Abstract
Purpose
Standardized prevalence and incidence data on carbapenem-resistant organisms (CRO) and, as a relevant subgroup, carbapenem-resistant Enterobacteriaceae (CRE) are scarce. CRO-surveillance within the German nosocomial infection surveillance system (KISS) aims to provide epidemiological surveillance data on CRO colonizations and infections.
Methods
CRO-surveillance is part of a KISS-module for the surveillance of multidrug-resistant organisms (MDRO). MDRO-KISS methods require surveillance of all patients admitted to the ward and standardized documentation of imported and ICU-acquired cases. Data on all MDRO-carriers including colonization and infection with MDRO are collected. All presented data were routine data collected from January 1st 2013 until December 1st 2013 in accordance with the German Protection against Infection Act (IfSG).
Results
341 ICUs submitted data on MDRO during the first year. In total, 5,171 cases of multidrug-resistant Gram-negative bacteria (MRGN) were identified. 848 were CRO (16 %). 325 CRO-cases were acquired within the ICU (38 %), and 373 CRO-patients had an infection (44 %). CRO-prevalence was 0.29 per 100 patients. Acquisition rate of MRGN was 1.32 per 1,000 patient days. This rate is more than doubled the acquisition rates of other MDRO under surveillance within MDRO-KISS (0.57 MRSA, 0.49 VRE). CRO-acquisition rate was 0.3 per 1,000 patient days. Incidence density of MRGN infections bacteria was 0.58 per 1,000 patient days (CRO 0.15/1,000 patient days).
Conclusions
To date, CRO are common in German ICUs and the relatively large proportions of ICU-acquired CRO and infections emphasize their potential to cause outbreaks. High MRGN infection rates and high ESBL prevalence data from clinical studies suggest a lack of MRGN identification in asymptomatic carriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the WHO’s global report on surveillance of antimicrobial resistance issued April 2014, the world is on the verge of entering a post-antibiotic era. However, the exact magnitude of the problem at both population and global levels is unclear and needs to be clarified. Current and reliable data are required to determine the scope of the problem, to systematically monitor trends and to inform and evaluate containment efforts [1].
In the last years, multidrug-resistant Gram-negative (MRGN) organisms have emerged as a major challenge in the care of hospitalized, and especially critically ill, patients [2]. Infections with carbapenem-resistant organisms (CRO) are particularly dangerous, as only few treatment options remain for patients and outcomes are generally poor [3, 4]. Participation in surveillance systems to measure and compare frequencies of bacterial resistance is essential to understand the extent of emerging and established multidrug-resistant organisms (MDRO) infections. However, most surveillance data available present proportions of resistant bacteria among tested isolates from routine testing at laboratories [1] which do not reflect the relative frequency of colonizations and infections with MDRO in the targeted patient population. True prevalence and incidence data are rare and require standardized methodology.
A voluntary method for the surveillance of healthcare-associated infections in Germany, the Krankenhaus-Infektionen-Surveillance-System (KISS), was established in 1997. In 2013, 1,403 hospitals in Germany participated in at least one KISS-module.
Several KISS-modules are designed to support MDRO-surveillance in different healthcare settings at the hospital and at the ward level. In response to the increase of MRGN (mainly ESBL-producers) in the general community and their growing diversity, the KISS-module for MDRO-surveillance in ICUs was revised. In contrast to former versions, the new MDRO-KISS allows for standardized surveillance of MRGN antimicrobial susceptibility data and production of beta-lactamases including carbapenemases.
Launched in January 2013, the primary objective of MDRO-KISS is to alert relevant stakeholders to the incidence trends of MDRO at a ward level.
The purpose of this report is to describe the prevalence of introduced and the incidence of ICU-acquired MRGN and CRO reported to the revised ICU-based module for MDRO-surveillance within KISS. Data on Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant Enterococcus (VRE) will be presented for comparison.
