Abstract
Background
Microbiological confirmation cannot be obtained in approximately two-third patients with tuberculous meningitis. In this study, we sought to identify epidemiological, clinical, cerebrospinal fluid, and imaging parameters that could indicate the possibility of microbiological confirmation among patients with suspected tuberculous meningitis.
Materials and methods
In this prospective observational study, patients with tuberculous meningitis were evaluated for clinical, laboratory (cerebrospinal fluid microscopy, culture, and polymerase chain reaction), and neuroimaging parameters. All patients received anti-tuberculosis drugs and corticosteroids. The patients were followed for a period of 6 months.
Results
Among 118 cases of tuberculous meningitis, there were 43 (36 %) definite (microbiologically confirmed) cases, 59 (50 %) probable and 16 (14 %) possible cases. Among 43 definite cases, tuberculosis polymerase chain reaction (PCR) was positive in 42 (35 %) patients, culture was positive in 1 case and microscopy, after Ziehl–Neelsen staining, was positive in 3 cases. Three factors, modified Barthel index score at admission ≤12 (p = 0.008), cerebrospinal fluid total cell count >100/mm3 (p = 0.016), and basal exudates on imaging (p = 0.015), were significantly associated with definite tuberculous meningitis. Among 20 patients who died within 6 months, 13 belonged to definite tuberculous meningitis group (p < 0.001). Stage III tuberculous meningitis (p = 0.004), baseline-modified Barthel index score ≤12 (p = 0.013), and positive tuberculosis PCR (p = 0.007) were independently associated with poor outcome on multivariate analysis.
Conclusion
Severe disability, cerebrospinal fluid cells >100 mm3, and basal exudates are significantly related to the presence of microbiologically confirmed definite tuberculous meningitis. Microbiologically confirmed tuberculous meningitis is associated with poorer outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningitis is the most serious and most common form of central nervous system tuberculosis. Correct diagnosis always remains a challenge. The majority of patients with tuberculous meningitis are diagnosed on the basis of clinical features, neuroimaging, and characteristic cerebrospinal fluid changes. The yield of diagnostic tests available for microbiological confirmation is terribly low. Mycobacterium tuberculosis cultures often take several weeks or longer to detect mycobacterial growth. A presumptive diagnosis of tuberculous meningitis, in cases with a negative cerebrospinal fluid (CSF) Ziehl–Neelsen staining, is often made before the results of CSF culture are available [1, 2].
Definite tuberculous meningitis is diagnosed following the demonstration of Mycobacterium tuberculosis in CSF either by Ziehl–Neelsen staining, polymerase chain reaction (PCR), or culture. Chiang and colleagues noted 9 % acid-fast bacilli positivity on microscopy and 35 % positivity for culture [3]. In a prospective observational study from Indonesia, only 11 % of CSF samples, taken from 207 suspected cases of tuberculous meningitis, were tested positive by Ziehl–Neelsen staining. A low percentage of CSF samples yielded growth of Mycobacterium tuberculosis by solid culture (36 %) and liquid culture (44 %). The diagnostic yield of IS6110-PCR was much higher (68 %) [4]. A study from Vietnam reported CSF sensitivities for Xpert MTB/RIF, Ziehl–Neelsen smear, and mycobacterial growth indicator tube culture, of 59.3 % (108/182), 78.6 % (143/182), and 66.5 % (121/182), respectively [5]. Xpert MTB/RIF is a fully automated diagnostic molecular test meant for rapid demonstration of Mycobacterium tuberculosis in various biological materials. It also detects rifampicin drug resistance.
The matter of early microbiological diagnosis of tuberculous meningitis is crucial because early treatment improves the prognosis. In many developing and poor countries, resources to confirm the diagnosis of tuberculous meningitis microbiologically are limited and are often not available in time. Clinicians, most of the time, have to rely on clinical parameters for the diagnosis. We sought to identify epidemiological, clinical, CSF, and imaging parameters that could indicate microbiological confirmation, among patients with suspected tuberculous meningitis. We also assessed the prognostic impact of microbiological confirmation.
