Abstract
Purpose
Kidney transplantation was recently introduced for the treatment of end stage renal disease (ESRD) in HIV-infected patients. We report the results of the first 28 procedures at our centre.
Methods
A retrospective study was conducted on HIV-infected patients evaluated for kidney transplantation between January 2005 and October 2016. Patients were selected and monitored by the kidney transplantation and infectious diseases teams, according to the national protocol.
Results
60 patients were evaluated; 32 entered the list and 28 were transplanted. Median CD4+ count was 337 cell/μL at transplantation and 399 cell/μL 12 months thereafter. HIV RNA was undetectable at transplantation in 27/28 patients and became undetectable within 24 weeks in the only patient starting antiretroviral combination therapy (cART) after surgery. Four patients experienced virological failure, but reached again undetectability after cART regimen change. At last available point of follow-up (median 126.1 weeks), HIV RNA was undetectable in all patients. Three patients experienced AIDS-defining events. We observed a cumulative number of 19 acute rejections in 16 patients (median time from transplantation to first rejection 5.2 weeks). Survival rate was 82.1%. To avoid pharmacokinetics (PK) interactions, cART regimen was changed from a protease inhibitor (PI)/non-nucleoside reverse transcriptase inhibitor (NNRTI)-based to an integrase inhibitor (InSTI)-based regimen in 11/20 alive patients with functioning graft.
Conclusions
Kidney transplantation appears to be safe in HIV-infected patients carefully selected. As previously reported, we observed a high incidence of acute rejection. We expect that the recent implementation of the immunosuppressive protocols will allow a better immunologic control. Moreover, the introduction of InSTI permits a better strategy of cART, with lower incidence of PK interactions with immunosuppressive drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
At the end of the 90s, the introduction of antiretroviral combination therapy (cART) has led to improved survival in HIV-infected patients [1]. While the frequency of AIDS-related events has consequently decreased, mortality due to organ failure (especially liver and less frequently kidney) has become a significant concern [2].
As recently as 15 years ago, HIV infection was considered as an absolute contraindication for organ transplantation [3]. The first experiences of solid organ transplantation in HIV-infected patients were liver transplants in cirrhotic patients co-infected with HCV [2, 4]. In the case of end-stage renal disease (ESRD), replacement therapies (hemodialysis and peritoneal dialysis) are an alternative to transplantation; thus kidney transplantation was not initially considered a therapeutic option for HIV-infected patients with ESRD. From the beginning of the 2000s, several reports of kidney transplantation in HIV-infected patients with satisfactory outcomes were published [5, 6].
On the basis of these positive results, a national protocol for kidney transplantation in HIV-infected patients was developed in 2004 in Italy by CNT (National Transplant Center).
We report the results of the first 28 kidney transplantation procedures in HIV-infected patients at our Institution, which joined the protocol in 2005.
Methods
A retrospective, observational, single-center study was conducted on HIV-infected patients evaluated for kidney transplantation between January, 1st 2005 and October 31st, 2016 at the Spedali Civili General Hospital, University of Brescia, Northern Italy. All candidates for kidney transplantation were selected by the nephrologist and infectious diseases teams after multidisciplinary discussion, according to the Italian CNT protocol. Specific inclusion criteria for HIV-infected patients were: CD4+ T cell count > 200/mm3, undetectable HIV RNA (if the patient was on cART) and presumable good compliance to follow up and therapy [7]. Immunosuppressive anti-rejection protocols were modified during the study period. The first 13 patients (transplanted from June 2007 until March 2012) received an induction therapy with basiliximab in two doses. Intravenous methylprednisolone was given in tapering doses and discontinued on day 5 after transplantation. Maintenance immunosuppression drugs included tacrolimus or cyclosporine microemulsion and enteric-coated mycophenolic acid [8]. A second group of patients (N = 5, transplantation from April 2012 to July 2014) received the same induction therapy, with a maintenance therapy with tacrolimus, enteric-coated mycophenolic acid and methylprednisolone. Ten patients transplanted between September 2014 and September 2016 received basiliximab, methylprednisolone and antilymphocyte serum as induction therapy and tacrolimus plus enteric-coated mycophenolic acid as maintenance therapy.
