Abstract
Purpose
Anaemia remains a serious concern among pregnant women, and thus, it is closely monitored from the onset of pregnancy through to delivery to help prevent adverse maternal and neonatal outcomes. In malaria-endemic settings, continuous low-level carriage of P. falciparum parasites is common and its contribution to maternal anaemia should not be underestimated. In this study, we evaluated the impact of adherence to malaria control measures [number of antenatal clinics (ANC) attended, supervised intake of sulphadoxine pyrimethamine (SP), and use of insecticide treated bed nets (ITNs)] on asymptomatic malaria and anaemia outcomes among pregnant women on ANC in hospitals in the Central region of Ghana.
Methods
The study was conducted during two seasons; October–November 2020 (dry season, n = 124) and May–June 2021 (rainy season, n = 145). Among the women, there was a high adherence to the control measures for both seasons (ANC ≥ 3 visits; ~ 82.0%, intake of SP; ~ 80.0% and ITNs use; ~ 75.0%).
Results
Asymptomatic P. falciparum carriage was high for both seasons (44.4% for the dry season; 46.9% for the rainy season). Correspondingly, the occurrence of anaemia was high for both seasons (57.3% for the dry season; 68.3% for the rainy season) and was strongly predicted by carriage of P. falciparum parasites. Despite the high adherence to ANC protocols, asymptomatic P. falciparum infection was common and contributed to the high burden of maternal anaemia.
Conclusions
Our findings emphasize the need for improved control measures that can clear asymptomatic/sub-microscopic P. falciparum infection and protect against malaria-induced anaemia among pregnant women attending ANC in malaria endemic-settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The level of exposure to malaria parasites remains high among women in endemic regions. In 2021, of an estimated 33.8 million pregnancies in Sub-Saharan Africa, close to 11.6 million (34%) were exposed to Plasmodium falciparum infection [1]. Within the Sub-Saharan Africa region, West Africa (where Ghana is located) recorded an even higher exposure of pregnancies to P. falciparum infection; thus 40% of an estimated 15.7 million pregnancies were exposed to malaria [1]. Clinical manifestations of malaria in pregnancy vary depending on the parasite species circulating, the transmission intensity and the levels of naturally-acquired immunity [2, 3]. In West Africa, where P. falciparum is the principal causative agent of malaria, malaria in pregnancy is often stealthy, but can nevertheless have dire consequences including maternal anaemia [4], intrauterine growth retardation [5, 6], preterm delivery [5, 6], low birth weight [1, 6], foetal loss [7] and perinatal mortality [6, 7]. Diagnosis of asymptomatic malaria is based on the detection of Plasmodium species in peripheral blood of an individual with axillary temperature < 37.5 °C who shows no malaria-related symptoms. Detection of asymptomatic malaria can be facilitated by the use of rapid diagnostic tests (RDTs), microscopy and molecular assays. In Sub-Saharan Africa, the prevalence of asymptomatic malaria in pregnancy ranges from 3.0 to 21.0% (using microscopy), 8.0 to 36.0% (using rapid diagnostic kits), and 20.0 to 40.0% (using molecular methods) [4].
Anaemia in pregnancy is a serious health concern due to the inability of the body’s red blood cells to meet physiological needs. Globally, anaemia affects 1.62 billion (24%) of the world’s population [8]. Among pregnant women in Sub-Saharan Africa, the prevalence of anaemia is reported to be 57.1%, with the highest rate of 61% recorded in West Africa [8]. Though the occurrence of anaemia in pregnancy is quite complex, it is more prevalent in Sub-Saharan Africa, predominantly owing to nutritional deficiencies in iron, folic acids and other micronutrients due to insufficient consumption of these nutrients before and during pregnancy [9]. That notwithstanding, within Sub-Saharan Africa, the contributions of infectious diseases including HIV, schistosomiasis and malaria to maternal anaemia is significant [4, 10, 11]. The pathogenesis of these infectious diseases, especially malaria, could increase erythrophagocytosis and decrease erythropoiesis leading to maternal anaemia and iron depletion [12]. During pregnancy, there is an increased demand for iron and folate, which are required for red cell mass and plasma volume expansion, and for the growth and development of foetal and utero-placental organs [13]. Deficiency in iron during pregnancy can therefore lead to adverse maternal and neonatal outcomes. Adverse maternal outcomes including pre-term deliveries, postpartum haemorrhage, and mortalities are all associated with maternal anaemia [14]. Also, adverse neonatal outcomes such as birth asphyxia, low birth weight, stillbirths and neonatal deaths are associated with maternal anaemia [15]. Consequently, babies born from anaemic mothers have an increased risk of mental and physical impairment [16]. Particularly, pre-term babies born from anaemic mothers have an increased risk of growth retardation within first year of life due to low iron stores [17].
