Abstract
We conducted a retrospective observational study at four German university hospitals of patients with laboratory-confirmed influenza in 2014/2015. Overall, a fatality rate of 8% was observed. Significantly more A(H1N1)pdm09 patients were admitted to ICU compared to those with A(H3N2). However, fatal outcome was not significantly increased among A(H1N1)pdm09 cases. Nosocomial infections were seen in 17% of cases. Systematic collection of data from hospitals will complement national influenza surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of each influenza epidemic on morbidity and mortality varies considerably as well as the predominant influenza virus. Surveillance is an important tool to monitor changes in influenza characteristics and to develop prevention strategies. In Germany, influenza surveillance is based on data collected by the Working Group on Influenza (AGI), which combines syndromic and virological surveillance of a representative sample of the population. Influenza surveillance is further supported by a mandatory notification system (Protection Against Infection Act, IfSG), which is in place to detect smaller outbreaks, e.g. in hospitals or in nursing homes. However, in the hospital setting data on influenza is not systematically collected [1]. In addition, much of our understanding of influenza is triggered by outbreak investigations or pandemics. Recent studies showed a dominance of influenza A(H1N1)pdm09 in the immediate post-pandemic seasons, but in 2013/14 influenza virus A(H3N2) took over according to national surveillance data. Of note, the case severity of A(H1N1)pdm09 infections in the post pandemic era appeared to be less severe compared to the pandemic year both among pediatric and adult patients [2].
We aimed to determine the clinical epidemiology of influenza, i.e. the prevalence and types of influenza virus, and to describe patient characteristics at four university hospitals in Germany in 2014/2015, a season which was dominated by novel drift variants of influenza A(H3N2). Of note, these variants resulted in reduced vaccine effectiveness and the overall burden of influenza was high compared to previous seasons [3].
Methods
Study population
A retrospective study was conducted at four German university laboratories: the Institute of Virology, University of Bonn Medical Centre (site A), the Institute of Medical Virology, University Hospital Frankfurt (site B), the Institute for Virology, Medical Center - University of Freiburg (site C), and the Institute of Clinical Microbiology and Hygiene, Regensburg University Medical Centre (site D). All sites are tertiary care centers with a total of 1232 (site A), 1302 (site B), 1610 (site C), and 833 (site D) beds, respectively. Patients with influenza-like illness (ILI) in combination with laboratory-confirmed influenza were included. Indication to test for influenza was done at the discretion of the treating physician. The criteria for ILI included sudden onset of symptoms, at least one of four systemic symptoms (fever, malaise, headache, myalgia), and at least one respiratory symptom (cough, sore throat, shortness of breath).
Each participating laboratory was asked to provide the number of laboratory-confirmed influenza cases and the influenza type and subtype if available. In addition, a predefined questionnaire was set up to provide basic clinical data of each case in a blinded manner. Specifically, the following information was requested: age and sex of the patient, date of specimen collection, admission status, and admission to intensive care unit (ICU), fatalities, immunosuppression, and nosocomial influenza. Fatalities include influenza-associated deaths only. Immunosuppression was defined due to steroid therapy, chemotherapy or immunosuppressive therapy. The information was retrieved from the hospital-based information system at each participating center. Nosocomial influenza was defined as symptom onset ≥72 h after admission to hospital and admission not related to respiratory symptoms. Analysis is limited to those patients where data were available. Unfortunately, we could not systematically retrieve information on influenza vaccination and administration of oseltamivir therapy or prophylaxis.
Laboratory methods
Specimens included upper and lower respiratory tract samples. Laboratory confirmation of influenza was done using reverse transcription PCR (RT-PCR) methods established at each participating laboratory. These included laboratory-developed methods as well as commercial assays to detect influenza virus and are available upon request.
Statistical analysis
We plotted the aggregated cases of each calendar week and compared our data with the data from the German mandatory notification system (Protection Against Infection Act, IfSG). Descriptive data were presented as frequencies (percentages) for categorical variables and as medians (interquartile range, IQR) for continuous variables. Statistical analysis was done using Chi square test and Mann–Whitney U-test as appropriate using Graphpad Prism 6 software (Graphpad, USA). A p value of < 0.05 was considered statistically significant for all tests.
