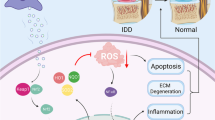
Abstract
Background:
Accumulating evidence supports the potential of exosomes as a promising therapeutic approach for intervertebral disc degeneration (IDD). Nevertheless, enhancing the efficiency of exosome treatment remains an urgent concern. This study investigated the impact of quercetin on the characteristics of mesenchymal stem cells (MSCs) and their released exosomes.
Methods:
Exosomes were obtained from quercetin pre-treated MSCs and quantified for the production based on nanoparticle tracking and western blot analysis. The molecules involved in the secretion and cargo sorting of exosomes were investigated using western blot and immunofluorescence analysis. Based on the in vitro biological analysis and in vivo histological analysis, the effects of exosomes derived from conventional or quercetin-treated MSCs on nucleus pulposus (NP) cells were compared.
Results:
A significant enhancement in the production and transportation efficiency of exosomes was observed in quercetin-treated MSCs. Moreover, the exosomes derived from quercetin-treated MSCs exhibited a greater abundance of antioxidant proteins, specifically superoxide dismutase 1 (SOD1), which inhibit the activation of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome in NP cells. Through in vitro and in vivo experiments, it was elucidated that exosomes derived from quercetin-treated MSCs possessed enhanced anti-inflammatory and antioxidant properties.
Conclusion:
Collectively, our research underscores an optimized therapeutic strategy for IDD utilizing MSC-derived exosomes, thereby augmenting the efficacy of exosomes in intervertebral disc regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The present treatment methods for intervertebral disc degeneration (IDD) typically aim at symptom relief but may not result in biological repair to the intervertebral discs [1]. Biological therapies that target IDD and repair of damaged cells have garnered long-term therapeutic effects [2]. Mesenchymal stem cells (MSCs) have been considered a potential biological strategy for IDD repair, owing to their proliferative and anti-apoptotic biological functions [3]. Extracellular vesicles (EVs) secreted by MSCs are known to mediate the biological effects of MSCs [4]. In comparison to cell-based therapies using MSCs, EVs offers potential advantages such as lower manufacturing costs, increased stability, and greater convenience in terms of sterilization, storage, and administration [5]. At present, EVs are widely recognized as a promising alternative to MSCs during intervertebral disc regeneration.
Exosomes, a type of EVs with a diameter ranging from 50 – 120 nm, play a crucial role in treating degenerative diseases, including processes such as musculoskeletal disorders, neurodegenerative, visual and auditory impairment [6,7,8,9]. Furthermore, the content of exosomes can serve as potential early diagnostic biomarkers and can be utilized to monitor diseases progression [10]. Notably, exosomes derived from MSCs have been found to carry various biological substances, such as proteins, RNAs, and lipids, to recipient cells, ultimately delivering therapeutic effects [8, 10]. Consequently, exosomes have emerged as promising targets for therapeutic purposes in the field of stem cell-based therapy [11]. Given that EVs secretion is a complex and multi-step process, which involves the transport, docking, and fusion of multivesicular bodies (MVBs) with the plasma membrane, the exact mechanisms governing the secretion of exosomes in MSCs still need to be elucidated.
A multitude of studies have demonstrated that the paracrine effects produced by aged cells undergo changes [12]. The senescence-associated secretory phenotype (SASP) is regarded as a significant indicator of cellular ageing. For instance, alterations in the protein or nucleic acid components of exosomes secreted by aged MSCs may exert a detrimental influence on target cells [13]. Reducing the senescent state of stem cells or rejuvenating them is beneficial for maintaining the therapeutic efficacy. One alternative approach is the use of senolytics. Quercetin is a kind of senolytics that is widely present in nature and is known to possess antioxidant properties [14]. To date, numerous studies have substantiated the anti-ageing effects of quercetin, which are thought to be mediated by alterations in intracellular signaling pathways [15]. Nevertheless, there is a paucity of research investigating the impact of quercetin treatment on the secretion of MSC-derived exosomes.
Inflammation has been established to have a significant role in the pathogenesis of IDD [16]. The progression of IDD involves a sustained activation of inflammasome, including NOD-like receptor thermal protein domain associated protein 3 (NLRP3), Absent In Melanoma 2 (AIM2) inflammasome and so on, resulting in the secretion of IL-1β and potentially cell damage [17]. Activation of caspase-1 cleaves gasdermin D (GSDMD), which mediates pyroptosis, a harmful process implicated in IDD [18]. Accumulating evidence supports the therapeutic potential of MSC-derived EVs in IDD [19, 20]. These EVs can effectively prevent excessive apoptosis or pyroptosis of nucleus pulposus (NP) cells and promote cell proliferation, likely through the activation of pro-survival signaling pathways. Multiple studies have verified the anti-inflammatory properties of MSC-derived EVs, which can mitigate inflammation and stimulate healthier extracellular matrix production in NP cells [20, 21]. The potential for EVs to regulate the inflammation provides a compelling rationale for the development of EV-based IDD treatment measures. Although numerous studies have demonstrated the efficacy of EVs in IDD, there is still considerable scope for improvement [19]. This may includes enhancing the level of powerful contents in EVs, promotion of the EVs release, and regulating processes involved in endocytosis or digestion.