Materials and methods
This study was conducted in the Department of Neurology, King George Medical University, Lucknow. Our institution is a tertiary care medical facility catering to approximately 100 million population of North India. The patients were enrolled from October 2012 to January 2014. Institutional Ethics Committee of the university approved the study. Written informed consent was obtained from all the patients or their legal guardians.
Inclusion criteria
In this prospective follow-up study, we enrolled consecutive patients with suspected tuberculous meningitis who were 14 years of age or older. These patients had presented with clinical evidence of tuberculous meningitis and had characteristic CSF abnormalities (mononuclear cell pleocytosis, low glucose levels, elevated protein levels). All included patients fulfilled the consensus tuberculous meningitis criteria. According to these criteria, patients were categorized into definite, probable, and possible tuberculous meningitis groups, depending on a system of diagnostic scores. Definite cases were defined by demonstration of tubercle bacilli in CSF either by microscopy, culture, or tuberculosis PCR. Tuberculous meningitis was considered probable, if patients scored 10 or more diagnostic points (when cerebral imaging is not available) or 12 or more diagnostic points (when cerebral imaging is available) along with exclusion of alternative differential diagnoses. Possible cases had a total diagnostic score of 6–9 points (when cerebral imaging is not available) or 6–11 points (when cerebral imaging is available) along with exclusion of alternative differential diagnoses [6]. We divided the patients into two groups; definite tuberculous meningitis (bacteriologically confirmed) and non-definite tuberculous meningitis (probable and possible cases).
Exclusion criteria
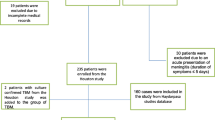
Patients with cryptococcal meningitis or any other cause for meningitis were excluded from the study (Fig. 1).
Evaluation
All included patients were subjected to a detailed clinical evaluation. A battery of routine hematological and biochemical tests like complete blood count with peripheral smear examination, erythrocyte sedimentation rate, blood sugar, blood urea nitrogen and serum creatinine, liver function tests, serum electrolytes, chest X-ray, and antibodies against human immunodeficiency virus by enzyme-linked immunosorbent assay were performed. CSF routine examination was done for cells, glucose, protein, and Gram’s staining including India ink preparation and corresponding blood sugar level.
Neuroimaging
Gadolinium-enhanced brain MRI was performed with a Signa Excite 1.5 tesla instrument (General Electric Medical Systems, Milwaukee, WI, USA). An experienced neuroradiologist unaware of treatment and patient outcome reviewed the MR scans. Neuroimaging features recorded were abnormal enhancement of the leptomeninges, tuberculomas, hydrocephalus (communicating or non-communicating), and infarcts.
Bacteriological confirmation
CSF microscopy for tuberculosis was done by Ziehl–Neelsen staining. CSF culture was done by solid culture method, on Lowenstein Jensen media. Drug susceptibility testing was done. Growth was monitored for 8 weeks and the mycobacterial species identified in positive cultures. PCR was done by real-time PCR. DNA amplification of mpt64 gene for Mycobacterium tuberculosis was done by real-time DNA detection technique [7].
Disease severity
All patients were assessed by Glasgow Coma Scale, modified tuberculous meningitis staging criteria by Thwaites and co-workers, and modified Barthel index scoring system. According to the staging criteria given by Thwaites and co-workers: patients with stage I disease had a Glasgow Coma Scale score of 15 with no focal neurologic signs, patients with stage II had a Glasgow Coma Scale score of 15 with neurological deficit (cranial nerve palsies) or Glasgow Coma Scale score of 11–14, and patients with stage III had Glasgow coma scale score <10 or multiple cranial nerve palsies, hemiplegia, or paraplegia [8]. Disability assessment was done using modified Barthel index, which is a 20-point scoring system. A score of ≤12 indicated a poor functional status (death scored as zero) while a score of >12 indicated a good functional status.