Ethics
Ethical clearance for the whole study was obtained at Freiburg University and individually at each participating study center.
Results
Descriptive influenza epidemiology
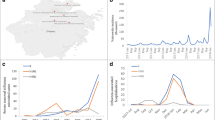
A total of 857 patients with laboratory-confirmed influenza at four university hospitals between 25 December 2014 and 3 May 2015 were included in this study (Table 1). The number of ordered influenza tests during the study period was 704 at site A, 992 at site B, 1984 at site C, and 1009 at site D, respectively, resulting in an influenza detection rate of 24% (site A), 12% (site B), 16% (site C), and 24% (site D). Overall, influenza A was detected in 671/857 (78%) patients (median age 58 years) and influenza B in 186/857 (22%) patients (median age 54 years). The detection of influenza A peaked around calendar week 8, whereas influenza B cases peaked shortly after around calendar week 9 (Fig. 1, panel A, B). We compared the temporal distribution of our cases with all cases notified to the German influenza surveillance system (IfSG, Fig. 1, panel A, B). The combined detection of influenza A and B at the four study sites preceded the peak of detection reported by the German influenza surveillance system by one to two weeks.
In a subset of patients (n = 341), information on the influenza A subtype was available [113/341 (33%) patients with A(H1N1)pdm09, and 228/341 (67%) patients with A(H3N2)]. For cases with subtype information available, influenza A(H3N2) was the most commonly detected subtype in the youngest (0–14 years of age) and the oldest (>60 years) age groups. In adolescent and middle aged patients most infections were caused by influenza B (Fig. 2).
The presence of A/Switzerland/9715293/2013-like virus in a limited number of samples from Freiburg was confirmed at the German National Reference Center for Influenza (data not shown).
Patient characteristics
The 476 male and the 381 female patients had a median age of 58 years (interquartile range (IQR) 40–72 years). A minority of 82/857 (9.6%) patients were ≤18 years of age. Next, patients with a complete dataset (n = 620) were analysed. A total of 434/620 (70%) patients were hospitalized and the rate ranged from 60% at study site D to 86% at study site B. A total of 186 patients were not admitted at one of our study sites as documented by the hospital-based information system. Overall, hospitalized patients (median age 62 years, IQR 48–74 years) were older compared to non-hospitalized patients (median age 48.5 years, IQR 31–65 years, Mann–Whitney U-test, p < 0.0001). A total of 188/620 (30%) patients were immunocompromised. In detail, the highest rate of immunosuppressed patients was seen at study site A (77%) followed by study site B (34%) (Table 1).
Case severity
A total of 149/620 (24%) patients required admission to an ICU (Table 1). The ICU admission rate for patients <18 years of age was 15% (8/52 patients). Clinically, 183/620 (29%) patients developed pneumonia, and 134/620 (22%) needed mechanical ventilation. A total of 52/620 (8%) fatalities were observed. Most fatalities occurred in cases ≥60 years of age, followed by those aged 35–59 years (Fig. 3). Among fatal cases, the majority had pneumonia [41/52 (79%)], required mechanical ventilation [40/52 (77%)], and 44/52 (85%) were admitted to ICU. Of note, 19/52 (37%) of fatal cases were observed in immunosuppressed patients. Next, we compared the proportion of fatalities in our study with data from the IfSG (Fig. 3). Interestingly, in the age group 35–59 years we observed more fatalities compared to national data (29 vs. 17%, p = 0.043), whereas in the oldest age group the opposite was observed (63 vs. 79%, p = 0.013).
Patient characteristics by influenza A subtype
Patients with A/H1N1pdm09 were significantly younger (54 vs. 62 years, p < 0.001), and were admitted to ICU more often compared to patients with A/H3N2 [30/74 (41%) vs. 35/142 (25%), p = 0.019] (Table 2). Remarkably, immunosuppression was less present in patients with A/H1N1pdm09 compared to those with A/H3N2 (27 vs. 40%, p = 0.072). No significant differences were observed in gender ratio of patients with A/H1N1pdm09 and A/H3N2, respectively.
