesity. These clinical ramifications become particularly salient in the preoperative evaluation of bariatric surgeries, where healthcare teams must carefully factor in the endemic nature of the infection to delineate optimal screening methodologies.
In this study, our objective was to conduct a systematic review of relevant studies and assess the prevalence of H. pylori in individuals with obesity. Additionally, we aimed to analyze the distribution of H. pylori prevalence across different geographical regions and investigate temporal trends in its prevalence.
Materials and methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline, which is widely recognized as the standard for transparent reporting of systematic reviews and meta-analyses [9]. Also, widely endorsed methodological guidelines were utilized to improve the quality of the study [10, 11]. The protocol of the systematic review was priorly published in a peer-reviewed scientific journal [12].
Search and data sources
The reviewers systematically searched PubMed, Scopus, and Web of Science for relevant studies, published since the inception of databases until May 19th, 2023. The search keywords included H. pylori, obesity, and relevant keywords. The syntax developed for each online database is provided in Supplementary Material 1.
Study selection and eligibility criteria
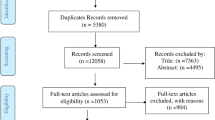
After automatic duplicate detection, the records were uploaded to the Rayyan online tool for management of systematic reviews where two reviewers (AS and NDE) independently screened them by titles and abstracts [13]. Controversial decisions were discussed to reach a consensus. In the next step, the full texts were assessed for eligibility. Observational and experimental studies were eligible for inclusion if they provided the statistics needed for the estimation of H. pylori prevalence (condition) among individuals with obesity (population) and had at least 30 sample participants from a representative population. Considering the variable definitions of obesity among the studies, the body mass index (BMI) of more than 30 kg/m2, generally, and more than 25 kg/m2, for Asian populations were acceptable [14]. To maximize the power of the analyses and avoid language and publication bias, we did not restrict the eligibility criteria to English papers [15]. Original articles, conference abstracts, and letters to editors were eligible for inclusion [16]. To avoid over-representation, when more than one study was available on a common series of patients, only the report with the largest sample size was included in the meta-analysis. A detailed PRISMA flow diagram presents the selection process in Fig. 1.
Data extraction and risk of bias assessment
Data were extracted into Excel spreadsheets by two independent reviewers (NDE and FN). The extracted data were later rechecked by a third reviewer (AS) for mistakes. The following data were extracted: study characteristics (first author, publication year, year(s) study conducted, language, and country), population characteristics (sample size, mean age, mean BMI, and male (%)), methodological considerations (detection methods, definition of the obesity, prior endoscopy, prior eradication, and definition of obesity), and the number of cases with H. pylori.
Risk of Bias (RoB) assessment was done by two independent reviewers (MAS and ET) using Joanna Briggs Institute (JBI) Critical Appraisal Checklist [17, 18]. For each domain, studies scored one point if they were assessed as low risk (“yes”). Overall risk assessment for each study was done using the sum of scores; if scored five or more they were considered low risk, otherwise high risk.
Statistical analyses
Stata MP Version 16 (StataCorp, College Station, TX, USA) was utilized for analyses. A p value < 0.05 was considered statistically significant. The DerSimonian–Laird random-effects model was used to pool the Freeman–Tukey double arcsine transformed effect sizes [19]. With reliable results, the Freeman-Tukey method is the preferred transformation in meta-analyses of proportions [20]. An I2 value > 75% indicated a statistically significant degree of heterogeneity [21].
Several subgroup analyses were applied to examine the potential sources of heterogeneity. WHO regional classification, geographical access to international waters, continents, global hemispheres, developed/developing condition, world bank income grouping, resource-richness of the countries, study periods, before/after the coronavirus disease of 2019 pandemic study period, mean age, mean BMI, prior eradication, overall RoB assessment, validity of the methods used for identification of the condition, and adequacy of the sample size were considered as potential moderators. Further subgroup analyses based on the peer-reviewed/non-peer-reviewed reports, the language of the report, and countries are available in Supplementary Material 2.