In this study, our aim was to examine the impact of exosomes secreted by MSCs (MSC-exos) in distinct functional states. The aging of MSCs in vitro may alter the characteristics of their exosomes. We discovered that MSCs treated with quercetin exhibited rejuvenation, which affected the quantity and quality of their secreted exosomes. Exosomes derived from quercetin-treated MSCs had superior efficacy in reducing the incidence of pyroptosis in NP cells. Further research has revealed that the generation and transport efficiency of exosomes in stem cells undergo changes after quercetin treatment. Moreover, antioxidant proteins are selectively loaded into the exosomes, enhancing their antioxidant capacity. Exosomes obtained from MSCs treated with quercetin have been demonstrated to relieve the progression of IDD both in vitro and in vivo. The findings of our study could offer novel insights for the employment of MSC-derived exosomes in IDD therapy.
2 Methods and materials
2.1 Reagents and antibodies
Quercetin, Hydrogen peroxide (H2O2), TNF-α, compound 59 and Nigericin were purchased from MCE Company (Shanghai, China). Primary antibodies against P53, P21, P16, Alix, CD81, CD63, CD9, NLRP1, NLRC4, AIM2, ASC, and Calnexin were purchased from Proteintech (Wuhan, China). Primary antibodies against Caspase-1, GSDMD-N, IL-1β, NLRP3, RAB27A, SOD1, SOD2, PRDX1, PRDX2, and β-actin were purchased from Abcam (Cambridge, MA, USA).
2.2 Cell culture
Human bone marrow MSCs were obtained from ATCC (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) containing 15% fetal bovine serum (FBS, Thermo Fisher, Waltham, MA, USA). Human NP tissues were obtained from patients undergoing lumbar fusion surgery. The degenerative levels of discs were evaluated by MRI Pfirrmann grades [22]. Samples with Pfirrmann Grade I or II were used in the experiments. NP tissues were cut into small pieces and digested by collagenase II for 2 h. NP cells in sediments were collected after centrifugation and then cultured in DMEM/F-12 with 15% FBS. The culture medium was changed every two days. Cells at passage 3 were usually used in the following experiments.
2.3 Exosomes isolation
Exosomes were isolated from the MSCs culture supernatant using ultracentrifugation method. Briefly, the medium was successively centrifuged at 500 g (10 min), 2500 g (30 min), and then 12000 g (1 h). The collected supernatant was then centrifuged at 100,000 g (90 min, Beckman Type 70 Ti, Beckman Coulter, Inc. Brea, CA, USA) twice. The final pellet was exosomes and resuspended by 100 μL PBS. For exosomes identification, the size and morphology of exosomes were observed by transmission electron microscopy (TEM). The diameter distribution and concentration of exosomes were assessed by nanoparticle tracking analysis (NTA) using NanoSight NS300 system (Malvern, UK). The protein levels of exosomes were measured using bicinchoninic acid (BCA) kit (Beyotime, Shanghai, China) according to the manufactural instructions.
2.4 Cytotoxicity assay
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan). After seeded in a 96-well plate, cells were treated with interests for certain time periods. Then, 10 % CCK8 solution was added into each well and incubated for 4 h at 37 °C in the darkness. The absorbance at a wavelength of 450 nm was measured using a spectrophotometer (BioTek, Winooski, VT, USA).
2.5 Western blot
Protein fraction was isolated from cells or exosomes using the RIPA buffer with 1% protease inhibitor cocktail (Beyotime). The protein was then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene difluoride (PVDF) bands. After washing with fast blocking buffer for 20 min, the bands were incubated with the primary antibodies overnight at 4 °C. After washing with buffer three times, the bands were then incubated with the secondary antibodies for 1h. After washing with buffer three times, the bands were prepared for exposure. The bands were incubated with chemiluminescence reagent and then captured via Bio-Rad system (Bio-Rad, Hercules, CA, USA).
2.6 β-galactosidase staining
The senescence-associated β-galactosidase was measured using Senescence β-Galactosidase Staining Kit (Beyotime). Cells were cultured in a 6-well plate and then treated with interests for certain time periods. After fixed by 2.5% paraformaldehyde, the cells were washed with PBS twice. The samples were incubated with β-galactosidase staining solution overnight at 37 °C. The images were captured under a microscope. The β-galactosidase-positive cells was counted at three random views by Image J software (National Institutes of Health, Bethesda, MD, USA).
