Abstract
Background
Low-frequency electromagnetic fields (EMFs) influence biological processes. This present study was aimed at the scientific literature on the use of EMFs in the mesenchymal stem cell differentiation process.
Materials and methods
The electronic search was carried out in PubMed and Web of Science, a database with a combination of the sinusoidal and pulsed low- and extremely low-frequency electromagnetic fields stimulation and mesenchymal stem cells differentiation, considering the period of publication until December 2021. The literature search identified 118 references in PubMed and Web of Science of which 46 articles were selected, respectively, according to the eligibility requirements.
Conclusion
The analysis of research indicated that EMFs are an easy-to-apply and practical way in cell therapy and tissue engineering when regulation of stem cells is required. Studies have shown that EMFs have positive effects on stem cell differentiation, accelerating its process regardless of the parameters and type of stem cells. However, the exact amplitude, frequency, duration of the electrical field, and application method remain elusive and need more study in future work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tissue engineering has gotten a lot of interest in the last few decades as a possible answer to clinical challenges and its purpose is to replace and restore biological tissue or organs [1]. Cells, bioreactors, scaffolds, tissue architecture techniques (3D printers), and chemical stimuli such as growth factors are the essential components of tissue engineering. Mesenchymal Stem cells are one of the most commonly used cell sources in tissue engineering because of their characteristics (self-renewal, differentiation, and immunomodulatory capacities) [2, 3]. Targeted differentiation of mesenchymal stem cells is significant. In light of recent improvements in stem cell differentiation, it has been demonstrated that chemical induction is not the only factor that influences stem cell fate. Physical factors such as electrical, and magnetic fields also play a significant role [4, 5]. Physical and mechanical stimuli are well recognized to affect biological systems, and the effects of electromagnetic fields (EMFs) have already been shown to play a significant role in this regard [6]. With the progress of electromagnetic theory in the last several decades, there has been an increased interest in the interaction between EMFs and many cell functions and behaviors. Low-frequency EMFs (0–100 Hz) have been shown to influence a variety of biological processes, including cell differentiation [7, 8], gene expression [9], protein secretion, proliferation, cell cycle [10], wound healing [11], and as a result, stem cell fate [12]. Furthermore, an essential component of the impact of these waves on the body, aside from mechanical and chemical factors, is the effective involvement of EMFs produced by cells in morphogenesis. These waves are produced during organ creation and direct the development of the fetus' primary organ in the early stages of development and formation [13]. Physiological activities, such as movements of the musculoskeletal system's structure, generate endogenous EMFs in living tissue. Mechanical stresses and currents due to human muscle vibrations have been observed during postural muscle activity (5–10 Hz) and walking (10 Hz) [12].
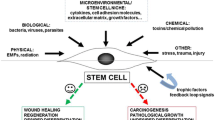
One of the biological processes which EMFs can influence is stem cell differentiation [7, 8]. The electrical characteristics of the plasma membrane are governed by the differences in the distribution of a few critical ions between intracellular and extracellular fluids, as well as their selective transport through the plasma membrane [7]. EMFs can influence ions influx and as a result ions concentration across the cell membrane and transmembrane potential (Fig. 1). In turn, this can induce physiological processes and affect stem cell fate by modulating epigenetic changes, gene expression, and differentiation pathways activation [13,14,15]. Calcium (Ca2+) is a crucial regulator of various cellular functions, and it has been shown that EMFs influences its influx and concentration by interacting with voltage-sensitive Ca2+ channels and the EMFs effect may differ depending on its parameters [12, 14, 16]. Calcium is a cyclic AMP activator, which is a critical component in the triggering of intracellular metabolic processes and it is well known that the differentiation process increases energy demand, and alters the mitochondria. It also has been shown that EMFs can induce free radicals (ROS) forming which can influence ATP production and other chemical reactions. Moreover, it has been also shown that EMFs can affect spindle microtubules because of tubulin dipoles and thus can influence asymmetric cell divisions leading to stem cell differentiation [14, 15]. In summary, EMFs can alter stem cell fate and determine their differentiation by influencing charges in cell components and thus cell communication by regulating the signals delivered to cells. However, the EMFs effect depends on its parameters and it is important to verify which can have a positive impact and stimulate mesenchymal stem cells (MSCs) differentiation.