Treatment
Patients were treated with anti-tuberculosis treatment protocol recommended by the World Health Organization. Patients were given two months of daily oral isoniazid (5 mg/kg of body weight; maximum, 300 mg), rifampicin (10 mg/kg; maximum, 600 mg), pyrazinamide (25 mg/kg; maximum, 2 g/day), and intramuscular streptomycin (20 mg/kg; maximum 1 gm/day) followed by 7 months of isoniazid and rifampicin at the same daily dose [9]. All patients received dexamethasone for 8 weeks. Patients received intravenous dexamethasone for 4 weeks (0.4 mg/kg body weight per day and then tapered off decreasing 0.1 mg/kg every week) and then oral treatment for 4 weeks, starting at a total of 4 mg per day and decreasing by 1 mg each week. Antiepileptic drugs were given to patients who had seizures. Pyridoxine was given orally 20–40 mg/day to all patients. Mannitol was used in patients showing features of raised intracranial pressure.
Follow-up
Every case was followed for a minimum of 6 months, from the start of treatment. Assessment of disability as per the modified Barthel index was done at baseline and at the end of 1st, 3rd, and 6th month of follow-up. Final modified Barthel index score >12 at the end of 6 months was regarded as a good outcome. Poor outcome was defined as death or modified Barthel index score ≤12 at 6 months.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Sciences, version 16 for windows (SPSS, Chicago IL, USA). Univariate analysis was used to identify predictors of definite tuberculous meningitis. Univariate analysis was performed by Chi square test for non-parametric data and student’s t test for independent variables for parametric data and relative risks with 95 % confidence interval were ascertained. For multivariate analysis, binary logistic regression was performed to see the impact of individual variables. Kaplan–Meier analysis was performed to estimate the event-free survival for the outcome in definite or non-definite tuberculous meningitis, using the Log Rank test. p value <0.05 was considered statistically significant. Statistical analysis was two-tailed. The variables, which were used for univariate analysis, included age, sex, fever, headache or vomiting, seizure, altered sensorium, focal deficit, diplopia, cranial nerve involvement, pulmonary tuberculosis, signs of meningeal irritation, papilledema, stage of tuberculous meningitis (stage 3), baseline functional disability status (modified Barthel index score ≤12), CSF cell count, protein, presence of acid-fast bacillus on smear examination, positive PCR, and a positive culture for Mycobacterium tuberculosis. Neuroimaging features like meningeal enhancement, basal exudates, hydrocephalus, tuberculoma, and infarct were also evaluated.
Results
Baseline clinical, laboratory, and neuroimaging characteristics of 118 patients with tuberculous meningitis are shown in Table 1. None of the patients were tested positive for human immunodeficiency virus antibodies. There were 43 (36 %) definite cases, 59 (50 %) probable, and 16 (14 %) possible cases of tuberculous meningitis. In the 43 definite cases, tuberculosis PCR was positive in 42 (35 %) cases including a case that was positive by culture; microscopy was positive in 3 cases. The single-positive Mycobacterium tuberculosis culture showed sensitivity to standard anti-tuberculosis drugs (Fig. 1).
Predictors of definite tuberculous meningitis
Three factors that were significantly associated with definite tuberculous meningitis included modified Barthel index score at admission ≤12 (p = 0.008), CSF total cell count >100/mm3 (p = 0.016), and basal exudates on imaging (p = 0.015).
Follow-up
In totality, 20 patients died in 6 months, of which 13 were from the definite group (p < 0.001).
At 6 months, 26 cases had poor outcome in the definite category (death or modified Barthel index score ≤12). Among 17 cases with good outcome (modified Barthel index score >12) in the definite category, 13 improved completely (modified Barthel index score of 20). Among probable and possible groups, 21 had a poor outcome (death or modified Barthel index score ≤12), and 54 improved (modified Barthel index score >12) (Table 2). Out of 54 patients in the good outcome group 42 achieved the score 20. Kaplan–Meier cumulative survival curve analysis showed that patients of definite tuberculous meningitis had significantly higher death and disability rates (p < 0.001). The mean survival of patients in the definite group was 139.37 days while it was 168.23 days in the other group (Fig. 2).
