Abstract
Purpose
The purpose of this study was to establish a baseline for measuring the impact of the programmatic management of drug-resistant TB program by following up on outcomes of all patients diagnosed with multidrug-resistant tuberculosis in Zambia between 2012 and 2014.
Methods
A cohort study of all the MDR-TB patients diagnosed at the national TB reference laboratory from across Zambia. MDR-TB was diagnosed by culture and DST, whereas outcome data were collected in 2015 by patient record checks and home visits.
Results
The total number of patients diagnosed was 258. Of those, 110 (42.6%) patients were traceable for this study. There were 67 survivor participants (60.9%); 43 (39.1%) were deceased. Out of the 110 patients who were traced, only 71 (64.5%) were started on second-line treatment. Twenty-nine (40.8%) patients were declared cured and 16.9% were still on treatment; 8.4% had failed treatment. The survival rate was 20.2 per 100 person-years of follow-up. Taking ARVs was associated with a decreased risk of dying (hazard ratio 0.12, p = 0.002). Sex, age, marital status and treatment category were not important predictors of survival in MDR-TB patients.
Conclusions
More than half of the patients diagnosed with MDR-TB were lost to follow-up before second-line treatment was initiated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) estimated that in 2015, there were 1.4 million deaths due to tuberculosis (TB) [1]. There were 250,000 deaths due to multidrug-resistant tuberculosis (MDR-TB), defined as TB due to a Mycobacterium tuberculosis strain that is resistant to at least rifampicin and isoniazid, or due to rifampicin-resistant tuberculosis (RR-TB) [1]. The emergence of MDR-TB has impacted negatively on the progress so far in global TB control [1]. High mortality among HIV-infected patients suffering from multi- and extensively drug-resistant tuberculosis M(X)DR-TB have raised concerns about TB control programs in sub-Saharan Africa [2,3,4]. Although a lot of progress has been made in the recent past to understand the burden of MDR-TB in sub-Sahara Africa, data are still limited; mainly due to limited surveillance systems and diagnostic capacity [4,5,6]. The region also has high rates of HIV prevalence and consequently, high TB/HIV co-infection rates [1, 7]. In 2015, WHO estimated that 480,000 people developed MDR-TB but only 37% of these were notified, even fewer were started on treatment and the treatment outcomes were poor with only close to half of these cases having successful outcomes [1, 8]. One of the reasons for fewer patients being started on second-line anti-TB treatment in many parts of the world could be due to the centralized approaches in treatment facilities and hence making it difficult for most people having access [1].
Programmatic management of drug-resistant TB (PMDT) in Zambia started in 2010 [9]. Culture and drug susceptibility testing (DST) for first-line anti-TB drugs were started in 1995; consequently, MDR-TB patients have been diagnosed in the country for the past 20 years [10]. The estimated prevalence of MDR-TB in Zambia is currently at 0.3% in new patients and 1.8% in previously treated patients [11]. Since 2008, there have been three main culture and DST laboratories that diagnose M(X) DR-TB, namely the National TB Reference Laboratory; the University Teaching Hospital in Lusaka and the Tropical Diseases Research Centre in Ndola [10]. However, only a few patients were started on second-line treatment at the latter two hospital facilities in the country.
To establish a baseline for measuring the impact of the programmatic management of drug-resistant TB (PMDT) program, we followed up on all the patients who were diagnosed from the three reference laboratories with the main objective to determine the outcomes of MDR-TB patients diagnosed in Zambia from 2012 to 2014 and their survival rate.
Methods
Design and population
This was a cohort study of all MDR-TB patients diagnosed across the country between 1st February 2012 and 1st February 2014, by the only three TB laboratories in Zambia that performed drug susceptibility testing. All patients recorded as MDR-TB, regardless of site, were enrolled in the study, that is, both pulmonary and extra-pulmonary MDR-TB patients.
A central data base was created that contained the demographic variables of these patients, the areas and health facilities where they had been referred from, and including their residential addresses if available. Normally in routine practice, the results were sent back to the referring facility through post mails to the attending clinicians to maintain the patients’ records confidential. Between January 2015 and October 2015, the confirmed MDR-TB patients by culture and DST (according to the diagnostic register) were traced back to the areas where they had been identified as presumptive MDR-TB patients. Research assistants in the respective provinces used details and home addresses from the registers to follow up the patients.