Multivariate meta-regression was applied considering the geographical coordinates of the country capitals of the studies (longitude and latitude), resource-richness, income, development, global hemisphere, prior H. pylori eradication, validity of the methods used, study year, overall RoB score, and male (%) as potential moderators. To reduce the bias caused by small studies, only studies with adequate samples sizes were included in the meta-regression. In this regard, the adequate sample sizes for examination of the association between potential covariate moderators and H. pylori prevalence among individuals with obesity was estimated at 370 using the method proposed by Naing et al. and the data provided by Lender et al. [22, 23]. Publication bias was not evaluated in this study, as it is not deemed pertinent to prevalence studies [24].
The robustness of the findings was examined using the leave-one-out method and subgroup analyses based on the overall RoB score, adequacy of the sample size, validity of the methods used to identify the condition, and peer-reviewed/non-peer-reviewed studies [16, 25].
Results
Study selection and characteristics
The search process generated 3138 records, 208 of which were included in the synthesis. Studies were from 53 countries (Fig. 2) of Europe (n = 74), North America (n = 50), Asia (n = 59), South America (n = 22), Oceania (n = 2), and Africa (n = 1). A total number of 472,511 individuals with obesity were evaluated for the presence of H. pylori. The included studies are cited in Supplementary Material 3. Supplementary Material 4 provides a summary of the study characteristics and settings. Supplementary Material 5 summarizes the identification methods used by each study to detect H. pylori infection. Definitions, findings, and overall RoB scores are provided in Supplementary Material 6.
(Top) World map of H. pylori prevalence. Countries filled with denser red color have higher prevalence. H. pylori, Helicobacter pylori. (Bottom) Bar chart demonstrating the estimated prevalence of H. pylori infection among individuals with obesity in each country. Detailed estimates are provided in Supplementary Material 2. H. pylori, Helicobacter pylori
Overall prevalence and geographical trends
As demonstrated in Table 1 and Fig. 3, the overall prevalence of H. pylori among individuals with obesity was 32.3% [95% CI 26.9%, 38.0%]. A detailed table demonstrating the meta-analysis of the overall pooled estimate is provided in Supplementary Material 7. Significant between-group differences were detected when classifying the studies based on the continent and WHO regional classification (p value < 0.01). Divided by the continents, South America had the highest prevalence of H. pylori infection with 46.9% [95% CI 40.3%, 53.6%], followed by Africa 38.5% [95% CI 31.5%, 45.8%], Asia 38.3% [95% CI 34.0%, 42.7%], Europe 36.9% [95% CI 32.4%, 41.5%], North America 15.2% [95% CI 10.9%, 19.9%], and Oceania 7.6% [95% CI 6.0%, 9.5%]. Divided by WHO regional classification, the prevalence of H. pylori among individuals with obesity was 47.1% [95% CI 40.0%, 54.3%] in Latin America and Caribbean, 43.8% [95% CI 23.6%, 65.2%] in South Asia, 40.3% [95% CI 34.4%, 46.4%] in Middle East and North Africa, 36.9% [95% CI 32.4%, 41.5%] in Europe and Central Asia, 30.3% [95% CI 23.5%, 37.5%] in East Asia and Pacific, and 13.4% [95% CI 9.5%, 17.9%] in North America. The difference between the prevalence of H. pylori among individuals with obesity in the global South and North, separated by Brandt Line [26, 27], was statistically significant with the global North 26.4% [95% CI 20.0%, 33.3%] and South 43.7% [95% CI 40.5%, 46.9%] (p value < 0.01). Also, significant between-group differences were observed when classifying the studies based on their access to international waters (p value < 0.01).