Provision of quality health services through antenatal clinics (ANC) is paramount in addressing malaria and anaemia in pregnancy [18]. Through ANC, pregnant women benefit from health care services such as health awareness, education, counselling, screening, diagnosis, treatment and monitoring. Together, these help in promoting both maternal and foetal well-being. In this regard, pregnant women, especially those within the second and third trimester, are encouraged to attend at least three ANC to enjoy maximum benefits [19]. In Ghana and other Sub-Saharan African countries, anaemia and malaria control strategies, including monitoring of maternal haemoglobin levels at each ANC visit, rapid diagnostic testing of malaria at each ANC, provision of insecticide treated bed nets, intermittent preventative therapies using sulphadoxine pyrimethamine (IPTp-SP) for malaria, provision of iron and folic acid supplementations and deworming, have been deployed through ANC and targeting pregnant women. However, across different health facilities in Ghana, these control strategies suffer from implementation challenges and some health facilities lack the capacity to execute some of the control strategies.
The main objective of malaria control strategies in pregnancy such as use of SP is to prevent adverse maternal and neonatal outcomes [20]. However, the current focus of its use has been on averting the rates of low birthweight due to maternal anaemia. According to the 2021 world malaria report, 74% of all pregnant women attended ANC at least once, 57% received at least one SP dose, with 46% and 32% receiving at least two and three doses respectively. This SP coverage is estimated to having averted over four hundred thousand low birthweights in 2020 alone [1]. However, several reports in Sub-Saharan Africa, including Ghana, have shown high prevalence of maternal anaemia despite IPTp-SP [4, 21]. Furthermore, some studies indicate that SP intake (irrespective of the number of doses) has limited effect against maternal anaemia [20, 22], although other studies in West Africa found reduced maternal anaemia after intake of at least three doses of SP [23, 24]. In any case, the burden of asymptomatic carriage of P. falciparum infection appears to be on the rise [4]. Additionally, control measures such as the use of IPTp-SP and ITNs have been reported to be ineffective in clearing P. falciparum infections during pregnancy [25,26,27]. This evidence points to a need for a re-evaluation of the impact of current malaria control measures on asymptomatic P. falciparum parasitaemia and anaemia among pregnant women. Malaria parasite transmission can either be highly seasonal or perennial with peaks during the rainy seasons, and it is unclear how this variation influences the impact of various control measures.
Methods
Study design, population and sampling sites
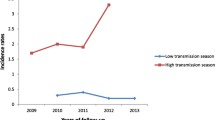
This was a cross-sectional study conducted at health facilities in the Central Region of Ghana at two different seasons; October–November 2021 (dry season) and May–June 2022 (Rainy season) (Fig. 1). The study involved pregnant women presenting at routine ANCs at health facilities in four communities; Abura Dunkwa, Moree, Effutu-Cape Coast, and Mankessim, all situated in the Central Region of Ghana.
Ethical approval
Approval for the study was granted by the ethical review committee of the Ghana Health Service (GHS-ERC 005/08/20) and the institutional review board of the Noguchi Memorial Institute for Medical Research (NMIMR-IRB CPN 005/20-21). Written informed consent was obtained from each study participant. For women below 18 years of age, additional consent was sought from their guardians or husbands.
Evaluation and recruitment
Women with gestational age of ≥ 14 weeks (second trimester and above), who were permanent residents in the study communities, showed no disease signs or symptoms and signed the informed consent were included in the study. During the sampling period, most pregnant women, who appeared for ANC visits were either in their second or third trimester. Considering that women in their first trimester do not receive SP as malaria prophylaxis, we decided to sample pregnant women within their second trimester and above. Midwives, Physician Assistants and Medical Officers examined the pregnant women, and all necessary tests required were done to determine participation eligibility and to allow routine diagnosis per each health facility procedure. Trained field workers explained the study procedures and requirements to participants in a language they understood, and a copy of the informed consent was given to them to complete. Once consented, qualified enumerators administered a structured questionnaire to the participants in their own language to obtain obstetric and socio-demographic information (age, gestational age, parity, number of ANCs, use of ITNs and supervised use of malaria prophylaxis). Blood samples were then drawn by an experienced phlebotomist with butterfly needles into heparin tubes and labelled appropriately. The remaining blood from the butterfly needles was spotted on Whatmann grade 3 filter paper to allow detection of asymptomatic P. falciparum carriage by PCR and also used to estimate haemoglobin (Hb) levels for each participant using HemoCue machine. Anaemia was defined as Hb < 11.0 g/dL [28].
Extraction of genomic DNA from dry blood spot samples
Three blood spots on Whatman filter paper were labelled with patients' study numbers, air-dried, and individually placed into plastic bags marked with the patients' initials. The bags were stored at room temperature. Genomic DNA was extracted using the Chelex method [29]. Briefly, a spot of about 100 μL blood was excised into a 1.5 mL microcentrifuge tube and soaked with 1 mL 0.5% saponin in 1 × PBS. Microcentrifuge tubes with contents were next vortexed and supernatant suctioned. Spots were washed twice with 1 × PBS and DNA was boiled out with 200 μL of 20% Chelex 100 solution (Bio-Rad Laboratories) prepared in nuclease free water. The DNA was stored at − 20 °C until it was used for the amplification reaction.