Methods
MDRO-KISS methods
To determine the prevalence of CROs and other MDROs we analysed data reported to MDRO-KISS from January 1st 2013 through February 15th 2014. The KISS database has been used for surveillance purposes for 17 years and its general methodology has been described previously [5]. Briefly, after completing KISS training, healthcare personnel collects and reports data on a monthly basis using standardized methods and definitions specific to the KISS-module selected. For the MDRO-module, at least 1 month of data per year must be submitted to maintain active status in KISS. Patients in the selected wards are monitored for colonization or infection with 1–3 types of MDRO: MRSA, VRE or MRGN. Microbiology data are provided by the hospitals’ clinical microbiology laboratory. Methodology for organism identification and antimicrobial susceptibility testing may vary between different laboratories, which are expected to adhere to ISO 20776-1 and to European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards, German antimicrobial susceptibility norms DIN 58940 [6] or to Clinical and Laboratory Standards Institute (CLSI) standards. MRGN are classified according to guidelines from the national committee for infection prevention (KRINKO) [7] situated at the German public health Institute (Robert Koch Institute, RKI). Enterobacteriaceae, Acinetobacter spp. and Pseudomonas spp. are classified according to their sensitivity into four classes of antimicrobial substances: acylureidopenicillins, 3rd and 4th generation cephalosporins, quinolones and carbapenems. Roughly, MRGN resistant to the first three antimicrobial classes are labelled 3MRGN, those with additional carbapenem resistance are labelled 4MRGN (CRO). Compared to the previous MDRO classification system based on the mechanism of resistance this is a new approach, as less resistant organisms are excluded from surveillance. If available, data on the production of beta-lactamases including Amp C, extended-spectrum beta-lactamases (ESBL) and carbapenemases (Klebsiella pneumoniae-carbapenemase, Metallo-beta-lactamases and OXA-48) are also collected. The annual participation in a questionnaire survey on screening policies and alert systems at the end of the year is compulsory.
Multidrug-resistant organisms carriage is considered to be ICU acquired if the microbiological sample is taken more than 72 h after admission to the ICU and the patient was not known to be colonized or infected with the MDRO previously. Infections are identified using standardized definitions that combine laboratory and clinical criteria and require antimicrobial treatment. Patient days from the selected wards are collected as denominator data.
Patient data are collected and stored anonymously and in accordance with national guidelines for data protection. All data collected by MDRO-KISS and included in this study were obtained during routine surveillance required by the German Protection against Infection Act (Infektionsschutzgesetz, IfSG) [8]. According to 23 of the IfSG, hospitals and clinics for ambulatory surgery are obliged to systematically collect and analyse data on hospital-acquired infections (HAIs) and antimicrobial-resistant pathogens. Ethical approval and informed consent are thus not required.
Statistical analysis
For participating ICUs and all reported MDRO, absolute frequencies and distributions were described by ICU and hospital types, sizes, and regions. Mean and median incidence densities of MDRO-cases per 1,000 patient days with the interquartile ranges (IQR) and the proportions of introduced and ICU-acquired cases were calculated from pooled data of all ICUs for all MDRO types. We calculated summary measures of select MDRO by organism type and ICU origin. Differences between ICUs were compared across MDRO types by means of the χ2 test for independence. Statistical significance was determined at a p value of 0.05.
Results
In total, 341 ICUs from 247 hospitals submitted data on MDRO during the first year. 330 ICUs submitted data for MRGN, 340 for MRSA and 319 for VRE. Basic characteristics for the 341 participating ICUs/hospitals are presented in Table 1. The majority of hospitals (n = 187, 76 %) participated with a single ICU, 18 % (n = 44) participated with 2, and 6 % (n = 16) with more than 2 ICUs. 51 ICUs (15 %) reported to have an established active surveillance screening for MRGN, in contrast to 121 (36 %) for MRSA and 26 (8 %) for VRE.
In total, 5,171 cases of multidrug-resistant Gram-negative bacteria (MRGN), 4,853 MRSA cases and 1,235 VRE cases were identified. Among the MRGN, 16 % were CRO (n = 848). 58 % of KISS ICUs (n = 199) identified cases of CRO infection or colonization and 25 % infection or colonization with carbapenem-resistant Enterobacteriaceae (CRE, n = 84).
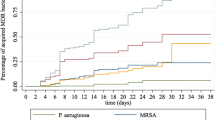
Total prevalence of MDRO is shown in Fig. 1, admission prevalence and acquisition rates are shown in Table 2. 28 % of the MRGN (n = 1457) and among these 38 % of the CRO-cases (n = 325) were acquired within the ICU.