Nosocomial cases
Finally, a total of 105/620 (17%) nosocomial cases were recorded. Of these, 19/105 (18%) were fatal, which contrasts with 33/515 (6%) fatalities among community acquired influenza cases. The lowest rate of nosocomial cases was seen at study site A (10%), and the highest at study sites B (23%) and D (19%), respectively. Nosocomial cases were observed across all age groups with the highest rate among patients >65 years of age (data not shown). No significant difference in the rate of nosocomial infections with respect to influenza A subtype was observed (Table 2).
Discussion
Retrospective analysis of influenza cases in 2014/2015 was performed at four large university hospitals in Germany to determine the epidemiology and the characteristics of influenza in a tertiary care hospital setting. The main result of the study was the finding that, among other observations, patients with A(H1N1)pdm09 were younger but more often admitted to ICU compared to patients with A(H3N2). However, the fatality rate between A(H1N1)pdm09 and A(H3N2) cases was not different.
The majority of our study sites was located in the southern part of Germany and was thus affected rather early by the influenza epidemic, which started to spread from southern Germany. This time frame might explain the earlier peak in our population compared to the IfSG data. Of note, nosocomial cases were observed at each study site with an overall rate of 17% underpinning the results of our recent single center study that identifies a rate of 20% [4]. In particular, the rate of nosocomial cases in our multi-center study varied from 10 to 23%. Interestingly, the lowest rate was observed at study site A, where the majority of patients were immunosuppressed. A likely explanation is a higher degree of awareness to prevent nosocomial infections among health care workers treating immunocompromised patients and a higher ratio of single bedrooms compared to wards for non-immunocompromised patients. Our rate of 10–23% nosocomial infections contrasts with a rate of 35.5%, which was recently reported from another large German university center in 2014/2015 [5]. In this context, it should be mentioned that a universal influenza case definition is not available and the indication to test for influenza was done at the discretion of the treating physician in both studies. Of concern, a high rate of fatalities (18%) was observed among nosocomial cases. In light of this high mortality among nosocomial cases, the prevention of nosocomial transmissions is therefore of utmost importance.
Although all four study sites are classified as tertiary care hospitals, the decision to test patients apparently differed among study sites, e.g. at site A the majority of influenza patients were immunosuppressed supporting the notion of a targeted testing strategy. Especially for immunocompromised patients ILI criteria have a poor positive predictive value [6]. Critically, a recent study demonstrated that influenza in ICU patients is frequently overlooked since influenza testing was not ordered by the physicians [7]. It is suggestive that financial constraints of hospitals in general and tertiary care centers in particular might negatively influence the decision to order influenza testing in patients. An integrated stewardship approach was recently proposed by Dik and colleagues to overcome the constraints of individual stakeholders within a hospital and warrants further study [8].
Overall, the age distribution of influenza cases displayed the well-known pattern from previous seasons. It is noteworthy that the majority of patients < 18 years of age were infected with A(H3N2) virus indicating that they were less susceptible to A(H1N1)pdm09 infection. This is most likely due to the relatively high attack rate during the 2009 influenza pandemic, the influenza seasons here after, and the vaccination strategies, which were recommended for children during the influenza pandemic [9].
Of note, the detection of influenza B peaked in the group of 15–34 years of age. The vast majority of influenza B virus circulating in 2014–2015 belonged to the Group 3 of the Yamagata lineage [10]. The seasonal influenza vaccine contained Group 2 Yamagata lineage virus and the 2014/2015 vaccine proved to be slightly less effective. Nevertheless, of all patients with influenza B only 17% required admission to an ICU and 7% died suggesting an overall milder course of the disease than influenza A.
The overall ICU admission rate of 24% at the four study sites was high and is consistent with our data from the single-center study (20%) [4]. In detail, patients with A(H1N1)pdm09 were younger and more frequently admitted to an ICU compared to patients with influenza A(H3N2). This is consistent with previous findings from the 2009 pandemic and post-pandemic influenza seasons [11, 12]. Noteworthy, a lower rate of immunosuppressed patients was seen among patients with A(H1N1)pdm 09 compared to those with A(H3N2). Current guidelines in Germany recommend yearly influenza vaccination amongst others for health care workers, persons older than 60 years of age, individuals with underlying chronic diseases, and immunosuppressed patients despite suggested inferior efficacy. Although we could not assess the influenza vaccination status of our patients it indicates at least partial protection mediated by the A(H1N1) vaccine and less protection due to the A(H3N2) variant as observed early in the season [3].