According to the multivariate meta-regression (Table 2), geographical latitude was negatively associated with the prevalence of H. pylori among individuals with obesity with β -0.008 [95% CI − 0.015, − 0.002] (p value 0.014). Geographical longitude was not significantly correlated with H. pylori prevalence with β 0.001 [95% CI − 0.001, 0.003] (p value 0.139).
Sociodemographic trends
Islamic countries had the highest prevalence of H. pylori among the individuals with obesity with 45.1% [95% CI 39.9%, 50.4%], followed by folk religions 44.9% [95% CI 37.4%, 52.6%], Judaism 33.7% [95% CI 22.9%, 45.4%], Hinduism 33.1% [95% CI 16.9%, 51.7%], Christianity 26.3% [95% CI 22.1%, 30.7%], and Buddhism 17.2% [95% CI 14.3%, 20.2%] (p value < 0.01). When classified by developmental status, studies from developed countries had a prevalence of 22.7% [95% CI 16.0%, 30.1%], whereas developing countries demonstrated a significantly higher prevalence of 43.9% [95% CI 41.0%, 46.8%] (p value < 0.01). Based on the world bank income classification of countries, studies from countries with a high income had 25.3% [95% CI 19.0%, 32.2%], whereas, studies from countries with a low and middle income had significantly higher prevalence 45.3% [95% CI 42.4%, 48.1%] (p value < 0.01). Figure 4 demonstrates Voronoi treemaps presenting the relative prevalence for each country classified based on geographical and sociodemographic classifications. The area of the polygon allocated to each country represents the relative estimated prevalence of H. pylori infection among individuals with obesity in that country. By employing a sequential process of dividing convex polygons, a Voronoi treemap is an exceptional means of visually representing hierarchical information. The area of the polygons within the Voronoi treemap is directly linked to the weights assigned to their respective nodes in the hierarchical structure [28].
Voronoi treemaps present the relative prevalence for each country classified based on geographical and sociodemographic classifications. The area of the polygon allocated to each country represents the relative estimated prevalence of H. pylori infection among individuals with obesity in that country. Classifications of the countries based on a continent, b developed versus developing, c global hemispheres, d world bank income groups, e the religion held by the majority of the population of the country, f resource-richness, and g WHO regional classification. H. pylori Helicobacter pylori, WHO world health organization
The meta-regression model of potential moderating factors of H. pylori prevalence is presented in Table 2 to explain the sources of heterogeneity in the effect estimates. Multivariate meta-regression showed that the prevalence is significantly influenced by geographical latitude.
Study characteristics and temporal trends
Studies with inadequate sample size demonstrated significantly higher prevalence than studies with adequate sample size with 36.0% [95% CI 31.8%, 40.3%] and 26.3% [95% CI 22.0%, 30.8%], respectively (p value < 0.01). On the other hand, studies that failed to employ validated identification methods for H. pylori infection demonstrated significantly lower prevalence than those that employed validated tools 24.4% [95% CI 16.8%, 32.8%] and 33.2% [95% CI 29.8%, 36.7%], respectively (p value 0.04).
Meta-regression and subgroup analysis based on the study period failed to demonstrate a statistically significant temporal trend for the prevalence of H. pylori among individuals with obesity (p values 0.769 and 0.130, respectively). Also, the between-group differences were not statistically significant between English and non-English, peer-reviewed and non-peer-reviewed, and high- and low-risk studies (p values 0.75, 0.33, and 0.05, respectively). Bubble plots demonstrating the distribution of studies based on their estimated effect size and study period are available in Supplementary Material 8.
RoB assessment and sensitivity analyses
The median RoB score was 8 points for the included studies. Assuming a score less than 5 as high risk, 10 studies were high risk for bias, whereas 198 studies were low risk for bias. Sensitivity analyses based on the overall risk of bias and leave-one-out demonstrated the robustness of our findings (Supplementary Material 9). However, assuming adequacy of sample size and validity of identification methods as the major component of bias, the findings differed significantly between the respective low- and high-risk studies. Furthermore, sensitivity analysis was done based on the peer-review status of studies (Supplementary Material 2). The proportional evaluation of the quality score in each JBI domain is presented in Fig. 5. A detailed evaluation of each study for RoB is presented in Supplementary Material 10.