37 % of the MRGN (n = 1929) and 44 % of the CRO-patients (n = 373) had an infection, see Fig. 2. Infections were pneumonia (n = 687, 36 %), urinary tract infections (n = 518, 27 %), surgical site infections (n = 266, 14 %), bacteremia (n = 168, 9 %), bronchitis (n = 121, 6 %), skin infections (n = 141, 7 %) and others (n = 161, 8 %). CRO were mainly Pseudomonas spp. (n = 516, 61 %), mostly Pseudomonas aeruginosa (n = 493, 58 %), followed by CRE (n = 212, 25 %) and Acinetobacter spp. (n = 120, 14 %) dominated by Acinetobacter baumannii (n = 115, 14 %). CRE were mainly Klebsiella pneumoniae (n = 108, 51 %), Escherichia coli (n = 27, 13 %) Enterobacter cloacae (n = 24, 11 %) and Enterobacter aerogenes (n = 19, 9 %), see Fig. 3.
In total, 81 carbapenemases were identified, 25 OXA-48 (15 K. pneumoniae, 6 A. baumannii, 2 E.coli, 1 K. oxytoca, 1 Pseudomonas spp., respectively), 36 KPC (24 K. pneumoniae, 5 P. aeruginosa, 2 A. baumannii, 2 K. oxytoca, 2 Pseudomoans spp., 1 E. cloacae) and 20 MBL (17 P. aeruginosa, 1 K. pneumoniae, 1 P. mirabilis, 1 A. baumannii).
ICUs from large hospitals with more than 400 beds reported almost twice the number of MRGN (ID 5.5 vs. 2.9, p < 0.001) and more than twice the number of CRO as ICUs from small hospitals (ID 0.94 vs. 0.41, p < 0.001). Incidence densities of MRGN and CRO in eastern (ID = 11.5; 2.0) and western (ID = 14.4; 1.9) Germany more than doubled that of northern (ID = 4.3; 0.8) and south-eastern (ID = 4.0; 0.6) regions (all p values < 0.001). The largest proportion of participating units were from south-western Germany with the lowest MRGN (ID = 0.85) and CRO (ID = 0.15) incidence densities (p < 0.001).
Discussion
Multidrug-resistant Gram-negative organisms cause almost three times as many HAIs in German ICUs as MRSA, and CRO alone have almost caught up with this number one HAI causing antimicrobial-resistant pathogen [2, 9]. This is particularly worrying as clinicians are increasingly forced to utilize carbapenems to treat patients with serious Gram-negative infections, which has laid ground for a greater prevalence of carbapenem-resistant Enterobacteriaceae species [10–12]. Although the total number of MRSA cases is similar, MRGN appear more likely to cause infections, which is at least partly caused by an underreporting of MRGN colonizations rather than a higher pathogenicity of MRGN. However, total numbers of MRGN infections reported by extensively trained infection control personnel with longstanding experience in the surveillance of nosocomial infections certainly qualify as credible data. Moreover, MDRO infections treated or requiring immediate antimicrobial treatment highlight the necessity of preventive infection control measures.
Admission prevalence of MRGN from clinical samples obtained at admission almost equals, and total MRGN prevalence surpasses MRSA. Active surveillance screening for MRGN at admission is still scarce and MRGN are often only identified by clinical sampling, so MRGN prevalence in ICUs may be considerably higher than KISS results suggest.
Current MRGN prevalence data are hard to find as the new German MRGN classification system was only implemented in 2013 [7]. Available ESBL data from high-risk settings indicate that colonization rates in ICUS range from 3 to 17 % [13–16] and up to 27 % in long-term care facilities [17]. In contrast to MRGN, these data include ESBL-producing isolates susceptible to quinolones. Since 2013 German microbiologic laboratories are not obliged to label ESBL-producing bacteria which do not classify as MRGN. Future comparisons of European and international ESBL-frequencies on the one hand and German MRGN on the other hand will be even more difficult.
Prevalence data for CRO are scarce. A point-prevalence-survey among 773 patients in 98 ICUs from October 2012 to February 2013 in Saxony, a federal state in the east of Germany, detected a CRO-prevalence of 1.2 % (n = 9) [16] by means of active surveillance screening, four times as many cases as reported from KISS-participants. Notably, the study was initiated by the Saxon State Ministry of Social Affairs and Consumer Protection following a large outbreak of infections with Klebsiella pneumoniae producing KPC-2 at one hospital in Saxony [18]. However, only 2 of the identified CRO were genetically related to the outbreak.
As adherence to specific susceptibility breakpoints is not compulsory in Germany, classification of MRGN according to resistance phenotype may vary depending on the susceptibility breakpoints applied [19]. There are important differences between susceptibility breakpoints proposed by the German DIN and EUCAST on the one hand and CLSI on the other hand [20] so that data may not be comparable. Data from the national network for the surveillance of antimicrobial resistance at the RKI (ARS) suggest that the majority (86 %) of 21 participating laboratories have changed methodology in the past three years from CLSI to EUCAST [21].