The national guidelines defined presumptive MDR-TB patients as all previously treated patients (re-treatment) or patients who were contacts of MDR-TB patients [9].
Once the patients had been traced, they were assessed clinically and interviewed. Patients who did not provide informed consent or were in prison at the time of the follow-up were excluded. In cases where patients were found to have died, the consenting next of kin was interviewed.
Patient screening and interviews
A team comprising an interviewer and an assistant data clerk traveled to the respective province to trace the patients who were recorded as diagnosed MDR-TB and to determine whether results had been obtained or not. This was done by checking the records of the clinics that sent the samples for DST to check if the patient had been registered for subsequent second- or first-line treatment. The national patient treatment cards, TB treatment registers and hospital record cards were reviewed. During the period under review, second-line treatment was provided at the designated health facilities in accordance with the national guidelines. A standardized regimen was used and when indicated an individualized regimen was provided. The MDR-TB patients were treated with an initial phase of treatment for a minimum of 8 months using injectable kanamycin, levofloxacin, ethionamide, cycloserine and pyrazinamide followed by a continuation phase for a minimum of 12 months using, levofloxacin, ethionamide, cycloserine and pyrazinamide [9]. Treatment outcomes of patients were defined in accordance with the NTP guidelines [9].
In case the patient was traced, a standardized structured questionnaire was administered by the interviewer after informed consent had been obtained. The questionnaires were designed according to different scenarios: (1) if the patient was found to be alive, symptom screening was conducted through a standard questionnaire, including history of cough, fever, night sweats, chest pain, haemoptysis, weight loss, and previous TB treatment before the recorded episode. In addition, sputum was collected and sent for microscopy, culture and DST using MGIT or Xpert MTB/RIF at the central reference laboratory according to national guidelines. (2) If the patient was found to be deceased, a verbal autopsy questionnaire was administered to an available next-of-kin respondent. Where the patient had died, as much information as possible was collected from case notes and interviews with relatives through the use of the verbal autopsy tool that was adapted from the World Health Organization/International Standard Verbal Autopsy questionnaire [12]. The verbal autopsy tool collected information pertaining to previous TB treatment before the recorded episode, history of cough, fever, chest pains, haemoptysis, weight loss, and history of other diseases.
Three attempts of visits were made and if by the third visit the patient was not found or confirmed dead, they were considered as lost to follow-up. Patients who were found but for whom there was no clinical information available were also excluded.
Data management and analysis
The information was entered using double data entry into the MS Excel database and analyzed using Stata version 14. Pearson’s Chi square test or Fisher’s Exact tests were used to compare categorical variables as appropriate. Censoring for participants who were traced took place on the date of the interview. Survival analysis was performed using the Kaplan–Meier method, while the Log-rank test was used to compare survival rates between groups. To identify predictors of mortality among MDR-TB patients, Cox proportional hazards regression was used with a backward elimination method for variables with p < 0.2. The Akaike and the Bayesian Information criteria methods were used to compare models. A p value less than 0.05 was considered statistically significant.
Ethical considerations
The study was approved by the Tropical Diseases Research Centre Ethics Committee, and the authority to conduct research was granted by the Ministry of Health.
Results
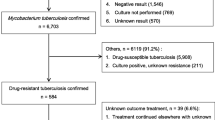
The cohort comprised 258 patients who were diagnosed with MDR-TB from 1st February 2012 to 1st of February 2014, from across the ten provinces of the whole country (Fig. 1). There were 110 (42.6%) out of 258 patients whose results were received at the referring facility and we were able to trace and contact them or next of kin. The results for the other 148 (57.4%) patients were not found in the health facility records, that is, they were lost before treatment initiation.
Of the 110 patients who were traced, 71 (64.5%) had been started on second-line treatment (and 11 had continued on first-line treatment (10 on Category II and 1 on Category I). For 28 (25.4%) patients, the treatment regimen was not indicated (Fig. 2).