Discussion
H. pylori infection is still one of the most common infections worldwide. The most recent evidence estimates that almost 48.9% of the global general population are infected with H. pylori [3]. To further extend the knowledge, several studies have been undertaken to investigate the prevalence of H. pylori infection in individuals with obesity. However, these studies have produced a wide range of estimates leading to inconclusive results. Moreover, the absence of a systematic review that comprehensively gathers and evaluates all the available evidence further contributes to the inconclusive nature of these estimations. The current study addresses the prevalence of H. pylori among people with obesity and associated study-level geographical, temporal, and sociodemographic factors by systemically reviewing the literature and pooling the eligible reports.
H. pylori prevalence in individuals with obesity versus general population
Our analyses showed that the global estimation of H. pylori prevalence among individuals with obesity was 32.3% (95% CI of 26.9%, 38.0%). In comparison with recent evidence [3], our findings suggest a lower prevalence of H. pylori infection among individuals with obesity than the general population. However, it seems that our findings replicate the previous findings from the general population regarding geographical and sociodemographic trends [3, 4]. In this regard, consensual evidence suggests that North America and Latin America have the lowest and highest regional prevalence, respectively. However, our findings do not necessarily replicate the temporal trends proposed by Li et al. [3]. They found that the prevalence of H. pylori decreased during the past decades in the general population, while our meta-regression and subgroup analyses suggest that the prevalence of H. pylori infection among individuals with obesity does not follow a statistically significant linear temporal trend.
A possible justification for the lower prevalence of H. pylori infection among the population with obesity than the general population is the heightened postprandial gastric acid secretion observed in individuals with obesity. It has been shown that hypergastrinemia can occur in individuals with obesity due to vagal dysfunction, even if it is not necessarily related to achlorhydria [29]. Transient gastric achlorhydria has been shown to be associated with primary H. pylori infection and increased survival and colonization of H. pylori in the stomach [30, 31]. Therefore, increased gastric acid production can decrease H. pylori virulence and its inoculation. Furthermore, in those with obesity, it has been reported that they have a more prominent maximal gastric acid response to intravenous gastrin administration and, also, a lesser stimulatory requirement to reach the secretory plateau. Consequently, the increased sensitivity to blood gastrin levels and accentuated postprandial gastric acid secretion may affect H. pylori colonization [32]. In addition, previous studies have shown that gastric emptying and proximal small intestine transit are accelerated in overweight and obese individuals [33]. This, in turn, may help in the clearance of H. pylori from the stomach by peristalsis and decrease its acquisition [34]. In addition, we propose that different gastric pre-H. pylori infection microbiota, gastric secretion of proteolytic enzymes, and mucosal environment immune activity in individuals with obesity may have a role in the H. pylori infection rate, compared to the general population.
Geographical trends
Latitude and H. pylori prevalence in individuals with obesity
We have found compelling evidence supporting an inverse relationship between geographical latitude and the prevalence of H. pylori infection among the subjects included in our study. However, it is not fully investigated and there are inconsistent findings, here, we gathered some existent explanations in this regard. As one moves farther from the equator, the amount of daily sunlight and the average temperature decrease, which is contrary to the favorable conditions for the growth of H. pylori. The best temperature for the optimum growth of H. pylori is 30 to 37 °C and at temperatures around 25 degrees, the growth is barely possible [35]. In addition, in a review study on the relationship of H. pylori with environmental conditions and geographical latitude, increased average daily sunlight time was found to correlate with higher H. pylori infection rates; their analysis, however, showed a negative trend with average annual temperature and latitude. They hypothesized that this variation in the prevalence of different geographical areas, in part, results from differences in the level of vitamin D synthesis [36]. Vitamin D synthesis is dependent on the exposure to sun and plays an important role in immunoregulation [26]. Prior investigations have also found that vitamin D is a protective factor against H. pylori infection, and those with vitamin D deficiency had a higher probability of eradication failure [37].