Follow-up after transplantation consisted of scheduled tri-monthly periodic assessments by the multidisciplinary nephrologist and infectious diseases team. Viro-immunological parameters were evaluated at 30 and 60 days after transplantation and every 3 months thereafter.
A database was generated with clinical and biochemical data of patients. A descriptive analysis of the characteristics of the patients and the distribution of events was performed.
Being an observational, retrospective and non-pharmacological study, specific informed consent is not needed and we are allowed to analyse recorded data due to informed consent signed by HIV-infected patients at the time of the admission in Outpatient Clinic (general authorization of the Italian Guarantor).
Results
A total of 60 patients were evaluated; 32 (53%) entered the list and among them, 28 (87.5%) were transplanted. Three patients were transplanted elsewhere and one patient was lost to follow-up after entering the list; 28 patients (47%) were excluded from the list because they did not match CNT criteria or were lost to follow-up. Baseline characteristics of patients are shown in Table 1. Only eight out of 28 patients were already on follow-up in Dialysis Unit of our Hospital, while the remaining were sent from other centres in Northern Italy (Bergamo, Cremona, La Spezia, Lodi, Mantova, Piacenza, Rimini, Rovigo, Trento, Verona and Vicenza). 13/28 patients (46.4%) were Italian, 14/28 (50%) were from Africa and 1/28 (0.6%) was Brazilian. Histopathologic diagnosis of kidney disease was available in 15/28 (53.6%) and 5/28 (17.8%) had diagnosis of HIVAN (HIV-associated nephropathy).
75% of patients were males. Median age at transplantation was 48 years (SD 10 years). Median time from list entrance to transplantation was 15 weeks (IQR 3.2–24.1 weeks, range 1.2–40.4 weeks). Cytomegalovirus (CMV) and Toxoplasma gondii serologies and HBV co-infection of recipients are shown in Table 1. 4/28 patients (14.3%) were co-infected with HCV;1/4 (25%, genotype 4c/4d, F4 fibrosis) was treated with ombitasvir/paritaprevir/ritonavir and ribavirin for 24 weeks before transplantation, obtaining sustained virological response (SVR); 3/4 with fibrosis < F3 were treated with directly acting antivirals (DAAs) according to genotype and AIFA (Italian Drugs Agency) guidelines after transplantation [9]. In particular, two patients harbouring 1b HCV genotype were treated with ledipasvir/sofosbuvir (in one case in combination with ribavirin) for 12 weeks after 170 and 15 weeks after transplantation, respectively. Both of them presented undetectable HCV-RNA 1 month after the end of treatment. One genotype 1b HCV-infected patient was treated with daclatasvir, sofosbuvir and ribavirin 122 weeks after transplantation, obtaining SVR.
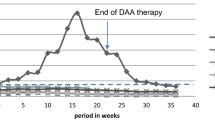
Median CD4+ count at transplantation was 337 cell/μL (IQR 235–441 cell/μL, range 172–841 cell/μL). We chose to admit to transplantation list a patient with CD4+ cell count < 200 cell/μL, taking into consideration good adherence, stable HIV RNA undetectability and high CD4+ percentage (durably > 14%). Median CD4+ T cell count during follow-up is shown in Fig. 1.
HIV RNA was undetectable at transplantation in 27/28 patients and became undetectable within 24 weeks in the patient starting cART after transplantation; four patients faced virological failure after transplantation, but obtained HIV RNA undetectability after cART regimen change. At last available point of follow-up (median 126.1 weeks, IQR 62.1–336.5 weeks, range 4.1–479.1 weeks), all patients had undetectable HIV RNA.
Three patients experienced AIDS-defining events after transplantation: one presented esophageal candidiasis and CMV pneumonia, one was diagnosed with cutaneous Kaposi’s sarcoma, which resolved after switch from cyclosporine to sirolimus and one patient faced cutaneous Kaposi’s sarcoma plus disseminated cytomegalovirus.
16/28 (57.1%) patients presented infectious complications after transplant; pneumonia and urinary tract infections were the most frequent diagnosis.
A cumulative number of 19 acute rejections in 16 patients (57.1%) was observed; the median time from transplantation to first rejection was 5.2 weeks (IQR 2.9–36.6 weeks, range 1.3–348.6 weeks). The number of rejections according to calendar year of transplantation is shown in Table 2. On October, 31st 2016 (end of follow up), 23/28 patients (82.1%) were alive and 20/28 (71.4%) were alive with functioning graft. Causes of death were invasive sinusal mucormycosis, sepsis in pancolitis, West Nile virus encephalitis, acute myocardial infarction and colorectal cancer. Median time from transplantation to death was 54.5 weeks (IQR 29.5–82 weeks, range 24–90 weeks).