Flow diagram of MDR-TB patients diagnosed in Zambia (2012–2014) and their outcomes. Rx treatment, CAT I Category I treatment (i.e. first-line treatment regimen), CAT II Category II treatment regimen (i.e. first-line treatment regimen for re-treatment TB cases), FLD First-line anti-TB drugs, SLD Second-line anti-TB Drugs, ‘not received’ not able to be accounted for at the referring facility, ‘received’ able to be accounted for at the referring facility. *There was no other treatment outcome data recorded on patients who were found to be deceased
There were 67/110 (60.9%) participants who were alive at the time of the interview. Forty-three (39.1%) were deceased. Their demographic characteristics are shown in Table 1.
The median age of the survivors was 36 years (IQR 28–45; range 14–82 years). The majority of the patients were male (62.8%) and more than 50% had at least a secondary education, although the majority (81.4%) were either unemployed or in informal employment. There were 39 (58.2%) patients that were HIV positive among the survivors (Table 1).
Among the 71 patients who were started on second-line treatment, 12 (16.9%) were recorded to be still on treatment at the time of interview; of these, three were found to be still bacteriologically positive and the other nine were bacteriologically negative. Twenty-nine (40.8%) patients had been declared cured and all were alive. Nine (12.7%) patients were recorded as ‘lost to follow-up’; however, one was traced and found alive and bacteriologically positive, whereas eight patients were found to be deceased. There were 12 (16.9%) patients who according to records were deceased and so was one (0.01%) patient recorded as transferred out. Among the 8 (11.3%) patients who were indicated as having stopped treatment due to adverse treatment effects, three were found to be alive and five had died. In addition, eight of the patients who had no treatment outcome recorded were also found to be bacteriologically positive on follow-up tests as shown in Fig. 2.
The overall survival of MDR-TB patients and the follow-up period was 212 person-years, during which 43 MDR-TB patients died, with a survival rate of 20.2 per 100 person-years of follow-up. More than 25% had died within 1 year of treatment and there was no difference (p = 0.35) in survival rates between patients who were on first-line treatment compared to those on MDR therapy (Fig. 3). The HIV co-infected MDR- TB patients’ rate of survival was less than their HIV negative counterparts (p = 0.013) as illustrated in Fig. 4.
Table 2 shows that taking ARVs was associated with an 88% decreased risk of dying (adjusted hazard ratio (aHR) 0.12, p = 0.002). Being HIV positive was also associated with a decreased risk of dying, after adjusting for the effect of taking ARVs and other risk factors (aHR 0.10, p = 0.04). Sex, age, marital status and treatment category were no significant predictors of survival in MDR-TB patients.
Discussion
This article underscores the fact that most of the MDR-TB patients diagnosed in Zambia were lost to follow-up even before they were started on treatment (Fig. 2). The loss to follow-up of more than half of the patients diagnosed with MDR-TB within a couple of years is cause for concern. The reason for this situation can be attributed to the fact that the reference laboratories from where culture and DST are performed are centralized in Zambia, and yet patients or specimens are referred from all over the country; in a country with limited resources to maintain and sustain a strong courier system for specimen referral and transportation, this poses a huge challenge [10]. In a study from South Africa examining reasons for loss to follow-up between time point of diagnosis and referral to a specialized DR-TB treatment centre, Nkosi and colleagues noted that a significant problem in the control of MDR-TB was the loss to follow-up after diagnosis and the delay in patient tracing [13]. There is need to strengthen patient flow and referral mechanisms to minimize loss of patients at this critical time [14]. Some of the other reasons that have been associated with low rates of traceable patients from other studies include; death after diagnosis, unknown addresses or inability to be contacted, migration from other provinces, incarceration and belief of being cured through other means, including poor health seeking behavior [13,14,15,16]. A systemic review by MacPherson et al. [17] showed that male sex, old age living in an urban area, diagnosis in a hospital or stationary clinic were associated high risk of pre-treatment loss to follow-up in most middle and low income countries and the main reason for the low rate of traceable patients in sub-Sahara Africa was death, although tracing of patients was sub-optimal.
It is well understood that pulmonary MDR-TB or XDR-TB can be transmitted just like drug susceptible TB and therefore, patients who are not traced and thus not put on treatment continue to transmit the disease in the communities [18].