In this review, optimal conditions and specific EMFs parameters (frequency, intensity, and time of exposure) are discussed for effective differentiation of MSCs to osteogenic, chondrogenic, and neurogenic lineages in an in vitro setting.
2 Methods and materials
For this review study, the electronic databases PubMed and the Web of Science using keywords (mesenchymal stem cells, differentiation, low-frequency & extremely low-frequency electromagnetic field) were searched. The inclusion criteria were the year of publication from January 2010 to December 2021, studies on mesenchymal stem cell differentiation by using low- and extremely low & extremely low-frequency. The method of selecting the papers was carried out by perusing the titles and abstracts of the studies and completed articles, when necessary, importing all results from Mendeley library and then removing duplicates. Studies on other effects of electromagnetic fields on cells were excluded.
The initial search in the PubMed and Web of Science databases found 118 articles, of which 52 were excluded because they did not meet the eligibility criteria and 20 duplicate reports were removed. After the analyzing of the titles and abstracts, 46 articles were selected for systematic review (Fig. 2).
3 Results
It has been confirmed that EMFs is a non-invasive and cost-effective method to facilitate or increase the differentiation of mesenchymal stem cells into different cell lines. The following review of the papers in detail.
3.1 EMFs and osteogenic differentiation
EMFs stimulation has been utilized successfully in the bone healing process for many years [10]. Aldebs et al. found that exposing human adipose-derived mesenchymal stem cells (hASCs) to a low-frequency pulsed electromagnetic fields (PEMFs) (15 Hz, 1 mT) for 8 h a day for 21 days in combination with super magnetic iron oxide nanoparticles (NPs) improved their osteogenic potential [17]. Wang et al. investigated the effect of EMFs (15 Hz/1 MT) on bone marrow MSCs (BM-MSCs) osteogenic differentiation. Low-frequency EMFs was found to improve osteogenic differentiation and bone repair in this investigation. Rabbit BM-MSCs were put onto a hydroxyapatite/collagen scaffold and subsequently stimulated with a 15 Hz/1mT low-frequency EMFs in their research. Low-frequency EMFs was found to improve osteogenic differentiation and bone repair in this investigation. Rabbit BM-MSCs were put onto a hydroxyapatite/collagen scaffold and subsequently stimulated with a 15 Hz/1mT low-frequency EMFs in their research. ALP activity and bone gene expression were used to measure MSC osteogenic differentiation. In addition, an in vivo assessment of a rabbit femur condyle defect model was carried out. The fate of MSCs influenced by EMF induction has been suggested as a possible therapeutic technique for bone tissue engineering based on this study. [18]. Coculture of human osteoblasts with hASCs and exposure to extremely low -frequency PEMFs were recommended by Ehnert et al. to promote osteogenic differentiation. The gene expression of hASCs subjected to two different very low- frequency PEMFs (16 and 26 Hz) was analyzed, and it discovered that exposure to 16 Hz PEMFs might result in bone formation, exposure to 26 Hz ELF-PEMF resulted in bone remodeling [19]. PEMFs therapy is shown to be an effective, non-invasive treatment for a Varous of clinical problems, particularly in the context of mesenchymal stem cell development. The effects of extremely low-frequency PEMFs on osteogenesis have been studied in certain research [19,20,21,22,23,24]. BM-MSCs tagged with super-paramagnetic iron oxide nanoparticles were used by Wu et al. to analyze the osteoblastogenesis influence (SPION). SPION-labeled rat BM-MSCs were subjected to a 50 Hz PEMFs at 1.1 mT. compared to a control group, low-frequency PEMFs exposure resulted in increased proliferation of SPION-labeled BM-MSCs [22]. Lim et al. conducted research on the effects of extremely low-frequency PEMFs on human alveolar bone-derived mesenchymal stem cells (hABMSCs) proliferation and differentiation. Osteogenesis is a complex series of events involving the differentiation of mesenchymal stem cells to produce new bone. The effects of extremely low-frequency PEMFs on cell proliferation, alkaline phosphatase (ALP) activity, and extracellular matrix mineralization were investigated in this work, as well as the expressions of vinculin, vimentin, and calmodulin (CaM) in hABMSCs throughout osteogenic differentiation. On day 5, PEMFs stimulation of hABMSCs increased proliferation by 15% relative to untreated cells. In addition, extremely low- frequency PEMFs considerably increased ALP expression during the early stages of osteogenesis and