2.7 EdU staining
Cells were cultured in a 6-well plate and then treated with interests for certain time periods. The EdU (10 μM) were incubated with cells for 2 h. The samples were then disposed using EdU Cell Proliferation Kit (Beyotime) according to the manufactural instructions. The images were captured under a fluorescence microscope (Olympus, Center Valley, PA, USA). The EdU-positive nucleus was counted at three random views by Image J software.
2.8 Quantitative real-time PCR (qRT-PCR)
RNA fraction was isolated from cells or exosomes using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and chloroform. Reverse transcription was performed using the cDNA Reverse Transcription Kit (Thermo Fisher) according to the manufactural instructions. The PCR reaction was then conducted with qRT-PCR using the SYBR Green qPCR kit (Thermo Fisher) according to the manufactural instructions.
2.9 Immunoprecipitation
Protein fraction was isolated using the NP-40 buffer with 1% protease inhibitor cocktail (Beyotime). The protein sample was incubated with a primary antibody overnight at 4 °C. After washing with buffer three times, the sample was incubated with the protein A/G Magnetic Beads (Beyotime) and rotating for 1 h at room temperature. After washing with buffer three times and isolated by magnetic force, the isolated proteins were then conducted with western blot assays.
2.9.1 Live and dead staining
Live and dead staining was performed using Calcein/PI Cell Viability Kit (Beyotime). Cells were cultured in a 6-well plate and then treated with interests for certain time periods. Calcein AM and PI solution was added to the plate, and incubated for 30 min at 37 °C in the darkness. The images were captured under a fluorescence microscope (Olympus). The red-staining cells was counted at three random views by Image J software.
2.9.2 RNA knockdown
Cells were seeded in 6-well plate with 50–60% confluency. Cells then were transfected with small interfering RNA (siRNA, 100 nm) with Lipofectamine 2000 siRNA transfection reagent (Beyotime). All the siRNAs were purchased by Sangon Biotech (Shanghai, China). The transfection efficiency was evaluated by qRT-PCR at 24 h after transfection.
2.9.3 Immunofluorescence
Cells were cultured in a 6-well plate with slides and then treated with interests for certain time periods. After washed with PBS twice, the slides were fixed by 2.5% paraformaldehyde fixation, and permeated by 1% Triton X-100. The samples were incubated with the primary antibody overnight at 4 °C, and following incubated with fluorescence conjugated second antibodies for 1 h in the darkness. The images were captured under a fluorescence microscope (Olympus) and the mean fluorescent intensity of images at three random views was calculated by Image J software.
2.9.4 In vivo experiments
A total of 60 Sprague-Dawley rats (3 months old) were randomly allocated to six groups with 10 rats per group. The rats were anaesthetized with pentobarbital (2% w/v, 40 mg/kg). The Co7/8 of the coccygeal vertebrae was used as the experimental level. The discs were injected into different drugs (TNF-α, compound 59 or Nigericin) or different types of exosomes (100 μg/ml) via a 32-gauge needle. The sham group was punctured but without injection. All animals were permitted unrestricted freedom of movement and were raised for 8 weeks. The animals were euthanized, and the discs were gathered thereafter. These discs were fixed, decalcified, dehydrated, and embedded in paraffin. The samples were further dissected into 4–μm slices. Subsequently, these sections were stained with hematoxylin-eosin, Alcian blue, Safranin O-fast green, or Masson. The extent of degeneration of the discs was evaluated based on a grading scale [23].
2.9.5 Statistical analysis
For each experiment, at least three replicates were performed and data were presented as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 9 software (La Jolla, CA, USA). Differences between groups were compared using were Student’s t-test or One-way analysis of variance (ANOVA), and p values < 0.05 was considered statistically significant.
3 Results
3.1 Quercetin improves the aging state of MSCs
Multiple studies have shown that Quercetin can alleviate cellular senescence. However, it is unclear whether Quercetin has an effect on the paracrine effects of MSCs. Therefore, we initially assessed the cell viability of MSCs following different doses or duration of quercetin treatment (Fig. 1A). The optimal dose and duration of quercetin (20 μM, 12 h) were applied in subsequent experiments. To induce senescence in MSCs, H2O2 was employed to simulate cellular stress condition. The MSCs at first passage (P1) were used as the young control group. Based on the results of the CCK-8 assay (Fig. 1B), we selected the final concentration of H2O2 (50 μM, 12 h). It was demonstrated that quercetin decreased the levels of aging markers P53, P21, and P16 (Fig. 1C). In subsequent experiments, it was demonstrated that treatment with quercetin increased the cell proliferative rate, as determined by EdU staining (Fig. 1D). Furthermore, the number of senescent MSCs was found to decrease following treatment with quercetin, as determined by β-galactosidase staining. These findings suggest that quercetin ameliorates the senescence of MSCs.