Regional differences
In line with studies on the general population, there is a wide variation in the prevalence of H. pylori between geographical regions and even between countries in each region [3]. We found that South America had the highest pooled prevalence of H. pylori in individuals with obesity, followed by Africa, Asia, Europe, North America, and Oceania. This variation may reflect the presence of differences in socioeconomic status, the level of sanitary infrastructure, access to clean foodstuff, and crowded living conditions. In this regard, we found that the developing countries have significantly higher H. pylori prevalence than the developed countries. The source of H. pylori infection can be contaminated drinking water, well water, seawater, and rivers [38]. This context may be the reason behind our finding that a positive correlation was found between higher H. pylori infection rates and access to international waters. In addition, using shared dishes and eating utensils could facilitate intrafamilial clustering of H. pylori infection. Therefore, with more crowded households, the possibility of its transmission from person to person increases as a result of close contact [39].
Other contributing factors
In bariatric surgery, prior eradication of H. pylori is a common practice. Many of the included studies were evaluations of the pre-, intra-, and postoperative prevalence of individuals who had undergone bariatric surgeries. Although not statistically significant in this study, prior eradicative treatments can change the findings. This result may be due to low quality and variable of reporting among the studies. Therefore, further investigations should be done. In addition, we observed a significant difference in the prevalence of H. pylori in individuals with obesity based on the official religion of the countries. This variation could be attributable to the differences in cultural, religious, and sanitary practices. Studies with larger sample sizes tend to provide more representative and reliable results. In our review, we found that studies with sufficient sample sizes reported a notably lower prevalence of H. pylori. This observation suggests a greater gap between the general population and the population affected by obesity. Consequently, it is crucial to consider various evaluations and exercise caution when drawing conclusions from the available evidence.
Strengths and limitations
To the best of our knowledge, this is the first study that estimates a global prevalence of H. pylori among individuals with obesity. The present study attempts to minimize language and publication biases by not restricting the eligibility criteria to a specific language, time period, and publication type. Furthermore, sensitivity analyses are done to measure the effect of these characteristics on final estimation. However, some important limitations are present in the present study. Our study was only successful in detecting studies from almost 26% of the countries globally (53/200). The H. pylori infection prevalence among individuals with obesity from the rest of the countries remains unestimated. Therefore, the geographical representation is incomplete, especially for developing and underdeveloped countries. Moreover, the included studies varied significantly regarding the time period, methodology, sample population, definitions, and identification methods, resulting in considerable heterogeneity. Similar to other meta-analyses of prevalence, our study indicated considerable heterogeneity, even between populations with close characteristics, suggesting local variations in risk factors of H. pylori infection. Our study fails to discover the sources of heterogeneity [11, 40,41,42].
Conclusion
To sum up, this systematic review and meta-analysis provides a comprehensive overview of H. pylori prevalence among individuals with obesity, H. pylori distribution based on geographical regions, and correlated sociodemographic trends. Our study confirmed that H. pylori prevalence in individuals with obesity is not greater than the prevalence among the general population. Failure to consider this point may cause overtreatment in obese people by physicians or arbitrary prescription of antibiotics, especially before bariatric surgeries. In addition, considering the higher prevalence of this infection in developing countries, the most important way to decrease this disease transmission is to provide sanitary distribution of water by governments. Also, educating people to avoid sharing eating utensils and encouraging them to personal hygiene issues will be effective in preventing infection. Conducting studies to evaluate the stomach microbiota in obese and overweight people before colonization with H. pylori, evaluating the effect of climatic conditions on the survival and growth of H. pylori, assessing worldwide and countries’ temporal trends, and exploring host factors predisposing to higher acquisition rates are recommended for further investigations.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Abbreviations
- H. pylori :
-
Helicobacter pylori
- GI:
-
Gastrointestinal
- JBI:
-
Joanna Briggs institute
- BMI:
-
Body mass index
- COVID-19:
-
Coronavirus disease of 2019
- RoB:
-
Risk of bias
References
Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics (in eng). Front Endocrinol. 2021;12:706978. https://doi.org/10.3389/fendo.2021.706978.