Detection of asymptomatic P. falciparum infection
A PCR assay was used to amplify the seryl-tRNA synthetase gene of P. falciparum in the extracted DNA. The total reaction volume of 10 μL consisted of 0.5 μL each of 10 μM forward and reverse primers, 5 μL Low ROX PerfeCTa SYBR green SuperMix (Quanta Biosciences Inc., Gaithersburg, USA), 2 μL nuclease free water and 2 μL DNA. Thermal cycling was performed on a Quant Studio 5 real-time PCR system. Cycling conditions were: initial denaturation at 95 °C for 5 min followed by 40 cycles of denaturation at 94 °C for 15 s and annealing and extension at 60 °C for 1 min. A melt curve stage of 95 °C for 10 s, 60 °C for 1 min and 95 °C was included. To confirm positive and negative samples from the amplification plots obtained, the PCR products were resolved on 1.5% agarose gel electrophoresis with an expected amplicon size of 120 bp for the amplified gene.
Study variables
Two main binary variables: asymptomatic carriage of P. falciparum (Yes or No) and anaemia (Yes or No), were assessed in this study. Factor variables such as socio-demographic information (age), obstetric characteristics (gestational age and parity), and ANC protocols (number of ANC visits, use of ITNs, intake of SP) were assessed as well. Age was stratified as < 20 years, 20–30 years, and > 30 years. Gestational age was grouped into second (14–27 weeks) and third trimester (≥ 28 weeks). Among the women, the gestational age ranged from 25 to 41 weeks. With regards to parity, the women were put into nulliparous (parity = 0), primiparous (parity = 1), secundiparous (parity = 2) and multiparous (parity ≥ 3) groups. Number of ANC visits by participants was stratified into 1, 2 and ≥ 3 visits, use of ITNs was stratified into Yes or No, while supervised intake of SP was stratified into 0, 1, 2, and ≥ 3 number of doses taken.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 9.3.1 (GraphPad Software, La Jolla, CA) and SPSS version 28.0. Both continuous and categorical variables were presented as numbers and percentages. Prevalence of asymptomatic P. falciparum infection and anaemia were estimated based on the proportion of women who tested positive by PCR and had Hb < 11.0 g/dL, respectively. Association between categorical data was evaluated using Chi-Square or Fisher’s exact test. For each outcome (asymptomatic malaria and anaemia), multinomial logistic regression analyses were carried out with predictor variables. Variance-inflation factors (VIF) were computed using linear regression to ensure there were no multi-collinearity between predictor variables. Additionally, correlations among predictor variables were evaluated using Pearson’s test. Variables with VIF of < 2.000 and Pearson’s correlation coefficient (r) of < 0.400 were considered. Two-tailed p-values < 0.05 were considered as statistically significant.
Results
Demographic and obstetric characteristics of participants
For the first sampling period (dry season, n = 124, Fig. 1), the age range of the women was 15–46 years. The majority (53.2%) were 20–30 years, while 35.5% were > 30 years, and 11.3% were < 20 years (Table 1). For the second sampling period (rainy season, n = 145, Fig. 1), the age range was 14–42 years. Most (49.7%) were 20–30 years, 36.6% were > 30 years while the remaining 13.8% were < 20 years. For both seasons, the majority (~ 80.0%) of women, who turned out for ANC were in their third trimester and many (~ 37.0%) were multiparous (Table 1). Comparison analysis showed the proportions of women in the different age (χ2 = 0.52; p = 0.772), trimester of pregnancy (χ2 = 0.13; p = 0.721) and parity (χ2 = 0.78; p = 0.854) groups were similar for both sampling periods (Table 1).
Adherence to malaria control measures among participants
In this study, high adherence to malaria control measures was evident from the high number ANC visits by participants (ANC visits ≥ 3), by the dominant supervised intake of at least three SP doses and by the widespread use of ITNs during pregnancy. For both sampling periods, the number of ANC visits ranged from 1 to 9, and a high coverage of ~ 82.0% was observed among the women (Table 1). Supervised SP intake ranged from 0 to 5 doses. High proportions of 75.0% and 80.0% of the women received at least one dose of SP during the dry and rainy seasons, respectively (Table 1). Out of this number, many, (35.5% and 44.8% for dry and rainy season respectively), received at least three doses of SP (Table 1). Use of ITNs was common, with 75.0% (dry season) and 62.1% (rainy season) claimed to be sleeping under ITNs (Table 1). Comparison analyses showed ANC coverage (χ2 = 0.80; p = 0.672) and SP intake (χ2 = 5.07; p = 0.167) were similar between the two seasons (Table 1). However, use of ITNs was lower in the rainy season compared to the dry season (χ2 = 5.14; p = 0.023, Table 1).