Quercetin ameliorates the senescence of MSCs in vitro. A CCK8 assay of MSCs viability after treating with quercetin (20 μM) under different time periods, or with a dose gradient for 12 h treatment. B CCK8 assay of MSCs viability after treating with H2O2 (50 μM) under different time periods, or with a dose gradient for 12 h treatment. C MSCs were treated with PBS, quercetin, or H2O2. MSCs at P1 passage (P1) was used a positive control. Western blot images of P53, P21 and P16, and the quantification of relative protein levels. D Representative EdU staining images and the quantification of EdU-positive (green) rate. E Representative β-galactosidase staining images and the quantification of β-galactosidase-positive rate. Data were presented as the mean ± SD (n ≥ 3). *p < 0.05, **p < 0.01, ***p < 0.001, and “ns” for not significant
3.2 Quercetin boosts the secretion of MSC-exos and alters their effects on NP cells
We then detected the secretion of exosomes in quercetin-treated MSCs. Quercetin was found to increase the concentration and number of exosomes released by MSCs (Fig. 2A). No significant changes in the morphology of exosomes derived from quercetin-treated MSCs (Quer-exos) were observed compared to those derived from normal MSCs (Con-exos), as shown in Fig. 2B. Quer-exos exhibited greater protein content (Fig. 2C). There were no notable differences in expression levels of exosomal markers Alix, CD81, and CD63 between Quer-exos and Con-exos (Fig. 2D). To assess the impact of MSC-exos, equivalent Quer-exos and Con-exos (50 μg/ml) were incubated with NP cells. Results indicated that both Quer-exos and Con-exos decreased the expression levels of pyroptosis-related proteins Caspase-1, GSDMD-N, and IL-1β (Fig. 2E, F). Additionally, Quer-exos and Con-exos reduced the death rate of TNF-α-treated NP cells (Fig. 2G, H). Based on these results, in resisting cell death related to pyroptosis, Quer-exos show a more significant therapeutic effect than Con-exos.
Quercetin promotes the secretion of exosomes and affects their effects on NP cells. A BCA assay of total protein levels in exosomes, and NTA analysis of particles number produced by each cell as calculated. The exosomes were isolated from equal amout MSCs treated with or without quercetin (Con-exos and Quer-exos). B Representative transmission electron microscope images of Con-exos and Quer-exos. C Coomassie blue and western blot images of equal Con-exos and Quer-exos. D Western blot images of Alix, CD81, and CD63 of equal Con-exos and Quer-exos. Calnexin was used as a negative control. E NP cells were treated with TNF-α and then treated with PBS, Con-exos or Quer-exos. Control NP cells were treated without TNF-α. Western blot images of Caspase-1, GSDMD-N, and IL-1β. F The quantification of relative protein levels. G Representative Calcein and PI staining images. H The quantification of PI-positive rate. Data were presented as the mean ± SD (n ≥ 3). *p < 0.05, **p < 0.01, and ***p < 0.001
3.3 Quercetin manages the transport and secretion of exosomes via a RAB27A-related mechanism
We assessed the expression levels of genes associated with exosome biogenesis and transport (Fig. 3A), and discovered a significant up-regulation of RAB27A in quercetin-treated MSCs. Our results additionally revealed an up-regulation in RAB27A and exosomal markers CD9 and CD63 in quercetin-treated MSCs (Fig. 3B, C). Furthermore, immunoprecipitation demonstrated the integration between RAB27A and CD63 (Fig. 3D). The co-localization between RAB27A and CD63 was observed via immunofluorescence staining (Fig. 3E), with an increased co-localization ratio following quercetin treatment (Fig. 3F). Small RNA was subsequently utilized to knockdown RAB27A expression (Fig. 3G). After the siRNA was used, the protein levels and particle number of exosomes were assessed by BCA assay and NTA analysis, which reveals a significant decrease in both the concentration and number of exosomes (Fig. 3H, I). These findings illustrate that RAB27A is responsible for the transportation and secretion of exosomes under the influence of quercetin.