significantly improved mineralization towards the middle of osteogenesis in just 2 weeks. compared the control, PEMFs improved vinculin, vimentin, and CaM expression. CaM discovered that PEMFs significantly changed the expression of osteogenesis-related genes. extremely low-frequency PEMFs were found to increase early cell proliferation and accelerate osteogenesis in hABMSC-mediated osteogenesis [25]. PEMFs has been shown in this research to be a feasible option for bone tissue engineering applications. Yan et al. discovered that extremely low-frequency EMFs had no obvious influence on human mesenchymal stem cell development (hMSC). They tested the effect of extremely low-frequency EMFs (50 Hz, 20mT) on hMSCs for 23 days and discovered that EMFs inhibited hMSCs proliferation and metabolism. The alkaline phosphatase (ALP) assay, calcium assay, ALP staining, and Alizarin red staining, however, do not support the regulating influence of EMFs on osteogenic differentiation of hMSCs [26]. It could be because of the high magnetic flux density (20mT), different from prior research that used 1.0 mT or 1.1 mT. Mirzaee et al. found that the presence of the conductive polymer might increase the beneficial effects of PEMFs on the osteogenic development of dental pulp stem cells dental pulp stem cells (DPSCs). According to the findings, polyaniline (PANI) and PEMFs improved the osteogenic differentiation ability of human DPSCs in a synergistic manner [27]. Their results indicate that using an PEMFs during this loading regime causes the early phases of bone tissue formation. Many studies have demonstrated that an EMF can help MSCs differentiate into osteogenic cells. However, depending on the experimental and environmental conditions, the experimental outcomes have changed. These differences can be compensated for by optimizing electromagnetic field characteristics in a single designated machine [28,29,30]. In vitro, Kang et al. confirmed that various electromagnetic field parameters (frequency and magnetic flux density) fundamentally affect osteogenic differentiation of adipose-derived stem cells (ASCs). ASCs was determined before osteogenic differentiation, and the EMF became homogenous at the center of the solenoid coil. Then, by measuring alkaline phosphate (ALP) mRNA expression, positive (30/45 Hz, 1 mT) and negative (7.5 Hz, 1 mT) osteogenic differentiation conditions were chosen. compare to the non-stimulated group, the expression of osteogenic markers (RUNX2, COL-I, OSX, and OC) was higher in the 30/45 Hz condition than in the 7.5 Hz condition. Those findings were confirmed by both positive and negative modulation of ALP activity and mineralized nodule development. he effects of EMFs on osteogenic differentiation varied depending on the electromagnetic field's parameters. This finding lays the groundwork for future research into influencing stem cell fate by adjusting EMFs settings [31]. The synergistic effect of EMFs with other stimulus elements has been studied extensively. The synergistic effects of PEMFs signal with iron-ion-doped tricalcium phosphate bone substitute on osteogenesis of hMSCs in vitro were summarized by Habib et al. They stimulated hMSCs using PEMF (15 Hz) for 4 h daily for up to 10 days, with ALP activity increasing at a higher rate when combined with magnetic nano bone substitutes (MNBS). The findings demonstrate the synergistic effects of PEMFs and MNBS on osteogenesis and suggest that PEMFs and MNBS could be used to promote bone repair [32]. The effects of PEMFs (0.2 mT, 15 Hz) and biochemical stimulation on MSCs and their osteogenic pattern were studied by Jazayeri et al. After 10 days of EMF stimulation at 6 h each day, they found that a combination of chemical components and electromagnetic fields improves osteogenesis. Furthermore, in animal models, the use of differentiated osteoblasts seeded on collagen scaffolds promotes the formation of new bone tissues [33]. Meshkian et al. found that cell adhesion, proliferation, and differentiation were all regulated as the Nanofibers were influenced by EMFs, which had synergistic effects on the bone formation process [34]. The scaffold was combined with EMFs in different combinations. Many of these studies have found that using EMFs in conjunction with the scaffold has a synergistic effect. In analyzing osseointegration in osteoporosis, Ye et al. used 3D printed porous Ti (PTi) scaffolds with optimum pore size and porosity matching bone tissue with PEMF as an exogenous osteogenic induction stimulus. PEMF boosted the expression of osteogenic genes (ALP, RUNX2, BMP-2) on the surface of PTi scaffolds in vitro, resulting in increased BM-MSC proliferation and osteogenic differentiation [35].