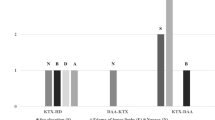
To avoid PK interactions, cART was modified from a PI/NNRTI-based to an InSTI-based regimen in 11/20 patients alive with functioning graft (65%); 7/11 were switched to raltegravir and 4/11 were switched to dolutegravir. 7/20 (35%) were on treatment with a cART regimen including both InSTI and PI/ritonavir (RTV) or efavirenz (EFV) at the end of follow-up, as reported in Table 3.
Discussion
This study provides an important clinical point of view from one of the few cohort of HIV-infected recipient of kidney transplantation worldwide. Our data showed a satisfying outcome of kidney transplantation with a survival rate of 82.1%.
Among transplanted patients, 15/28 had histopathologic diagnosis available and five patients were affected by HIVAN. As previously described, definitive diagnosis of HIVAN requires kidney biopsy even if clinical criteria may be useful in excluding rather than establishing the diagnosis [10]. Protocols considering a prompt execution of kidney biopsy, if not contraindicated, every time HIVAN is suspected are therefore mandatory. In fact, progression of HIVAN to ESRD is rapid, and in the absence of treatment, mortality approaches 100% within 6 months of the onset of uraemia.
The survival rate of our patients was 82.1% and functioning grafts at the end of follow-up were 71.4%. A recent report from the Italian national transplantation registry showed a survival rate of 95% and a graft survival rate of 85% between 2006 and 2014 [11]. However, data on immunological status of recipients are not available and we speculate that different baseline characteristics could have lead to different outcomes. Stock et al. [12] reported a survival rate of 94.6% after 1 year from transplantation (88.2% after 3 years) in a multicentric trial (150 patients) and in a recently published review, survivors were 93% within the first year of transplantation (254 patients) [13]. The worse outcome of our patients must be put into relation to a longer observation period; in fact survival rate after 1 year from transplantation was 100% in our cohort.
With regard to rejection, most studies observed a higher number of events in HIV-infected patients than in HIV-negative. In the present study, the rate of rejection was 57.1%, with a median onset time of 5.2 weeks from transplantation. Even if rejection was controlled successfully with steroid therapy, these results, as previously reported, suggest a possible scenario where the immune system damaged by HIV infection has a worse response to immunosuppressive treatment in respect of the general population, even in patients without a severe immunological dysfunction at the time of transplantation [8]. A crucial point is therefore the selection of optimal immunosuppressive protocols to prevent rejection in HIV-infected patients. A recent study from the United Kingdom included 33 HIV-infected patients, which underwent induction with interleukin-2 (IL-2) receptor antibody and were maintained on triple immunosuppression. Acute rejection rate was 44% [14].
As many as 57.1% of transplanted patients presented infectious complications after transplantation and three developed neoplasms (two skin Kaposi’s sarcoma and one colorectal cancer). An infectious disease incidence of 29% after transplantation was previously reported [13] and the incidence of post-transplant neoplasms has been described as not different from the incidence in HIV-negative patients.
Viro-immunological outcome of transplanted patients was satisfactory, suggesting the efficacy of co-administration of cART and immunosuppressive therapy. However, a significant drug–drug interaction is encountered between cART and calcineurin inhibitors (CNIs), which are cornerstones of immunosuppressive therapy schemes. In particular, NNRTIs induce and PIs inhibit CYP3A4, which is involved in CNIs clearance, resulting in need of frequent therapeutic drug monitoring and in a complex management of doses, possibly determining rejection. The advent of InSTI raltegravir and dolutegravir, which are free from such PK interactions, has dramatically changed the management of HIV-infected transplanted patients and recent reports pointed out the need of switching cART regimens from NNRTI/PI-based to this class of antiretrovirals, to optimize immunosuppression [15,16,17].