Based on WHO recommendations, TB control programs usually report on cohorts of TB patients from those who were “enrolled for treatment” for the purposes of recording and reporting. Therefore, patients lost to follow-up before starting treatment are usually not accounted for. Some studies from across the globe have highlighted the high loss to follow-up among MDR-TB patients before initiation of treatment and hence have advocated for more careful cohort analysis starting from all diagnosed patients rather than only those who are started on treatment [19,20,21]. This study underscores that need, and calls for similar studies to be undertaken in other countries in the region to ascertain the magnitude of the problem. In fact, one of the reasons for inadequate access to diagnosis and treatment of MDR-TB in many countries is that the network for PMDT is usually too centralized [1].
Among the patients who were started on second-line treatment during the 2-year period, 29% were found to have died by the time of the interviews, implying that there is an urgent need for improvement in patient diagnosis, treatment, and management. This study, however, did not assess all the patients started on treatment in 2012–2014 but rather all patients started on treatment among those diagnosed during this period. It is envisaged that such cohort analysis is conducted within routine PMDT services.
There were 258 patients diagnosed during the 2-year period of the study, which was far below the expected number of cases according to the estimated prevalence of MDR-TB in Zambia. The prevalence of 1.1% for MDR-TB in Zambia entails the number of cases per annum is expected to be approximately 600 and thus in a 2-year cohort enrolment period, close to 1200 patients should have been diagnosed and enrolled for second-line treatment. Efforts need to be made to improve on case detection and diagnosis [10, 11]. Although Zambia has started the use of new diagnostics and technologies, they need to be scaled-up and expanded to improve the status quo; the use of the Xpert MTB/RIF and technologies such as the Genotype MTBDRplus assay have shown to improve detection of MDR-TB in different settings and hence should be utilized [22,23,24,25,26,27].
Only one patient diagnosed with MDR-TB during the study period was a child less than 15 years of age, thereby emphasizing the need to improve diagnosis in children as currently there is limited diagnostic capacity for childhood TB and MDR-TB [28, 29]. However, the other reason could also be that there are fewer children with MDR-TB, although this is unlikely given the comparative figures from the surrounding countries [1]. An autopsy study conducted in Zambia by Bates et al. showed that childhood TB was missed in a number of patients [30]; including some who had rifampicin resistance (RR), and thus it is possible that some of these patients could have had MDR-TB.
For those who were started on SLD, the cure rate was 41%; a low treatment success rate which is not so different from what was pertaining in the region, especially in South Africa [31,32,33].
Unfortunately, there are only a limited number of MDR-TB cohorts from Africa that have been described [34,35,36]. However, globally, there were only 52% of MDR-TB patients who were successfully treated amongst the patients enrolled on treatment in the 2012 cohort, falling short of the 2015 target of 75% or more; implying, therefore, that a lot needs to be done to address this challenge [1, 37].
Nonetheless, with more efforts the treatment success rate can still be improved, considering the fact that 17% of the patients were still on treatment at the end of the study period. For instance, Loveday et al. [38] showed in South Africa that employing a community-based approach for care was effective in increasing the treatment success rate. A study in Ethiopia showed that it was possible to improve outcome of treatment through concerted efforts from cooperating partners and national TB programs through various interventions such as training volunteers and treatment supporters, regular monthly home visits and monitoring by trained staff, provision of food supplements, transportation and accommodation for patients, capacity building of staff, strengthening health systems and using a combination of hospital-based care and ambulatory care, including management of side effects with ancillary drugs that were readily available [39]. Although there are multiple challenges in delivering appropriate MDR-TB treatment in the region and the evidence base being limited, some studies elsewhere have also shown that addressing the non-adherence issues by MDR-TB patients through improving health care worker’s attitude towards patients, decentralization of services, providing sufficient and timely financial assistance and other enablers may improve treatment outcomes [40,41,42,43].
There were discrepancies between the records at the health facilities and the findings of this study for some of the patients which were important to note; for instance, 11% of patients who had actually died were recorded as lost to follow-up in the treatment registers. Such findings underscore the need to ensure that the PMDT in Zambia is strengthened including the reporting and recording. Patient follow-up and tracing of lost to follow-up is cardinal to improve case holding and eventually patient outcomes. A substantial proportion of patients remained bacteriologically positive, thereby adding to the transmission risk in the population.