Another issue is the phenotypic susceptibility of bacteria that contain antimicrobial resistance determinants, e.g. a KPC or MBL gene. The true incidence of resistance may be underestimated by surveillance systems that report only resistant isolates [19]. German microbiological laboratories are required to report carbepenem-susceptible pathogens that harbour carbapenemases as 4MRGN (CRO). However, only few KISS-participants reported identification of carbapenemases, as probably many of the designated microbiological laboratories have not yet established routine specific testing for carbapenemases. The German national reference centre for MRGN reported 3,156 CRO in 2013 from ICUs and Non-ICUs including 1,237 carbapenemase-producers [22] which indicates an underreporting of carbapenemases to the KISS-system. Thus, the proportion of carbapenemase-producing organisms (CPO) among CRO in Germany remains unknown.
Large university hospitals reported significantly more MDRO-cases than smaller hospital types, which is not surprising as these hospitals often treat patients at a higher risk for MDRO carriage. Among 35 large university hospitals in Germany, 20 participated in MDRO-KISS, so this group is clearly overrepresented. MDRO incidence in ICUs from eastern and western Germany is more than doubled that of northern and southern regions, corroborating prior KISS data [23].
KISS is designed to allow for nationwide surveillance of hospitals, but as a voluntary system it does not cover all German ICUs. ICU-beds from MDRO-KISS-participants in 2013 represent 17 % (n = 4,423) of all 26.162 ICU-beds in Germany [24]. Extrapolation of MDRO-KISS-data suggests approximately 30,000 MRGN and 5,000 CRO-cases in all German ICUs in 2013, which is a low estimate considering the lack of active surveillance screening for MRGN. Extrapolated MDRO-KISS infection data would imply 1,453 MRGN infections and 305 CRO infections in German ICUs.
Limitations
We acknowledge that data retrieved from voluntary KISS-participants may bear the risk of selection bias as ICUs may engage in KISS for a reason, e.g., prolonged outbreaks or high MDRO rates. Non-representativeness and biased sampling are important pitfalls for the interpretation and comparison of results [1]. However, an analysis of representativeness of surveillance data within ICU-KISS in 2010 found infection rates of ICU-KISS to be representative for German ICUs [25]. Moreover, MDRO data collected on a voluntary basis and published in aggregate form only guarantee certain credibility, as hospitals do not have to fear damage for their reputation. KISS comprises the largest group of German ICUs contributing a broad range of data to a standardized surveillance system of MDRO.
Conclusions
High MRGN infection rates and high ESBL prevalence data from clinical studies suggest a lack of MRGN identification in asymptomatic carriers. In light of increasing numbers of carbapenem-resistant or even pan-resistant organisms, credible data on MRGN colonization rates are urgently needed. Current data indicate a massive underreporting of CRO, which may only be overcome by legislative means, for example, mandatory admission screening. The data presented here provide a benchmark for the comparison of German MRGN prevalence data and to analyse the impact of infection control policies.
References
Antimicrobial resistance: global report on surveillance 2014. World Health Organization; 2014
Maragakis LL. Recognition and prevention of multidrug-resistant Gram-negative bacteria in the intensive care unit. Crit Care Med. 2010;38:S345–51. doi:10.1097/CCM.0b013e3181e6cbc5.
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho, F et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38:1930–45. doi:10.1007/s00134-012-2695-9.
Lubbert C, Becker-Rux D, Rodloff AC, Laudi S, Busch T, Bartels M, et al. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis. Infection. 2014;42:309–16. doi:10.1007/s15010-013-0547-3.
Gastmeier P, Sohr D, Schwab F, Behnke M, Zuschneid I, Brandt C, et al. Ten years of KISS: the most important requirements for success. J Hosp Infect. 2008;70:11–6. doi:10.1016/s0195-6701(08)60005-5.
DIN 58940-4, 4 (2004).
KRINKO. Kommission für Krankenhaushygiene und Infektionsprävention: hygienemaßnahmen bei Infektionen oder Besiedlung mit multiresistenten gramnegativen Stäbchen. Bundesgesundheitsbl. 2012;55:1311–54.