Predictors of outcome
At six months, eight factors were found to be associated with poor outcome on univariate analyses; baseline-modified Barthel index score ≤12 (p < 0.001), stage III (p < 0.001), cerebral infarct (p = 0.009), hydrocephalus (p = 0.003), hemiparesis (p = 0.031), paraparesis (p = 0.016), altered sensorium (p < 0.001), and positive tuberculosis PCR (p = 0.001). Multivariate analysis showed that baseline-modified Barthel index score ≤12 (p = 0.013), positive tuberculosis PCR (p = 0.007), and stage III (p = 0.004) were independently associated with poor outcome (Table 3).
Discussion
In this study, approximately one-third of patients with suspected tuberculous meningitis had microbiological confirmation of their clinical diagnosis. Our analysis revealed that three factors (severe disability at presentation, CSF white cells >100 mm3, and basal exudates were associated with microbiological confirmation. Thwaites and co-workers reported that five clinical and laboratory features (age, length of history, white blood cell count, total CSF white cell count, and CSF neutrophil proportion) were predictive of a diagnosis of tuberculous meningitis and were reliable in differentiating it from bacterial meningitis [10].
We found an abysmally low yield of acid-fast bacilli on microscopy (2.5 %, 3/118) and culture (0.8 %, 1/118) in CSF samples. We do not have an explanation for the extremely low positivity of CSF ZN staining (3/118) and Mycobacterium tuberculosis culture (1/118) in our study. The possibility of inadequate centrifugation of CSF samples, and/or insufficient examination of microscopy slides by our laboratory staff cannot be excluded as contributors to the low output. Several modifications in microbiological methods have been suggested to increase the yield. It has been found that large volume of CSF, if carefully examined, can yield a positive result in over 90 % of cases. With repeat examination of sequential CSF, tubercle bacilli can be detected in up to 87 % of cases by microscopy and up to 83 % by culture [11]. In a recent study, Feng and co-workers observed increased positivity of Ziehl–Neelsen stain by simple modification of the stain method and an ESAT-6 intracellular stain. Acid-fast bacteria and ESAT-6-expressing leukocytes were detected by light microscopy [12].
Neuroimaging in patients with tuberculous meningitis, frequently, demonstrates basal meningeal enhancement, infarcts, hydrocephalus, and tuberculomas. Combining together, these features are considered suggestive of tuberculous meningitis [13]. Botha and co-workers clearly demonstrated that, among prominent CT features of tuberculous meningitis, basal meningeal enhancement is the most specific (69.2 %) imaging feature [14]. The patients with positive Ziehl–Neelsen stain (3 in number) had extensive cranial basal meningeal enhancement in the form of optochiasmatic arachnoiditis in addition to lumbosacral arachnoiditis. On the basis of our findings, we suggest that a combination of optochiasmatic arachnoiditis and lumbosacral arachnoiditis can be considered a surrogate imaging characteristic suggestive of tuberculous meningitis, even before or in the absence of microbiological confirmation.
We observed that patients with definite tuberculous meningitis had poorer outcomes (death or modified Barthel index score ≤12) at 6 months. A higher proportion of these patients presented at stage III was compared with the probable and possible category although the difference was not statistically significant. Prognostic factors that have been shown to adversely affect the survival and disability include the magnitude of delay in initiating anti-tuberculosis treatment, occurrence of complications, HIV infection, and meningitis caused by multidrug-resistant Mycobacterium tuberculosis. A better anti-tuberculosis regimen with a higher drug penetration in CSF than the standard antituberculous regimen may be desirable in these patients. Higher dose of rifampicin has been shown to improve the outcome in patients with severe tuberculous meningitis. Six-month mortality was significantly less in patients who were given high-dose rifampicin intravenously (33 % higher dose) [15]. A short intensified treatment regimen consisting of a higher isoniazid and rifampin dosages, longer administration of pyrazinamide, and substitution of ethambutol with ethionamide has been shown to produce a higher concentration of anti-tuberculosis drugs in the CSF. This short intensified treatment was found to be safe and effective in children with drug-susceptible tuberculous meningitis [16]. These newer anti-tuberculosis regimens should be subjected to rigorous randomized controlled trials before they can be recommended for every-day practice. Benefit of adding a fluoroquinolone (levofloxacin or moxifloxacin) should also be investigated.