Our study also shows that patients were more likely to die in the intensive phase of treatment than during the continuation phase. The reason for this could be due to the fact that patients report late for treatment and it may take a long time for them to stabilize, thereby increasing their risk to die, especially if they have co-infections with HIV which may complicate the outcomes, sometimes due sepsis [44]. The treatment regimen for MDR-TB during this period was 6 months of an intensive phase then followed by 18 months of the continuation phase, thereby making the whole treatment duration to be not less than 24 months. Although Zambia now recommends a 20-month treatment regimen, much shorter regimens as recommended by the WHO are advocated for [1, 9]. However, intensified monitoring of patients through a strong PMDT and patient support system is cardinal to reduce mortality [45]. In addition, we strongly recommend a decentralized system of patient management, while ensuring capacity is built at all levels of care.
MDR-TB patients who were on anti-retroviral therapy were found to have better outcomes and survival rates than those who were not on ART; therefore, underscoring the fact that ensuring co-infected people to be on treatment is important to reduce morbidity and mortality in these patients. There was no difference in survival rates between MDR-TB patients starting second-line treatment and those continuing first-line treatments (Fig. 4). However, the number of patients on first-line treatment was very small and this may account for this finding. We recommend further similar studies with more numbers to explore the actual reasons for this, especially that presumptive MDR-TB patients in most times may continue on first-line treatment as they wait to be initiated on second-line therapy.
The limitation of the study was that only MDR-TB patients were followed up and patients with rifampicin resistance were not included and neither were patients with polydrug resistance included. There were no XDR-TB cases diagnosed during this period.
Conclusions
Our study shows that in Zambia more than half of the patients diagnosed with MDR-TB patients are lost to follow-up even before treatment has been instituted; underscoring the fact that PMDT needs strengthening. The status quo must be challenged as a matter of urgency to improve treatment outcomes of MDR-TB patients in Zambia.
References
World Health Organization, Global TB Report 2016. Geneva, Switzerland.
Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80.
Shah N, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, et al. Worldwide emergence of extensively drug-resistant tuberculosis (XDR TB): global survey of second-line drug resistance among Mycobacterium tuberculosis isolates. Emerg Infect Dis. 2007;13:380–7.
Lukoye D, Ssengooba W, Musisi K, Kasule GW, Cobelens FG, Joloba M, et al. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health. 2015;25:291. doi:10.1186/s12889-015-1614-8.
Andrews RJ, Shah NS, Gandhi N, Moll T, Friedland G, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: implications for the HIV epidemic and anti-retroviral therapy rollout in South Africa. JID. 2007;196:S482–90.
Berhan A, Berhan Y, Yizengaw D. A meta-analysis of drug resistant tuberculosis in Sub Saharan Africa: how strongly associated with previous treatment and HIV co-infection? Ethiop J Health Sci. 2013;23:271–82.
Kapata N, Chanda-Kapata P, O’Grady J, Schwank S, Bates M, Mukonka V, et al. Trends of Zambia’s tuberculosis burden over the past two decades. Trop Med Int Health. 2011;16:1404–9.
Moodley R, Godec RT. Short-course treatment for multidrug-resistant tuberculosis: the stream trials. Eur Respir Rev. 2016;25:29–35. doi:10.1183/16000617.0080-2015.
MOH. Guidelines for the programmatic management of drug-resistant tuberculosis in Zambia. Lusaka: Ministry of Health; 2015.
Kapata N, Chanda-Kapata P, Bates M, Mwaba P, Cobelens F, Grobusch MP, et al. Multidrug-resistant TB in Zambia: review of national data from 2000 to 2011. Trop Med Int Health. 2013;18:1386–91.
Kapata N, Mbulo G, Cobelens F, de Haas P, Schaap A, Mwamba P, et al. The Second Zambian National Tuberculosis Drug Resistance survey—a comparison of conventional and molecular methods. Trop Med Int Health. 2015;20:1492–500.
World Health Organization. International Standard Verbal Autopsy questionnaire. 1999. http://www.who.int/healthinfo/statistics/verbal_autopsy_standards2.pdf. Accessed 15 Mar 2014.
Nkosi D, Janssen S, Padanilam X, Louw R, Menezes CN, Grobusch MP. Factors influencing specialist care referral of multidrug- and extensively drug-resistant tuberculosis patients in Gauteng/South Africa: a descriptive questionnaire-based study. BMC Health Serv Res. 2013; 13:268. http://www.biomedcentral.com/1472-6963/13/268. Accessed 10 Jan 2017.