Conclusions
In line with other experiences, our data show that with careful selection of patients and multidisciplinary evaluation, kidney transplantation can be safely performed with good outcome in HIV-infected patients. Moreover, with the advent of InSTI-based cART regimens, drug–drug interactions between cART and immunosuppressants have been dramatically reduced. Nevertheless, further studies are needed to optimize immunosuppressive therapy regimens for HIV-infected patients, with the aim of reducing the high rate of rejections after transplantation.
References
Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14:195–207.
Smith CJ, Ryom L, Weber R, D: A: D Study Group, et al. Trends in underlying cause of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–8.
Spital A. Should all human immunodeficiency virus-infected patients with end-stage renal disease be excluded from transplantation? The views of US transplant centres. Transplantation. 1998;65:1187–91.
Miro JM, Aguero F, Laguno M, et al. Liver transplantation in HIV/hepatitis coinfection. J HIV Ther. 2007;12:24–35.
Trullas JC, Cofan F, Tuset M, Ricart MJ, Brunet M, Cervera C, Manzardo C, López-Dieguez M, Oppenheimer F, Moreno A, Campistol JM, Miro JM. Renal transplantation in HIV-infected patients: 2010 update. Kidney Int. 2011;79:825–42.
Nashar K, Sureshkumar KK. Update on kidney transplantation in human immunodeficiency virus infected recipients. World J Nephrol. 2016;5:300–7.
Protocollo del Centro Nazionale Trapianti-18 giugno 2004. Programma pilota di terapia sostitutiva con trapianto da donatore cadavere dell’insufficienza renale cronica in soggetti con infezione da HIV. http://www.trapianti.salute.gov.it/imgs/C_17_normativa_6_allegato.pdf. Accessed 17 Jan 2016.
Bossini N, Sandrini S, Casari S, Tardanico R, Maffeis R, Setti G, Valerio F, Forleo MA, Nodari F, Cancarini G. Kidney transplantation in HIV-positive patients treated with a steroid-free immunosuppressive regimen. Transpl Int. 2014;27:1050–9.
http://www.agenziafarmaco.gov.it/it. Accessed 20 Dec 2016.
Atta MG, Choi MJ, Longenecker JC, Haymart M, Wu J, Nagajothi N, Racusen LC, Scheel PJ Jr, Brancati FL, Fine DM. Nephrotic range proteinuria and CD4 count as noninvasive indicators of HIV-associated nephropathy. Am J Med. 2005;118:1288.
Morabito V, Grossi P, Lombardini L, et al. Solid organ transplantation in HIV+ recipients: Italian experience. Transpl Proc. 2016;48:4242–430.
Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A, Millis JM, Lyon GM, Brayman K, Slakey D, Shapiro R, Melancon J, Jacobson JM, Stosor V, Olson JL, Stablein DM, Roland ME. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363:2004–14.
Landin L, Rodriguez-Perez JC, Garcia-Bello MA, Cavadas PC, Thione A, Nthumba P, Blanes M, Ibañez J. Kidney transplants in HIV-positive recipients under HAART. A comprehensive review and meta-analysis of 12 series. Nephrol Dial Transplant. 2010;25:3106–15.
Gathogo EN, Hamzah L, Hilton R, Marshall N, Ashley C, Harber M, Levy JB, Jones R, Boffito M, Khoo SH, Drage M, Bhagani S, Post FA. Kidney transplantation in HIV-positive adults: the UK experience. Int J STD AIDS. 2014;25:57–66.
Waki K, Sugawara Y. Implications of integrase inhibitors for HIV-infected transplantation recipients: raltegravir and dolutegravir (S/GSK 1349572). Biosci Trends. 2011;5:189–91.
Azar MM, Malinis MF, Moss J, Formica RN, Villanueva MS. Integrase strand transferase inhibitors: the preferred antiretroviral regimen in HIV-positive renal transplantation. Int J STD AIDS. 2017;28:447–58.
Touzot M, Pillebout E, Matignon M, Tricot L, Viard JP, Rondeau E, Legendre C, Glotz D, Delahousse M, Lang P, Peraldi MN. Renal transplantation in HIV-infected patients: the Paris experience. Am J Transplant. 2010;10:2263–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Izzo, I., Casari, S., Bossini, N. et al. Effectiveness of kidney transplantation in HIV-infected recipients under combination antiretroviral therapy: a single-cohort experience (Brescia, Northern Italy). Infection 46, 77–82 (2018). https://doi.org/10.1007/s15010-017-1092-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-1092-2