Quercetin regulates the RAB27A-mediated transport and secretion of exosomes in MSCs. A Relative mRNA levels of RAB7, RAB27A, RAB27B, SNAP23, SNAP25, VAMP7, and VAMP8 in MSCs treated with or without quercetin. B Western blot images of CD9, CD63, and RAB27A in MSCs treated with or without quercetin. C The quantification of relative protein levels. D Immunoprecipitation of CD63 and RAB27A in MSCs treated with or without quercetin. IgG was used as a control. E Representative images of RAB27A and CD63 fluorescent staining in MSCs treated with or without quercetin. F The quantification of colocalization ratio. G MSCs were treated with quercetin and then treated with si-RAB27A or scrablemed siRNAs (si-scr). Western blot images of RAB27A and the quantification of relative protein levels. H BCA assay of total protein levels in exosomes. I NTA analysis of particles number produced by each cell as calculated. Data were presented as the mean ± SD (n ≥ 3). *p < 0.05, **p < 0.01, ***p < 0.001, and “ns” for not significant
3.4 Selective loading of antioxidant proteins in MSC-exos upon the treatment of quercetin
We then detected the content of antioxidant proteins in exosomes. Some common antioxidant proteins, namely SOD1, SOD2, PRDX1, and PRDX2, were identified in MSC-exos (Fig. 4A). Our results revealed that the levels of certain antioxidant proteins, particularly SOD1 (Fig. 4B), were elevated in Quer-exos, probably contributing to the enhanced anti-inflammatory properties of Quer-exos in NP cells. Moreover, the integration between RAB27A and SOD1 was shown by the immunoprecipitation results (Fig. 4C), which may indicate the SOD1 loading during the biogenesis of exosomes. The co-localization between SOD1 and RAB27A was visualized through immunofluorescence staining (Fig. 4D), observing a notably amplified co-localization ratio subsequent to the quercetin treatment (Fig. 4E). These outcomes indicate that RAB27A binds with SOD1 and regulates the loading of SOD1 into exosomes.
Quercetin treatment results in the selective loading of SOD1 in exosomes. A Western blot images of SOD1, SOD2, PRDX1 and PRDX2 in exosomes. The exosomes were isolated from MSCs treated with or without quercetin (Con-exos and Quer-exos). B The quantification of relative protein levels. C Immunoprecipitation of RAB27A and SOD1 in MSCs treated with or without quercetin. IgG was used as a control. D Representative images of SOD1 and RAB27A fluorescent staining in MSCs treated with or without quercetin. E The quantification of colocalization ratio. Data were presented as the mean ± SD (n ≥ 3). **p < 0.01, ***p < 0.001, and “ns” for not significant
4 MSC-exos primarily affect pyroptosis of NP cells by regulating the NLRP3 inflammation-mediated pathway
We identified the activation of inflammasome in NP cells treated with TNF-α. It was shown that TNF-α led to the activation of NLRP3 inflammasome (Fig. 5A). Both Quer-exos and Con-exos significantly reduced the levels of NLRP3 expression (Fig. 5B). We employed Compound 59 (C59), an NLRP3 inhibitor, and Nigericin (Ni), an specific NLRP3 activator, to investigate the role of NLRP3 inflammasome in the pyroptosis of NP cells. Co-treatment with C59 resulted in an enhanced effect of Quer-exos and Con-exos on the pyroptosis of NP cells, decreasing the levels of GSDMD-N, Caspase-1 and IL-1β more significantly (Fig. 5C, D), and reducing the death rate of NP cells (Fig. 5E, F). However, co-treatment with Nigericin abrogated the effects of Quer-exos and Con-exos on the pyroptosis of NP cells, leading to an increase in the levels of GSDMD-N, Caspase-1, and IL-1β (Fig. 6A, B), and an elevation of the death rate of NP cells (Fig. 6C, D). These findings indicate that Quer-exos and Con-exos impact the pyroptosis of NP cells by modulating the NLRP3 inflammasome activation.
MSC-exos reduce the activation of NLRP3 inflammasome and ameliorate the pyroptosis of NP cells. A NP cells were treated with TNF-α and then treated with PBS, Con-exos or Quer-exos. Western blot images of NLRP3, NLRP2, NLRC4, AIM2 and ASC. B The quantification of relative protein levels. C NP cells were treated with TNF-α and then treated with PBS, Con-exos or Quer-exos. The Con-exos or Quer-exos treated NP cells were co-treated with Compound 59 (C59). Western blot images of NLRP3, Caspase-1, GSDMD-N and IL-1β. D The quantification of relative protein levels. E Representative Calcein and PI staining images. F The quantification of PI-positive rate. Data were presented as the mean ± SD (n ≥ 3). *p < 0.05, **p < 0.01, ***p < 0.001, and “ns” for not significant
Activation of NLRP3 inflammasome weakens the therapeutic effects of MSC-exos on NP cells. NP cells were treated with TNF-α and then treated with PBS, Con-exos or Quer-exos. The Con-exos or Quer-exos treated NP cells were co-treated with Nigericin (Ni). A Western blot images of NLRP3, Caspase-1, GSDMD-N and IL-1β. B The quantification of relative protein levels of NLRP3, Caspase-1, GSDMD-N, and IL-1β. C Representative Calcein and PI staining images. D The quantification of PI-positive rate. Data were presented as the mean ± SD (n ≥ 3). *p < 0.05, **p < 0.01, and ***p < 0.001
4.1 Exosomes derived from rejuvenated MSCs can effectively alleviate the progression of IDD
We compared the effects of Quer-exos and Con-exos in vivo based on a rat disc degeneration model. Histological staining of the rat disc was used to assess the degree of IDD, as represented in Fig. 7A. Additionally, distribution and sparsity of cells were revealed through Hematoxylin-eosin staining (Fig. 7B), and Alcian blue staining was used to visualize extracellular matrix deposition (Fig. 7C). Safranin O-fast green staining revealed proteoglycan contents (Fig. 8A), while Masson staining identified different types of collagenous fibers (Fig. 8B). Our findings indicated that C59 hinders the progression of IDD, whereas Nigericin promotes IDD (Fig. 7A), which highlights the role of NLRP3 inflammasome on IDD in vivo. Collectively, our results illustrate that both Quer-exos and Con-exos exhibited a positive effect on IDD progression, while Quer-exos demonstrating a superior therapeutic outcome which may serve as the possible optimization for IDD treatment.