Cox H, Dickson-Hall L, Ndjeka N, Hoog A, Grant A, Cobelens F, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017;14:e1002238. doi:10.1371/journal.pmed.1002238.
Xu Z, Xiao T, Li Y, Yang K, Tang Y, Bai L. Reasons for non-enrollment in treatment among multi-drug resistant tuberculosis patients in Hunan Province, China. PLoS One. 2017;12:e0170718. doi:10.1371/journal.pone.0170718.
Ade S, Trébucq A, Harries AD, Ade G, Agodokpessi G, Wachinou P, et al. Follow-up and tracing of tuberculosis patients who fail to attend their scheduled appointments in Cotonou, Benin: a retrospective cohort study. BMC Health Serv Res. 2016;16:5. doi:10.1186/s12913-015-1219-z.
MacPherson P, Houben MGJR, Glynn RJ, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92:126–38. doi:10.2471/BLT.13.124800.
Lange C, Abubakar I, Alffenaar JC, Bothamley G, Caminero JA, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44:23–63. doi:10.1183/09031936.00188313.
Khaliaukin A, Kumar AMV, Skrahina A, Hurevich H, Rusovich V, Gadoev J, et al. Poor treatment outcomes among multidrug-resistant tuberculosis patients in Gomel Region, Republic of Belarus. PHA. 2014;4:S24–8.
Khann S, Eang MT, Rajendra YP, Satyanarayana S, Nagaraja SB, Kumar AMV. Linkage of presumptive multidrug resistant tuberculosis (MDR-TB) patients to diagnostic and treatment services in Cambodia. PLoS One. 2013;8:e59903.
Chadha SS, Sharath BN, Reddy K, Jaju J, Vishnu PH, Rao S, et al. Operational challenges in diagnosing multi-drug resistant TB and initiating treatment in Andhra Pradesh, India. PLos One. 2011;6:e26659.
Singh UB, Pandey P, Mehta G, Bhatnagar AK, Anant Mohan A, Goyal V, et al. Genotypic, phenotypic and clinical validation of GeneXpert in extra-pulmonary and pulmonary tuberculosis in India. PLos One. 2016;11:e0149258. doi:10.1371/journal.pone.0149258 (eCollection2016).
Metcalfe ZJ, Makumbirofa S, Makamure B, Sandy C, Bara W, Mason P, et al. Xpert MTB/RIF detection of rifampin resistance and time to treatment initiation in Harare, Zimbabwe. Int J Tuberc Lung Dis. 2016;20:882–9. doi:10.5588/ijtld.15.0696.
Ade S, Adjibodé O, Wachinou P, Toundoh N, Awanou B, Agodokpessi G, et al. Characteristics and treatment outcomes of retreatment tuberculosis patients in Benin. Tuberc Res Treat. 2016;2016:1468631. doi:10.1155/2016/1468631 (Epub 2016 Mar 24).
Stagg HR, White PJ, Riekstiņa V, Cīrule A, Šķenders G, Leimane V, et al. Decreased time to treatment initiation for multidrug-resistant tuberculosis patients after use of Xpert MTB/RIF test, Latvia. Emerg Infect Dis. 2016;22:482–90. doi:10.3201/eid2203.151227.
Nathavitharana RR, Hillemann D, Schumacher SG, Schlueter B, Ismail N, Omar SV, et al. Multicenter noninferiority evaluation of Hain GenoType MTBDRplus version 2 and Nipro NTMMDRTB line probe assays for detection of rifampin and isoniazid resistance. J Clin Microbiol. 2016;54:1624–30. doi:10.1128/JCM.00251-16.
Nikolayevskyy V, Balabanova Y, Simak T, Malomanova N, Fedorin I, Drobniewski F, et al. Performance of the Genotype® MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin Pathol. 2009;9:2. doi:10.1186/1472-6890-9-2.
Hicks RM, Padayatchi N, Shah NS, Wolf A, Werner L, Sunkari VB, et al. Malnutrition associated with unfavourable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis. 2014;18:1074–83. doi:10.5588/ijtld.14.0231.
Seddon JA, Hesseling AC, Godfrey-Faussett P, Schaaf HS. High treatment success in children treated for multidrug-resistant tuberculosis: an observation cohort study. Thorax. 2014;69:458–64. doi:10.1136/thoraxjnl-2013-203900 (Epub 2013 Sep 24).