Prevalence of asymptomatic malaria
The prevalence of asymptomatic P. falciparum infection was 44.4% (95% CI 35.4–53.6%) in the dry season and 46.9% (95% CI 36.8–55.4) in the rainy season (Fig. 1), and the rates were similar for both seasons (χ2 = 0.17; p = 0.677). For each season, the proportion of women with asymptomatic parasitaemia was similar across the different age, gestation and parity groups (χ2 < 6.45; p > 0.05 for all comparisons) (Fig. 2). That notwithstanding, in the dry season, parasite carriage rates were highest among younger (57.1%, Fig. 2a) and secundiparous women (68.2%, Fig. 2a). In the rainy season, the rates were highest among younger (65.0%, Fig. 2b) and nulliparous women (54.1%, Fig. 2b). For both seasons, the prevalence of parasite carriage was not significantly related to ANC coverage or SP intake (χ2 < 3.60; p > 0.05 for all comparisons, Fig. 3). Despite this observation, in the dry season, parasites were most commonly found in women with low ANC coverage (one ANC visit; 63.6%, Fig. 3a) and in those who did not receive SP (58.1%, Fig. 3a). Women who did not sleep under ITNs more often carried parasites than those who did (61.3% in the dry season; Fig. 3a and 54.5% in the rainy season; Fig. 3b); this association was only significant in the dry season (χ2 = 4.80; p = 0.028 for, Fig. 3a).
Comparison of prevalence of asymptomatic carriage of P. falciparum across age, gestational age and parity groups among pregnant women during the a dry season; sampling 1—October–November 2020, and b rainy season; sampling 2—May–June 2021. Proportions were compared by Chi-square analysis, with χ2 indicating the chi-square value and p representing the p-value which is considered significant at < 0.05 (2 tailed)
Association of prevalence of asymptomatic carriage of P. falciparum with number of antenatal visits, use of insecticide-treated nets and SP intake among pregnant women during the a dry season; sampling 1—October–November 2020, and b rainy season; sampling 2—May–June 2021. Proportions were compared using Chi-square or fisher’s exact test, with χ2 indicating the chi-square value and p representing the p-value which is considered significant at < 0.05 (2 tailed)
Prevalence of anaemia
Among the women, anaemia was diagnosed in 57.3% (95% CI 48.1–66.1%) and 68.3% (95% CI 60.0–75.7%) respectively in the dry and rainy seasons (Fig. 1). The proportions of women diagnosed with anaemia were similar in both seasons (χ2 = 3.49; p = 0.062). The occurrence of anaemia was significantly associated with asymptomatic parasite carriage (χ2 = 7.53; p = 0.006; for dry season; Supplementary Fig. 1a, χ2 = 7.33; p = 0.005; for rainy season; Supplementary Fig. 1b). With regard to demographic and obstetric distribution, anaemia was highest (range 48.1–78.6%) across all age, gestation and parity groups in both sampling periods (Fig. 4). The proportions of women with anaemia were similar across the different age, gestation and parity groups (χ2 < 3.12; p > 0.05 for all comparisons in both sampling periods, Fig. 4). With regards to adherence to ANC protocols, anaemia rates were highest among women with ≥ 3 ANC visits (58.4% in the dry season; Fig. 5a and 70.8% in the rainy season; Fig. 5b), among those who used ITNs (53.8% in the dry season; Fig. 5a and 72.2% in the rainy season; Fig. 5b) and among those who received ≥ 3 doses of SP (59.1% in the dry season; Fig. 5a and 58.5% in the rainy season; Fig. 5b). Comparisons showed that the proportions of women with anaemia were similar in those using and not using ITNs (χ2 < 1.90; p > 0.05 for all comparisons in both sampling periods, Fig. 5) and irrespective of intake or non-intake of SP (χ2 < 6.20; p > 0.05 for all comparisons in both sampling periods, Fig. 5). However, the comparison analyses revealed that, in the dry season, a significantly higher proportion of women on their first ANC visit and those with ≥ 3 ANCs had anaemia (Fig. 5a).