Therapeutic effects of exosomes on disc degeneration in vivo. The discs were injected with TNF-α, TNF-α with Con-exos, TNF-α with Quer-exos, Nigericin (Ni), or TNF-α with Compound 59 (C59). The sham group was punctured but without injection. A Histological grades of different groups. B Representative images of Hematoxylin-eosin staining. C Representative images of Alcian blue staining. Data were presented as the mean ± SD (n = 10). **p < 0.01, ***p < 0.001, and “ns” for not significant
5 Discussion
In this study, it was discovered that MSCs treated with quercetin displayed reduced susceptibility to senescence. Additionally, alterations were observed in the exosomes secreted by the cells. RAB27A expression was found to be enhanced in the MSCs, leading to greater exosome production. Furthermore, SOD1 was selectively loaded into the exosomes. This led to exosomes from quercetin-treated MSCs having increased levels of antioxidant proteins, which better suppressed TNF-induced inflammasome activation in NP cells, thus reducing cell pyroptosis. In vivo experiments have demonstrated that the activation of inflammasome hastens the progression of IDD. However, exosomes obtained from quercetin-treated MSCs have shown to be beneficial in mitigating the development of IDD. Further, these exosomes are notably more effective than traditional MSCs in terms of therapeutic impact (Fig. 9).
The diagram depicts the concept of this research. Quercetin stimulates the rejuvenation of MSCs and triggers the formation of vesicles mediated by Rab27a. SOD1 is specifically loaded into MVBs, which are then released as exosomes. These exosomes efficiently suppress the activation of the NLRP3 inflammasome in NP cells, thus safeguarding them against pyroptosis and inflammation during intervertebral disc degeneration. MVB, multivesicular bodies; MSC, Mesenchymal stem cell; NP, nucleus pulposus
As a promising therapy that has previously been used to treat many degenerative diseases, mesenchymal stem cells have the capacity to develop into a wide variety of cell types [24]. MSCs have the ability to self-renew and repair tissues, with a wide range of prospects in the field of regenerative medicine [25]. There have been clinical studies that have explored the effectiveness of MSCs in the treatment of IDD. Takashi et al. found that intradiscal transplantation of MSCs into degenerated discs prevents IDD by promoting the ECM synthesis of NP cells [26]. In another study, the combination of photo polymerizable biogel scaffold with MSCs into NP region has also produced satisfactory results, with increased cellularity and less severe disc degeneration [27]. However, there are some challenges and uncertainties in the current treatment of disc degeneration with MSCs [28]. First, the cell source and purity, as well as the functionality of MSCs may affect the therapeutic efficacy. Second, the optimal MSCs transplantation modality has not yet been determined, including whether auxiliary materials such as bioscaffolds or carriers are required. In addition, the long-term postoperative outcomes and safety need to be further evaluated as the cell differentiation in vivo is difficult to control.
EVs as the alternative of MSCs inherit the therapeutic benefits of MSCs while avoiding the problems associated with cell source [29, 30]. EVs are cell-free fraction and have applied in the treatments of IDD [20, 31,32,33]. Previous studies have revealed that MSC-derived exosomes could exert anti-oxidant and anti-inflammatory to ameliorate IDD [20]. Liao et al. also found that MSC-derived exosomes could regulate endoplasmic reticulum stress to ameliorate cell apoptosis during the progression of IDD [33]. Generally, EVs, including exosomes, have potentials in the treatment of IDD. EVs have diverse biologically active substances that can promote tissue repair and play an immune and inflammatory modulating role [34]. EVs can also be used as a drug-carrying vehicle to deliver drugs to the focal area in a targeted manner [35]. Consistent with these studies, our research indicates that exosomes derived from MSCs could protect against cell pyroptosis and play a siginifcant anti-inflammatory role in NP cells. Currently, in the field of EV research, the original mechanism of EVs, and typing and classification of EVs still remains unclear. Besides, there are more factors affecting the function of EV, therefore more relevant studies are still needed to conduct.