Bates M, Shibemba A, Mudenda V, Chimoga C, Tembo J, Kabwe M, et al. Burden of respiratory tract infections at post mortem in Zambian children. BMC Med. 2016;14:99. doi:10.1186/s12916-016-0645-z.
Shean KP, Willcox PA, Siwendu SN, Laserson KF, Gross L, Kammerer S, Wells CD, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. Int J Tuberc Lung Dis. 2008;12:1182–9.
Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N, et al. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis. 2010;14:413–9.
Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lund Dis. 2015;19:969–78. doi:10.5588/ijtld.15.0123.
Oladimeji O, Isaakidis P, Obasanya OJ, Eltayeb O, Khogali M, Van den Bergh R, et al. Intensive-phase treatment outcomes among hospitalized multidrug-resistant tuberculosis patients: results from a Nationwide Cohort in Nigeria. PLos One. 2014;9:e94393. doi:10.1371/journal.pone.0094393.
Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9153 patients. PLos Med. 2012;9:e1001300.
Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLos One. 2009;4:e6914.
World Health Organization. The global plan to stop TB: 2011–2015. Geneva: World Health Organization, 2010. http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011–2015.pdf. Accessed 2 Jun 2014.
Loveday M, Wallengren K, Brust J, Roberts J, Voce A, Margot B, et al. Community-based care vs. centralised hospitalisation for MDRTB patients KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2015;19:163–71. doi:10.5588/ijtld.14.0369.
Meressa D, Hurtado MR, Andrews RJ, Diro E, Abato K, Daniel T, et al. Achieving high treatment success for multidrug resistant TB in Africa: initiation and scale-up of MDR TB care in Ethiopia—an observational cohort study. Thorax. 2015;70:1181–8. doi:10.1136/thoraxjnl-2015-207374.
Mitnick DC, Rodriguez AC, Hatton LM, Brigden G, Cobelens F, Grobusch MP, et al. Programmatic management of drug resistant tuberculosis: an updated research Agenda. PLoS One. 2016;11:e0155968. doi:10.1371/journal.pone.0155968.
Holtz TH, Lancaster J, Laserson KF, Wells CD, Thorpe L, Weyer K. Risk factors associated with default from multidrug- resistant tuberculosis treatment, South Africa, 1999–2001. Int J Tuberc Lung Dis. 2006;10:649–55.
Gler MT, Podewils LJ, Munez N, Galipot M, Quelapio MID, Tupasi TE. Impact of patient and program factors on default during treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:955–60. doi:10.5588/ijtld.11.050.
Tupasi ET, Garfin GAMC, Kurbatova VE, Mangan JM, Orillaza-Chi R, Naval LC, et al. Factors associated with loss to follow-up during treatment for multidrug-resistant tuberculosis, the Philippines, 2012–2014. Emerg Infect Dis. 2016;22:491. doi:10.3201/eid2203.151788.
Huson MA, Kalkman R, Stolp SM, Janssen S, Alabi AS, Beyeme JO, van der Poll T, Grobusch MP. The impact of HIV on presentation and outcome of bacterial sepsis and other causes of acute febrile illness in Gabon. Infection. 2015;43:443–51. doi:10.1007/s15010-015-0753-2 (Epub 2015 Mar 11).
WHO 2016. The Shorter MDR-TB regimen. http://www.who.int/tb/short_MDR_regimen_factsheet.pdf. Accessed 25 Jul 2017.
Acknowledgements
The authors wish to acknowledge the field staff and collaborators who assisted with data collection; follow-up of participants and overall study implementation.
Author information
Authors and Affiliations
Contributions
Author contributions
Conceptualization: NK, GC, PCK, AZ. Methodology: NK, GC, FC, WN. Data analysis: PCK, MB, MPG, PK, PM. Writing original draft: NK, FC, MPG, MT, SM, PCK. Writing review and editing: NK, MPG, FC, PM, AZ.
Corresponding author
Ethics declarations
Funding
The study was partially funded by the Government of the Republic of Zambia through the Ministry of Health, research unit.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kapata, N., Grobusch, M.P., Chongwe, G. et al. Outcomes of multidrug-resistant tuberculosis in Zambia: a cohort analysis. Infection 45, 831–839 (2017). https://doi.org/10.1007/s15010-017-1054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-1054-8