Comparison of prevalence of anaemia across age, gestational age and parity groups among pregnant women during the a dry season; sampling 1—October–November 2020, and b rainy season; sampling 2—May–June 2021. Chi-square or fisher’s exact test was used to evaluate differences between proportions. On the graphs, χ2 and p indicate the chi-square value and p-value respectively. Significant statistical associations were considered at p < 0.05 (2 tailed)
Association of occurrence of anaemia with number of antenatal visits, use of insecticide-treated nets and doses of SP intake among pregnant women during the a dry season; sampling 1—October–November 2020, and b rainy season; sampling 2—May–June 2021. Comparisons were evaluated using Chi-square or fisher’s exact test. On the graphs, χ2 indicate the Chi-square value while p represent the p-value which is considered significant at < 0.05 (2 tailed)
Predictors of asymptomatic malaria among women during the dry and rainy seasons
Multinomial logistic regression analysis was used to evaluate the relative contributions of age, gestation, parity, number of ANC visits, SP intake, ITN use and anaemia as predictors of asymptomatic P. falciparum among the women (Table 2). Of the predictors, anaemia (in the rainy season only) or parity, ITN use and anaemia (in the dry season) were associated with asymptomatic malaria (Table 2). In the dry season, there was a significant increase in risk of asymptomatic malaria in secundigravidae (AOR = 5.080, 95% CI 1.440–17.915, p = 0.011, Table 2) and anaemic women (AOR = 3.286, 95% CI 1.353–7.979, p = 0.009, Table 2), while women who used ITNs had a significantly reduced risk of asymptomatic malaria (AOR = 0.376, 95% CI 0.150–0.940, p = 0.036, Table 2). In the rainy season, women who were anaemic had a significantly increased risk of asymptomatic malaria (AOR = 3.283, 95% CI 1.451–7.427, p = 0.004, Table 2).
Predictors of anaemia among women during the dry and rainy seasons
Similarly, multinomial logistic regression analysis was used to evaluate the relative contributions of age, gestation, parity, number of ANC visits, SP intake, ITN use and asymptomatic malaria as predictors of anaemia among the women (Table 3). In the dry season, only asymptomatic parasite carriage contributed to anaemia as infected women had a significantly increased risk of anaemia (AOR = 3.303, 95% CI 1.358–8.032, p = 0.008, Table 3). In the rainy season, there was a significantly increased risk of anaemia in women who did not receive SP (AOR = 3.895, 95% CI 1.077–14.084, p = 0.038, Table 3), and in those who had asymptomatic malaria (AOR = 3.247, 95% CI 1.428–7.380, p = 0.005, Table 3).
Discussion
This study was conducted to assess the impact of malaria control strategies including increased numbers of ANC visits, supervised intake of SP during ANC visits and use of ITNs and associated factors (age, gestation and parity) on low level or asymptomatic P. falciparum carriage and anaemia among pregnant women on ANC in the Central region of Ghana during the dry and rainy seasons. These variables are useful in assessing the effectiveness of the control strategies in both the dry season where parasite transmission is at its lowest and in the rainy season where it peaks.
Generally, the women sampled in the two seasons were similar with regard to their demographic (age) and obstetric characteristics (gestation and parity). The majority of the women were within the 20–30 years and > 30 years age brackets, which is unlike other studies in Ghana where most women appearing for ANC were younger (< 20 years) [22, 30]. Available evidence suggests that pregnant women < 20 years of age are less likely to show up for subsequent ANC, thereby not benefiting from health interventions [30]. Based on the age category, it was not surprising that most of the women who participated in this study were multiparous and ANC visits coverage of ~ 82.0% was observed. This high ANC coverage resulted in high IPTp-SP coverage (intake of at least one dose of SP; 75.0% dry season and 80.0% rainy season) relative to the 74% coverage recently reported in the 2021 world malaria report [1]. There was an improvement in the women who received at least three doses of SP (35.5% in dry season and 44.8% in the rainy season) in comparison to the 32% recently reported [1]. Interestingly, the use of ITNs among the study participants was higher (75.0% in the dry season and 62.1% in the rainy season) compared to the 43% use previously reported among Ghanaian pregnant women [31]. The higher ITN use reported in this study could be ascribed to the frequent ANC visits, which has been shown to be a determinant of ITN utilization among Ghanaian pregnant women [32].
Though the transmission of malaria has declined significantly in recent years, malaria still remains a major public health burden in Ghana [33]. Generally, reliable and accessible area-specific information on malaria transmission is not available for the selected communities in the Central region of Ghana where this study was conducted. That notwithstanding, the study sites (Moree, Abura Dunkwa, Effutu-Cape Coast and Mankessim) are characterized by high P. falciparum transmission intensity among pregnant women as evidenced in this study and a previous study by Nwaefuna et. al. in 2015 [34]. The prevalence of asymptomatic P. falciparum carriage peaked at 44.4% in the dry season and 46.9% in the rainy season. The rates reported here are slightly higher relative to 30% asymptomatic parasite carriage rates reported among pregnant women in Sub-Saharan Africa in general [4]. The rates were high irrespective of age, gestation, and parity for the two sampling seasons, showing that the women were consistently carrying parasites and at risk of adverse consequences at any time of the year. These underscore the need to maintain effective control measures throughout the entire course of pregnancy. However, the highest risk of asymptomatic parasite carriage in the study occurred among secundiparous women during the dry season. This risk factor has been reported among African women during low parasite transmission periods [35, 36], mainly culminating from parasites carried into pregnancy until first contact with ANC visit [25, 26]. Anaemia was a very prevalent condition among the women of all ages, gestation and parities, and this observation is consistent with studies conducted in other parts of Ghana and Africa [4, 35]. The rates of anaemia were higher for the two seasons, peaking at 57.3% in the dry season and at 68.3% in the wet season indicating a corresponding impact of asymptomatic P. falciparum carriage across the two seasons. The strong dependency of anaemia on asymptomatic parasitaemia was not surprising because it is consistent with several other reports among pregnant women in Sub-Saharan Africa [4, 35]. Asymptomatic malaria, which is often chronic can culminate into increased erythrophagocytosis and decreased erythropoiesis [12]. These changes contribute to maternal anaemia, leading to adverse outcomes, including mortality during pregnancy or postpartum, and dire neonatal outcomes such as foetal/infant mortality [12]. Moreover, pregnant women with asymptomatic malaria infection usually go undetected and untreated, serving as silent reservoirs for the transmission of natural P. falciparum infection to the general population [37, 38], thus, jeopardizing efforts to eliminate malaria.