Camilleri M, Malhi H, Acosta A. Gastrointestinal complications of obesity (in eng). Gastroenterology. 2017;152(7):1656–70. https://doi.org/10.1053/j.gastro.2016.12.052.
Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(6):553–64. https://doi.org/10.1016/S2468-1253(23)00070-5.
Zamani M, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection (in eng). Aliment Pharmacol Ther. 2018;47(7):868–76. https://doi.org/10.1111/apt.14561.
Hooi JKY, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis (in eng). Gastroenterology. 2017;153(2):420–9. https://doi.org/10.1053/j.gastro.2017.04.022.
Baradaran A, et al. The association between Helicobacter pylori and obesity: a systematic review and meta-analysis of case-control studies (in eng). Clin Diabetes Endocrinol. 2021;7:15. https://doi.org/10.1186/s40842-021-00131-w.
Chen Y, Blaser MJ. Association between gastric helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205(8):1195–202. https://doi.org/10.1093/infdis/jis106.
Liu J, et al. The synergy of Helicobacter pyloriand lipid metabolic disorders in induction of Th17-related cytokines in human gastric cancer. J Cancer Metast Treat. 2017;3:169–76.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews (in eng). BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
E. Aromataris and Z. Munn, JBI Manual for Evidence Synthesis: JBI, 2020. https://synthesismanual.jbi.global.
Borges Migliavaca C, et al. How are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol. 2020;20:96. https://doi.org/10.1186/s12874-020-00975-3.
Sadeghi A, Dehdari Ebrahimi N. Global prevalence of Helicobacter pylori infection among individuals with obesity: A protocol for a systematic review and meta-analysis. Health Sci Rep. 2023;6:e1505. https://doi.org/10.1002/hsr2.1505.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.
World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 2000.
Pieper D, Puljak L. Language restrictions in systematic reviews should not be imposed in the search strategy but in the eligibility criteria if necessary (in eng). J Clin Epidemiol. 2021;132:146–7. https://doi.org/10.1016/j.jclinepi.2020.12.027.
Scherer RW, Saldanha IJ. How should systematic reviewers handle conference abstracts? A view from the trenches. Syst Rev. 2019;8:264. https://doi.org/10.1186/s13643-019-1188-0.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data (in eng). Int J Evid Based Healthc. 2015;13(3):147–53. https://doi.org/10.1097/xeb.0000000000000054.
Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence (in eng). Int J Health Policy Manag. 2014;3(3):123–8. https://doi.org/10.15171/ijhpm.2014.71.
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–11. https://doi.org/10.1214/aoms/1177729756.
Doi SA, Xu C. The Freeman-Tukey double arcsine transformation for the meta-analysis of proportions: recent criticisms were seriously misleading (in eng). J Evid Based Med. 2021;14:259–61. https://doi.org/10.1111/jebm.12445.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis (in eng). Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Lender N, et al. Review article: associations between Helicobacter pylori and obesity - an ecological study. Aliment Pharmacol Ther. 2014;40(1):24–31. https://doi.org/10.1111/apt.12790.
Naing L, Winn T, Nordin R. Pratical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1:9–14.
Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias (in eng). J Clin Epidemiol. 2014;67(8):897–903. https://doi.org/10.1016/j.jclinepi.2014.03.003.
Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed, Wiley, Chichester, 2019.