Low yield of mycobacterial culture, lack of a group comprising HIV co-infected patients, and deficient data regarding multidrug resistance were major limitations of our study. The diagnosis of probable or possible tuberculous meningitis is determined by a diagnostic scoring system and such cases may have other diagnoses. Although HIV infection does not alter the neurological features of tuberculous meningitis, it significantly reduces the survival rate [17]. Presence of HIV co-infected patients in our study would have shed more light on this aspect. Drug-resistant tuberculous meningitis is a serious and fast-increasing clinical problem which is associated with a high mortality [18].
In conclusion severe disability, cerebrospinal fluid cells >100 mm3, and basal exudates are significantly related to the presence of microbiologically confirmed definite tuberculous meningitis. Microbiologically confirmed tuberculous meningitis is associated with poorer outcome.
References
Graham SM, Donald PR. Death and disability: the outcomes of tuberculous meningitis. Lancet Infect Dis. 2014;14:902–4.
Chin JH. Tuberculous meningitis: diagnostic and therapeutic challenges. Neurol Clin Pract. 2014;4:199–205.
Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:947–57.
Chaidir L, Ganiem AR, Vander Zanden A, Muhsinin S, Kusumaningrum T, Kusumadewi I, et al. Comparison of real time IS6110-PCR, microscopy, and culture for diagnosis of tuberculous meningitis in a cohort of adult patients in Indonesia. PLoS ONE. 2012;7:e52001.
Nhu NT, Heemskerk D, Thu do DA, Chau TT, Mai NT, Nghia HD, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52:226–33.
Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803–12.
Bhanu NV, Singh UB, Chakraborty M, Suresh N, Arora J, Rana T, et al. Improved diagnostic value of PCR in the diagnosis of female genital tuberculosis leading to infertility. J Med Microbiol. 2005;54(Pt 10):927–31.
Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51.
World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. (WHO/HTM/TB/2009.420) World Health Organization, http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf (2010). Accessed 25 Sept 2014.
Thwaites GE, Chau TT, Stepniewska K, Phu NH, Chuong LV, Sinh DX, et al. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet. 2002;360:1287–92.
Thwaites GE, Chau TT, Farrar JJ. Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol. 2004;42:378–9.
Feng GD, Shi M, Ma L, Chen P, Wang BJ, Zhang M, et al. Diagnostic accuracy of intracellular Mycobacterium tuberculosis detection for tuberculous meningitis. Am J Respir Crit Care Med. 2014;189:475–81.
Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12:999–1010.
Botha H, Ackerman C, Candy S, Carr JA, Griffith-Richards S, Bateman KJ. Reliability and diagnostic performance of CT imaging criteria in the diagnosis of tuberculous meningitis. PLoS ONE. 2012;7:e38982.
Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, van der Ven AJ, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13:27–35.
Turkova A, Seddon JA, Nunn AJ, Gibb DM. Phillips PP. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J. 2014;33:993.
Thwaites GE, Duc Bang N, Huy Dung N, Thi Quy H, Thi Tuong Oanh D, Thi Cam Thoa N, et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192:2134–41.
Garg RK, Jain A, Malhotra HS, Agrawal A, Garg R. Drug-resistant tuberculous meningitis. Expert Rev Anti Infect Ther. 2013;11:605–21.
Conflict of interest
None for all the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jha, S.K., Garg, R.K., Jain, A. et al. Definite (microbiologically confirmed) tuberculous meningitis: predictors and prognostic impact. Infection 43, 639–645 (2015). https://doi.org/10.1007/s15010-015-0756-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-015-0756-z