Currently, the most common malaria control strategies employed during pregnancy are ITNs, IPTp-SP, and supplementation of iron and folic acids during ANC visits. In this regard, pregnant women within the second to third trimester are recommended to have at least three ANC contacts to enjoy the maximum benefits [19]. Despite the high ANC coverage and high adherence to the malaria control strategies among the women studied here, asymptomatic parasite carriage and maternal anaemia were still found to be very common. SP intake irrespective of the number of doses did not influence asymptomatic carriage of P. falciparum infection (Table 2), leaning credence to previous studies implying the ineffectiveness of SP in clearing infections during pregnancy [27]. That notwithstanding, increased (≥ 3) SP intake was associated with maternal anaemia outcomes only in the rainy season where women who took less than three doses had an increased risk of anaemia (Table 3). This observation is corroborated by studies with seasonal stratifications that observed a beneficial effect of increased (≥ 3) SP intake on maternal anaemia [23], which is unlike studies without such seasonal stratifications [22, 39]. Thus, it appears that SP protects against malaria-induced maternal anaemia during seasons with intense parasite transmissions probably because women may carry higher parasitemia unlike in lower transmission seasons. This further buttresses the reported ineffectiveness of SP in clearing infections [27]. ITNs by their nature are supposed to prevent new infections rather than clearing them [25, 26], and this was evident as its use appeared to be protective only in the dry season (Table 2), mainly because of its increased utilization during this period (Table 1). Considering that pregnant women in malaria endemic settings can control and chronically propagate P. falciparum infection during pregnancy [2, 40], the use of ITNs as a preventative measure is limited as they cannot clear chronic infections. In this regard, the widespread use of ITNs among our study participants did not influence anaemia outcomes.
Limitations and strengths of the study
The study did not assess nutritional determinants and other co-morbidities as risk factors of anaemia in pregnancy and this could potentially influence the outcomes of the findings presented here. That notwithstanding, malaria-induced maternal anaemia is a well appreciated condition observed in Sub-Saharan Africa and as presented here. System-based studies are therefore needed to understand the contributory effect of malaria-induced anaemia in maternal anaemia. Such studies will also help to monitor and understand the effectiveness of malaria control measures such as chemoprophylaxis with SP and other partner drugs. Additionally, the study could not account for P. falciparum positive participants who potentially could have had delayed clearance of the parasite DNA after SP intake but not peripheral asymptomatic infection as captured here [41]. However, considering chronic and persistent P. falciparum infections are widespread in hyper-endemic malaria settings [2, 40], such as our study area, the aforementioned limitation might potentially not influence the outcomes of this study. Our findings provide useful information that can influence policy formulation and implementation on several fronts. Firstly, it stresses the need for the use of more sensitive point-of-care diagnostics to enable the detection of asymptomatic/sub-microscopic P. falciparum infections among pregnant women during ANC visits. Additionally, it prompts the use of alternative and suitable treatment regimens that can clear asymptomatic and chronic P. falciparum infections among pregnant women on ANC. Furthermore, it backs calls on the need for vaccines that can protect pregnant women against the adverse effects of placental malaria including malaria-induced maternal anaemia. Lastly, it stresses the need for nutritional and diet remedies. ANC care givers should intensify education and awareness on diet during pregnancy. Measures should be put in place to ensure compliance in consumption of foods fortified with iron, folic acid, and other micronutrients among pregnant women. Also, there should be strategies to ensure the availability and compliance in consumption of iron and folic acid tablets for women.
Conclusion
Our data suggests the presence of a high burden of asymptomatic carriage of P. falciparum and anaemia among pregnant women in the Central region of Ghana despite high ANC coverage, increased intake of SP doses and high use of ITNs. The findings imply that SP and ITN use are not effective tools in fighting asymptomatic P. falciparum carriage among pregnant women across different transmission seasons. This demonstrates a pressing need for alternative measures to control asymptomatic/sub-microscopic malaria in pregnancy and its corresponding impact on maternal anaemia.
Data availability
Data supporting the findings of this work are captured here. Raw data can be made available upon reasonable request.