The functional status of donor MSCs affects the nature of secretory EVs. For example, cellular senescence is a biological process that leads to a largely irreversible cessation of cell proliferation, and senescence-associated secretory phenotype (SASP) is the hallmark of senescence [36]. Basisty N et.al. found that cellular senescence alters the proteomic profile of EVs [37]. Based on their proteomic data, it was revealed that senescent cells released a greater number of EVs and the profile of protein cargoes in EVs were also largely distinct from the soluble protein fraction of cells. EVs secreted by aging cells may have negative effects on the recipient cells. Previous studies showed that aged skeletal muscle secreted senescence-associated EVs to induce senescence of bone marrow via the delivery of miRNA cargo [38]. Moreover, EVs secreted by senescent vascular smooth muscle cells influenced differentiation of monocytes to facilitate their pro-inflammatory characteristics, which indicated the EVs as an important inducer of inflammation in atherosclerosis [39]. Therefore, keeping a fine state of donor cells will be beneficial for maintaining the quality of secretory EVs, which is meant to ensure the effectiveness of their treatment that is of great significance in EV-based tissue regeneration and repair. Besides, previous studies have demonstrated that aged cancer cells seem have stronger secretion ability to secret more EVs, which mediates pro-tumorigenic function of surrounding cells [40, 41]. Lei et. al. also found that senescent MSCs secrete higher levels of microvesicles with smaller size than younger cells [42]. However, our results showed that rejuvenated MSCs using specific drugs secret more exosomes with a stronger antioxidant capacity. This difference may be due to the fact that drug-treated younger cells have a stronger secretion ability than aging cells, while the specific mechanisms involved still need to be explored.
At present, there still are some problems during EV-based therapy, one of the issues being the low yield. Although there are currently some technical methods to promote EV secretion, the overall effect is not satisfactory [43]. Previous studies have demonstrated that environmental factors, such as hypoxia, pH and mechanical stress, could alter the EV secretion, as well as the property of EVs. Wang et.al. found that mechanical stress contributed to the fusion of autophagic vesicles with multivesicular bodies, resulting in the increased release of EVs [44]. Other chemical compounds also have been used in stimulating EV secretion. One study has revealed that metformin treatment could stimulate the release of EVs via the activation of autophagy, as well as the increase of anti-oxidant proteins in EVs [31]. In the present study, we used a natural drug quercetin, which also promotes the release of exosomes in MSCs. Further results showed that anti-oxidant proteins are enriched in quercetin-induced exosomes. Our results may recommend the quercetin as the effective drug for boosting exosomes secretion. However, the concentration and duration of these drugs still need to be explored to ensure safety. In the practical application process, we need to ensure both the quantity and quality of EVs. Maybe it is reasonable to optimize the functional state of donor cells to promote the secretion of EVs under the premise of not altering the therapeutic efficacy of EVs.
There still some limitations of this present study. The limited exosome isolation technology accounts for the low purity of the exosomes obtained. Our study utilizes exosomes obtained through ultracentrifugation method, while optimized purification methods are needed in the future to confirm the conclusion. This research focuses on the alterations in antioxidant proteins present in the exosomes. Although the data supports the credibility, further research is needed to investigate additional potential molecular mechanisms. This study employed solely a model of disc degeneration in rats, while conducting additional models of IDD would enhance the credibility of our experiments.
In conclusion, pre-treatment of MSCs with quercetin enhances exosome production and modifies the therapeutic impact of their exosomes. Enhancing the functional condition of donor MSCs is instrumental in obtaining beneficial paracrine substances. Exosomes derived from quercetin-treated MSCs exhibit notably increased levels of antioxidant proteins, and their anti-inflammatory capabilities are significantly improved. This optimization is a crucial factor in boosting the therapeutic effect of exosomes in IDD. This study aims to enhance comprehension of exosomes in IDD treatment and to introduce novel approaches for exosome application.
Data availability statement
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
References
Huang YC, Urban JP, Luk KD. Intervertebral disc regeneration: do nutrients lead the way? Nat Rev Rheumatol. 2014;10:561–6.
Kamali A, Ziadlou R, Lang G, Pfannkuche J, Cui S, Li Z, et al. Small molecule-based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11:27–47.
Hajiesmailpoor A, Mohamadi O, Farzanegan G, Emami P, Ghorbani M. Overview of stem cell therapy in intervertebral disc disease: clinical perspective. Curr Stem Cell Res Ther. 2023;18:595–607.
Tsiapalis D, O’Driscoll L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells. 2020;9:991.
Makarova J, Turchinovich A, Shkurnikov M, Tonevitsky A. Extracellular mirnas and cell-cell communication: problems and prospects. Trends Biochem Sci. 2021;46:640–51.
Wei A, Shen B, Williams L, Diwan A. Mesenchymal stem cells: potential application in intervertebral disc regeneration. Transl Pediatr. 2014;3:71–90.
Piazza N, Dehghani M, Gaborski TR, Wuertz-Kozak K. Therapeutic potential of extracellular vesicles in degenerative diseases of the intervertebral disc. Front Bioeng Biotechnol. 2020;8:311.
Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases. J Biol Chem. 2016;291:26589–97.