The rate of influenza-associated deaths comprises an important indicator of the impact and severity of epidemic influenza. A total of 274 deaths related to influenza were reported by mandatory notification system to the German Public Health Institute, Robert-Koch-Institute, in 2014/2015 and 169/274 were considered as influenza-associated deaths. As a limitation, it should be noted that the decision to notify a death as influenza-associated is done at the discretion of the local public health office based upon available data. This contrasts with a total of 52 influenza-associated deaths at our four study sites. Of note, a higher percentage of fatalities among those aged 35–60 years was observed compared to data according to the IfSG. This supports the notion that a considerable proportion of fatalities might remain unnoticed by national surveillance systems, but this warrants further studies. It is speculative if fatality rates in non-university hospitals are lower, because a high proportion of severely ill patients will be transferred to referral university hospitals due to the possibility of extracorporeal membrane oxygenation (ECMO) and their activities in transplantation, treatment of patients with malignancies, and on immunosuppressive therapy.
The main limitation of our study was the retrospective design. In addition, a considerable proportion of samples were not influenza A subtyped. It should be noted that active screening may further increase the number of influenza cases as seen in a recent study [7]. Importantly, we believe that our study may set the basis to prospectively monitor influenza in the hospital setting and adds value to the already existing influenza surveillance in Germany.
References
Babcock HM, Merz LR, Dubberke ER, Fraser VJ. Case-control study of clinical features of influenza in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29:921–6.
Rao S, Torok MR, Bagdure D, et al. A comparison of H1N1 influenza among pediatric inpatients in the pandemic and post pandemic era. J Clin Virol. 2015;71:44–50.
Pebody RG, Warburton F, Ellis J, et al. Low effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/2015 mid-season results. Euro Surveill. 2015;20:21025.
Huzly D, Kurz S, Ebner W, Dettenkofer M, Panning M. Characterisation of nosocomial and community-acquired influenza in a large university hospital during two consecutive influenza seasons. J Clin Virol. 2015;73:47–51.
Hagel S, Ludewig K, Moeser A, et al. Characteristics and management of patients with influenza in a German hospital during the 2014/2015 influenza season. Infection. 2016;44:667–72.
Claus JA, Hodowanec AC, Singh K. Poor positive predictive value of influenza-like illness criteria in adult transplant patients: a case for multiplex respiratory virus PCR testing. Clin Transplant. 2015;29:938–43.
Giannella M, Rodriguez-Sanchez B, Roa PL, et al. Should lower respiratory tract secretions from intensive care patients be systematically screened for influenza virus during the influenza season? Crit Care. 2012;16:R104.
Dik JW, Poelman R, Friedrich AW, et al. An integrated stewardship model: antimicrobial, infection prevention and diagnostic (AID). Future Microbiol. 2016;11:93–102.
WHO. Influenza (seasonal). 2014. 09.06.2016. Available from http://www.who.int/mediacentre/factsheets/fs211/en/.
AG Influenza. Bericht zur Epidemiologie der Influenza in Deutschland Saison 2014/152015 16.06.2016. Available from https://influenza.rki.de/Saisonberichte/2014.pdf.
Bauernfeind S, Bruennler T, Ehrenstein B, et al. Pandemic and post-pandemic influenza A (H1N1) seasons in a tertiary care university hospital-high rate of complications compared to previous influenza seasons. Infection. 2013;41:145–50.
Minchole E, Figueredo AL, Omenaca M, et al. Seasonal influenza A H1N1pdm09 virus and severe outcomes: a reason for broader vaccination in non-elderly, at-risk people. PLoS ONE. 2016;11:e0165711.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Ethical clearance for the whole study was obtained at Freiburg University and individually at each participating study center.
Conflict of interest
No conflict of interest to declare by all authors.
Additional information
Robert Heyd, Anna Maria Eis-Hübinger, and Annemarie Berger have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Heyd, R., Eis-Hübinger, A.M., Berger, A. et al. Retrospective analysis of clinical and virological parameters of influenza cases at four university hospitals in Germany, 2015. Infection 45, 349–354 (2017). https://doi.org/10.1007/s15010-017-1008-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-1008-1