Lees N. The Brandt line after forty years: The more North-South relations change, the more they stay the same? Rev Int Stud. 2021;47(1):85–106. https://doi.org/10.1017/S026021052000039X.
Themrise K, Seye A, Catherine K, Madhukar P. How we classify countries and people—and why it matters. BMJ Glob Health. 2022;7: e009704. https://doi.org/10.1136/bmjgh-2022-009704.
Nocaj A, Brandes U. Computing voronoi treemaps: faster, simpler, and resolution-independent. Comput Graph Forum. 2012;31:855–64. https://doi.org/10.1111/j.1467-8659.2012.03078.x.
Sasaki H, et al. Hypergastrinemia in obese noninsulin-dependent diabetes: a possible reflection of high prevalence of vagal dysfunction (in eng). J Clin Endocrinol Metab. 1983;56:744–50. https://doi.org/10.1210/jcem-56-4-744.
Sachs G, Scott D, Weeks D, Melchers K. Gastric habitation by Helicobacter pylori: insights into acid adaptation. Trends Pharmacol Sci. 2000;21:413–6.
Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori (in eng). Gastroenterology. 1996;111:886–900. https://doi.org/10.1016/s0016-5085(96)70056-2.
Wisén O, Rössner S, Johansson C. Gastric secretion in massive obesity. Evidence for abnormal response to vagal stimulation (in eng). Dig Dis Sci. 1987;32:968–72. https://doi.org/10.1007/bf01297185.
Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility (in eng). Ann Transl Med. 2013;1:14. https://doi.org/10.3978/j.issn.2305-5839.2012.11.01.
Testerman TL, Mobley HLT. Adherence and colonization. In: Mobley HLT, Hazell SL, editors. Helicobacter pylori physiology and genetics. Washington (DC): ASM Press; 2001.
Versalovic J. Manual of clinical microbiology. American Society Mic Series; 2011
Lu C, Yu Y, Li L, Yu C, Xu P. Systematic review of the relationship of Helicobacter pylori infection with geographical latitude, average annual temperature and average daily sunshine. BMC Gastroenterol. 2018;18:50. https://doi.org/10.1186/s12876-018-0779-x.
Yang L, He X, Li L, Lu C. Effect of vitamin D on Helicobacter pylori infection and eradication: a meta-analysis (in eng). Helicobacter. 2019;24: e12655. https://doi.org/10.1111/hel.12655.
Aziz RK, Khalifa MM, Sharaf RR. Contaminated water as a source of Helicobacter pylori infection: a review (in eng). J Adv Res. 2015;6:539–47. https://doi.org/10.1016/j.jare.2013.07.007.
Kayali S, et al. Helicobacter pylori, transmission routes and recurrence of infection: state of the art (in eng). Acta Bio-med Atenei Parmensis. 2018;89:72–6. https://doi.org/10.23750/abm.v89i8-S.7947.
Higgins JPT. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158–60. https://doi.org/10.1093/ije/dyn204.
Migliavaca CB, et al. Meta-analysis of prevalence: I(2) statistic and how to deal with heterogeneity (in eng). Res Synth Methods. 2022;13:363–7. https://doi.org/10.1002/jrsm.1547.
Barker TH, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21:189. https://doi.org/10.1186/s12874-021-01381-z.
Funding
None.
Author information
Authors and Affiliations
Contributions
AS conceptualized, supervised, performed the search and analysis, and visualized the results. AS and NDE screened the records for eligibility. NDE and FN extracted the data. MAS and ET assessed the quality of the studies. AS and ET provided the draft. NDE revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the present study was done in the absence of any financial and personal competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadeghi, A., Nouri, F., Taherifard, E. et al. Estimates of global and regional prevalence of Helicobacter pylori infection among individuals with obesity: a systematic review and meta-analysis. Infection 52, 1223–1234 (2024). https://doi.org/10.1007/s15010-024-02244-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-024-02244-7