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507.
Liu Y, Wang Y, Lv Q, Li X. Exosomes: from garbage bins to translational medicine. Int J Pharm. 2020;583:119333.
Alcaraz MJ, Compan A, Guillen MI. Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells. 2019;9:98.
Ahmadi M, Rezaie J. Ageing and mesenchymal stem cells derived exosomes: molecular insight and challenges. Cell Biochem Funct. 2021;39:60–6.
Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G, et al. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY). 2019;11:7996–8014.
Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288:518–36.
Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D, et al. Therapeutic application of quercetin in aging-related diseases: sirt1 as a potential mechanism. Front Immunol. 2022;13:943321.
Navone SE, Marfia G, Giannoni A, Beretta M, Guarnaccia L, Gualtierotti R, et al. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol Histopathol. 2017;32:523–42.
Chao-Yang G, Peng C, Hai-Hong Z. Roles of nlrp3 inflammasome in intervertebral disc degeneration. Osteoarthritis Cartilage. 2021;29:793–801.
Zhao K, An R, Xiang Q, Li G, Wang K, Song Y, et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the nlrp3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021;54:e12941.
DiStefano TJ, Vaso K, Danias G, Chionuma HN, Weiser JR, Iatridis JC. Extracellular vesicles as an emerging treatment option for intervertebral disc degeneration: therapeutic potential, translational pathways, and regulatory considerations. Adv Healthc Mater. 2022;11:e2100596.
Xia C, Zeng Z, Fang B, Tao M, Gu C, Zheng L, et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1–15.
Dai Z, Xia C, Zhao T, Wang H, Tian H, Xu O, et al. Platelet-derived extracellular vesicles ameliorate intervertebral disc degeneration by alleviating mitochondrial dysfunction. Mater Today Bio. 2023;18:100512.
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–8.
Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi ZL, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33:1925–34.
Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transplant. 2018;27:349–63.
Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272.
Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206.
Vaudreuil N, Henrikson K, Pohl P, Lee A, Lin H, Olsen A, et al. Photopolymerizable biogel scaffold seeded with mesenchymal stem cells: safety and efficacy evaluation of novel treatment for intervertebral disc degeneration. J Orthop Res. 2019;37:1451–9.
Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–56.
Liu H, Li R, Liu T, Yang L, Yin G, Xie Q. Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front Immunol. 1912;2020:11.
Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14:136.
Liao Z, Li S, Lu S, Liu H, Li G, Ma L, et al. Metformin facilitates mesenchymal stem cell-derived extracellular nanovesicles release and optimizes therapeutic efficacy in intervertebral disc degeneration. Biomaterials. 2021;274:120850.
Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, et al. Mesenchymal stem cells deliver exogenous mir-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261–76.
Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K, et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084–100.
Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 2023;23:236–50.
Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–43.
Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22.
Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599.
Fulzele S, Mendhe B, Khayrullin A, Johnson M, Kaiser H, Liu Y, et al. Muscle-derived mir-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging (Albany NY). 2019;11:1791–803.
Głuchowska A, Cysewski D, Baj-Krzyworzeka M, Szatanek R, Węglarczyk K, Podszywałow-Bartnicka P, et al. Unbiased proteomic analysis of extracellular vesicles secreted by senescent human vascular smooth muscle cells reveals their ability to modulate immune cell functions. Geroscience. 2022;44:2863–84.
Han L, Long Q, Li S, Xu Q, Zhang B, Dou X, et al. Senescent stromal cells promote cancer resistance through sirt1 loss-potentiated overproduction of small extracellular vesicles. Cancer Res. 2020;80:3383–98.
Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through epha2. Nat Commun. 2017;8:15729.
Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, et al. Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics. 2017;7:2673–89.
Wiest EF, Zubair AC. Challenges of manufacturing mesenchymal stromal cell-derived extracellular vesicles in regenerative medicine. Cytotherapy. 2020;22:606–12.
Wang K, Wei Y, Liu W, Liu L, Guo Z, Fan C, et al. Mechanical stress-dependent autophagy component release via extracellular nanovesicles in tumor cells. ACS Nano. 2019;13:4589–602.
Author information
Authors and Affiliations
Contributions
Shuai Peng conceived and designed this study, as well as writing the manuscript. Xiangyang Liu conducted the experiments. Lei Chang performed the data acquisition. Bin Liu collected the samples. Mingyan Zhang performed the data analysis. Yan Mao also conducted the experiments. Xiongjie Shen conceived this study and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Hunan Provincial People’s Hospital (No. 184).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, S., Liu, X., Chang, L. et al. Exosomes Derived from Rejuvenated Stem Cells Inactivate NLRP3 Inflammasome and Pyroptosis of Nucleus Pulposus Cells via the Transfer of Antioxidants. Tissue Eng Regen Med (2024). https://doi.org/10.1007/s13770-024-00663-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13770-024-00663-z