References
World Health Organization. World malaria report 2021. Geneva: World Health Organization; 2021. p. 42–3. Licence: CC BY-NC-SA 3.0 IGO.
Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, Joshi H. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J. 2012;11:1–15.
Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–17.
Yimam Y, Nateghpour M, Mohebali M, Abbaszadeh Afshar MJ. A systematic review and meta-analysis of asymptomatic malaria infection in pregnant women in Sub-Saharan Africa: a challenge for malaria elimination efforts. PLoS One. 2021;16: e0248245.
Menendez C, Ordi J, Ismail M, Ventura P, Aponte J, Kahigwa E, Font F, Alonso P. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–5.
Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35.
Van Geertruyden J-P, Thomas F, Erhart A, D’Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg. 2004;71:35–40.
McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12:444–54.
Grum T, Brhane E, Hintsa S, Kahsay G. Magnitude and factors associated with anemia among pregnant women attending antenatal care in public health centers in central zone of Tigray region, northern Ethiopia: a cross sectional study. BMC Pregnancy Childbirth. 2018;18:1–7.
Adam I, ALhabardi NA, Al-Wutayd O, Khamis A. Prevalence of schistosomiasis and its association with anemia among pregnant women: a systematic review and meta-analysis. Parasites Vectors. 2021;14:1–10.
Correa-Agudelo E, Kim H-Y, Musuka GN, Mukandavire Z, Miller FD, Tanser F, Cuadros DF. The epidemiological landscape of anemia in women of reproductive age in sub-Saharan Africa. Sci Rep. 2021;11:1–10.
Matangila JR, Lufuluabo J, Ibalanky AL, Inocêncio da Luz RA, Lutumba P, Van Geertruyden J-P. Asymptomatic Plasmodium falciparum infection is associated with anaemia in pregnancy and can be more cost-effectively detected by rapid diagnostic test than by microscopy in Kinshasa, Democratic Republic of the Congo. Malar J. 2014;13:1–10.
Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016.
Stephen G, Mgongo M, Hussein Hashim T, Katanga J, Stray-Pedersen B, Msuya SE. Anaemia in pregnancy: prevalence, risk factors, and adverse perinatal outcomes in Northern Tanzania. Anemia. 2018;2018: 1846280.
Debella A, Eyeberu A, Getachew T, Atnafe G, Geda B, Dheresa M. Perinatal outcomes in anemic pregnant women in public hospitals of eastern Ethiopia. Int Health. 2022;15:274–80.
Kidanto HL, Mogren I, Lindmark G, Massawe S, Nystrom L. Risks for preterm delivery and low birth weight are independently increased by severity of maternal anaemia. S Afr Med J. 2009;99:98–102.
Mehrotra M, Yadav S, Deshpande A, Mehrotra H. A study of the prevalence of anemia and associated sociodemographic factors in pregnant women in Port Blair, Andaman and Nicobar Islands. J Fam Med Prim Care. 2018;7:1288.
Nyamtema AS, Jong AB-D, Urassa DP, Hagen JP, van Roosmalen J. The quality of antenatal care in rural Tanzania: what is behind the number of visits? BMC Pregnancy Childbirth. 2012;12:1–5.
World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience: summary: highlights and key messages from the World Health Organization’s 2016 global recommendations for routine antenatal care. Geneva: World Health Organization; 2018.
Mosha D, Chilongola J, Ndeserua R, Mwingira F, Genton B. Effectiveness of intermittent preventive treatment with sulfadoxine–pyrimethamine during pregnancy on placental malaria, maternal anaemia and birthweight in areas with high and low malaria transmission intensity in T anzania. Trop Med Int Health. 2014;19:1048–56.
Mikomangwa WP, Oms M, Aklillu E, Kamuhabwa AA. Adverse birth outcomes among mothers who received intermittent preventive treatment with Sulphadoxine-Pyrimethamine in the low malaria transmission region. BMC Pregnancy Childbirth. 2019;19:1–11.
Agyeman YN, Newton S, Annor RB, Owusu-Dabo E. Intermittent preventive treatment comparing two versus three doses of sulphadoxine pyrimethamine (IPTp-SP) in the prevention of anaemia in pregnancy in Ghana: a cross-sectional study. PLoS One. 2021;16: e0250350.
COSMIC Consortium. Community-based malaria screening and treatment for pregnant women receiving standard intermittent preventive treatment with sulfadoxine-pyrimethamine: a multicenter (The Gambia, Burkina Faso, and Benin) cluster-randomized controlled trial. Clin Infect Dis. 2019;68:586–96.
Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, MacArthur JR, Luntamo M, Ashorn P, Doumbo OK. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604.
Berry I, Walker P, Tagbor H, Bojang K, Coulibaly SO, Kayentao K, Williams J, Oduro A, Milligan P, Chandramohan D. Seasonal dynamics of malaria in pregnancy in West Africa: evidence for carriage of infections acquired before pregnancy until first contact with antenatal care. Am J Trop Med Hyg. 2018;98:534.