Infektionsschutzgesetz vom 20. Juli 2000 (BGBl. I S. 1045), das durch Artikel 5 Absatz 2 des Gesetzes vom 20. April 2013 (BGBl. I S. 868) geändert worden ist, Artikel 5 Absatz 2 (2000).
Meyer E, Schroder C, Gastmeier P, Geffers C. The reduction of nosocomial MRSA infection in Germany: an analysis of data from the Hospital Infection Surveillance System (KISS) between 2007 and 2012. Deutsches Arzteblatt int. 2014;111:331–6. doi:10.3238/arztebl.2014.0331.
Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–36. doi:10.1016/s1473-3099(09)70054-4.
Marquez P, Terashita D, Dassey D, Mascola L. Population-based incidence of carbapenem-resistant Klebsiella pneumoniae along the continuum of care, Los Angeles County. Infect Control Hosp Epidemiol. 2013;34:144–50. doi:10.1086/669087.
Meyer E, Gastmeier P, Deja M, Schwab F. Antibiotic consumption and resistance: data from Europe and Germany. Int J Med Microbiol. 2013;303:388–95. doi:10.1016/j.ijmm.2013.04.004.
Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, Vehreschild MJ. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients—review of the literature from a clinical perspective. Crit Rev Microbiol. 2014;. doi:10.3109/1040841X.2013.875515.
Liss B, Vehreschild J, Cornely O, Hallek M, Fätkenheuer G, Wisplinghoff H, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40:613–9.
Reddy P, Malczynski M, Obias A, Reiner S, Jin N, Huang J, et al. Screening for extended-spectrum β-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin Infect Dis. 2007;45:846–52.
Ehrhard I, Karaalp AK, Hackel T, Holl G, Rodewald N, Reif U, et al. Prevalence of carbapenemase-producing bacteria in hospitals in Saxony, Germany. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2014;57:406–13. doi:10.1007/s00103-013-1914-z.
Heudorf U, Gustav C, Mischler D, Schulze J. Healthcare associated infections (HAI), antibiotic use and prevalence of multidrug-resistant bacteria (MDRO) in residents of long-term care facilities: the Frankfurt HALT plus MDRO project 2012. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2014;57:414–22. doi:10.1007/s00103-013-1927-7.
Lippmann N, Lubbert C, Kaiser T, Kaisers UX, Rodloff AC. Clinical epidemiology of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2014;14:271–2. doi:10.1016/S1473-3099(14)70705-4.
Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro surveillance. 2008;13:19045.
Hombach M, Mouttet B, Bloemberg GV. Consequences of revised CLSI and EUCAST guidelines for antibiotic susceptibility patterns of ESBL- and AmpC beta-lactamase-producing clinical Enterobacteriaceae isolates. J Antimicrobiol Chemother. 2013;68:2092–8. doi:10.1093/jac/dkt136.
ARS. RKI: Antibiotika Resistenz Surveillance. 2014; https://ars.rki.de/Dateneinfuehrung.aspx Accessed 31.07.2014.
Kaase M, Anders A, Pfennigwerth N, Gatermann S. Report of the national reference laboratory for multidrug-resistant gramnegative bacteria on carbapenemases in Germany in 2013. ECCMID; Barcelona 2014.
Maechler F, Schwab F, Geffers C, Gropmann A, Gastmeier P. Infection control policies and ICU-acquired ESBL-cases in Germany: a cross-sectional questionnaire survey of 224 ICUs. ECCMID; Barcelona 2014.
Krankenhausstatistik-Grunddaten [database on the Internet]. Statistisches Bundesamt, Zweigstelle Bonn. 2014; http://www.gbe-bund.de/. Accessed: 27.06.2014.
Zuschneid I, Rucker G, Schoop R, Beyersmann J, Schumacher M, Geffers C, et al. Representativeness of the surveillance data in the intensive care unit component of the German nosocomial infections surveillance system. Infect Control Hosp Epidemiol. 2010;31:934–8. doi:10.1086/655462.
Acknowledgments
We thank all nurses and physicians in the participating hospitals who provided their MDRO data to KISS. Data have been generated as part of the routine work of the nosocomial infection surveillance system (NRZ KISS).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maechler, F., Peña Diaz, L.A., Schröder, C. et al. Prevalence of carbapenem-resistant organisms and other Gram-negative MDRO in German ICUs: first results from the national nosocomial infection surveillance system (KISS). Infection 43, 163–168 (2015). https://doi.org/10.1007/s15010-014-0701-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-014-0701-6