Ofori MF, Lamptey H, Dickson EK, Kyei-Baafour E, Hviid L. Etiology of placental Plasmodium falciparum malaria in African women. J Infect Dis. 2018;218:277–81.
Desai M, Gutman J, Taylor SM, Wiegand RE, Khairallah C, Kayentao K, Ouma P, Coulibaly SO, Kalilani L, Mace KE. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin Infect Dis. 2016;62:323–33.
World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011.
Anabire NG, Aryee PA, Abdul-Karim A, Abdulai IB, Quaye O, Awandare GA, Helegbe GK. Prevalence of malaria and hepatitis B among pregnant women in Northern Ghana: comparing RDTs with PCR. PLoS One. 2019;14: e0210365.
Ampofo GD, Tagbor H, Bates I. Effectiveness of pregnant women’s active participation in their antenatal care for the control of malaria and anaemia in pregnancy in Ghana: a cluster randomized controlled trial. Malar J. 2018;17:1–15.
Severe Malaria Observatory. Knowledge sharing for severe malaria, Ghana; Malaria prevention; 2020. https://www.severemalaria.org/countries/ghana#&gid=4&pid=1%0A. Cited 31 Oct 2022.
Dun-Dery F, Kuunibe N, Meissner P, Winkler V, Jahn A, Müller O. Determinants of the use of insecticide-treated mosquito nets in pregnant women: a mixed-methods study in Ghana. Int Health. 2022;14:619–31.
Awine T, Malm K, Bart-Plange C, Silal SP. Towards malaria control and elimination in Ghana: challenges and decision making tools to guide planning. Glob Health Action. 2017;10: 1381471.
Nwaefuna EK, Afoakwah R, Orish VN, Egyir-Yawson A, Boampong J. Effectiveness of intermittent preventive treatment in pregnancy with sulphadoxine-pyrimethamine against submicroscopic falciparum malaria in central region, Ghana. J Parasitol Res. 2015;2015: 959427.
Rouamba T, Samadoulougou S, Ouédraogo M, Hien H, Tinto H, Kirakoya-Samadoulougou F. Asymptomatic malaria and anaemia among pregnant women during high and low malaria transmission seasons in Burkina Faso: household-based cross-sectional surveys in Burkina Faso, 2013 and 2017. Malar J. 2021;20:1–13.
Thompson JM, Eick SM, Dailey C, Dale AP, Mehta M, Nair A, Cordero JF, Welton M. Relationship between pregnancy-associated malaria and adverse pregnancy outcomes: a systematic review and meta-analysis. J Trop Pediatr. 2020;66:327–38.
Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–39.
Galatas B, Bassat Q, Mayor A. Malaria parasites in the asymptomatic: looking for the hay in the haystack. Trends Parasitol. 2016;32:296–308.
Orish VN, Onyeabor OS, Boampong JN, Afoakwah R, Nwaefuna E, Acquah S, Sanyaolu AO, Iriemenam NC. Prevalence of intermittent preventive treatment with sulphadoxine-pyrimethamine (IPTp-SP) use during pregnancy and other associated factors in Sekondi-Takoradi, Ghana. Afr Health Sci. 2015;15:1087–96.
Desai M, Ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104.
Homann MV, Emami SN, Yman V, Stenström C, Sondén K, Ramström H, Karlsson M, Asghar M, Färnert A. Detection of malaria parasites after treatment in travelers: a 12-months longitudinal study and statistical modelling analysis. EBioMedicine. 2017;25:66–72.
Acknowledgements
The research was supported by DANIDA (grant MAVARECA-II; 17-02-KU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency. NGA was supported by a MAVARECA-II PhD studentship. We thank all the study participants and the numerous midwives, medical doctors, physician assistants, medical laboratory scientist and nurses in the various health facilities (Abura Dunkwa District Hospital, Moree Health Centre, Effutu Health Centre, Cape Coast and Mercy Women’s Catholic Hospital, Mankessim) for their enormous support. Special thank you to the Department of Immunology, Noguchi Memorial Institute for Medical research field team members including Rev. Alex Danso-Coffie and Sophia Ampaw for supporting in participant recruitment and enrolment.
Author information
Authors and Affiliations
Contributions
MO and NGA conceived the idea, and designed the study together with LH, MdPQ and GAA. NGA, BA, AP and EKB conducted data collection and data curation. NGA conducted data analysis and interpretation of findings, and wrote the first draft manuscript. MO, LH, MdPQ and GAA critically reviewed the manuscript. All authors read, improved and approved the final manuscript. LH and MO secured the funding and supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anabire, N.G., Aculley, B., Pobee, A. et al. High burden of asymptomatic malaria and anaemia despite high adherence to malaria control measures: a cross-sectional study among pregnant women across two seasons in a malaria-endemic setting in Ghana. Infection 51, 1717–1729 (2023). https://doi.org/10.1007/s15010-023-02058-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02058-z