Finally, EMFswith the suitable characteristics can help to stimulate osteogenesis. EMFs and PEMFs are potentially low-cost and widely applicable tissue engineering techniques that can heal and develop new bone. The studies discussed above are summarized in Table 1.
3.2 EMFs and chondrogenic differentiation
Cartilage is known for its low self-maintenance capacity, and currently, there are no efficient methods to improve cartilage repair [36]. Tissue engineering opens a new path to overcoming these limitations.
BM-MSCs exposed to low intensity (2mT) and extremely low- frequency (15 Hz) PEMFs for 10 min each day is ideal for chondrogenic differentiation, according to Parate et al. This finding emphasizes the complexities of calcium homeostasis during early chondrogenesis, as well as the limitations imposed on PEMFs-based healing approaches aiming at increasing MSCs chondrogenesis. The efficacy of optimized PEMFs for future cartilage regenerating techniques was suggested in this study [37]. Mayer-Wagner et al. developed a bioreactor system that allows the influence of low-frequency EMFs and simulated microgravity (SMG) in vitro chondrogenesis of human mesenchymal stem cells in 3D culture to be studied independently or in combination under controlled conditions. Gene expression was not affected by a single low-frequency EMFs. The expression of COLXA1 and COL2A1 was reduced by a single SMG. In comparison to SMG, low-frequency EMF/SMG resulted in considerably greater COL2A1 expression. In comparison to SMG, low-frequency EMF/SMG increased COLXA1 insignificantly. When compared to control culture levels treated with growth factors, the combination therapy EMFs/SMG was not substantially better. COL2A1 expression was maintained by EMFs/SMG, which had been decreased by SMG [38]. In a three-dimensional (3D) MSCs alginate bead, Kavand et al. investigated the ability of EMFs with frequencies of 25 Hz and 50 Hz to control cartilage gene expressions. Six groups of cell-alginate constructs were tested and treated for 21 days. TGF-beta 1 treatment had a higher impact on COL2 and SOX9 gene expression in MSCs than PEMFs treatments alone, according to real-time polymerase chain reaction (PCR) data. COL2 was found to have a larger transcriptional tendency to change after PEMF stimulation, however, there were no significant differences in SOX9 gene expressions compared to the control group under the stated electromagnetic parameters used in this investigation. PEMFs increased extracellular matrix molecule deposition, and glycosaminoglycans stained favorably with Alcian blue [39]. Using magnetoresponsive stem cell spheroids (MR-SCS) 3D culture, Yoo et al. explored whether low-frequency EMF stimulates chondrogenic differentiation [40]. Mayer-Wanger et al. discovered that exposing the cultures to low-frequency EMFs (15 Hz, 5mT) for 45 min every 8 h boosted collagen type II (Col -II) expression and GAG content, but not Aggrecan or SOX9 expression. Under electromagnetic stimulation, the Collagen type X gene expression was reduced. Based on these findings, it's been proposed that EMFs could be used to induce and maintain hMSCs chondrogenesis [41]. Low frequency PEMFs can stimulate chondrogenic differentiation of rat BM-MSCs in vitro, according to Oiu et al. the adhesion approach was used to extract the rat BM-MSCs, and the third generation of rat BM-MSCs was randomly divided into three groups: low-frequency PEMFs, chondrocyte-induced, and control. The whole medium PEMFs groups were subjected to 50 Hz, 1mT PEMFs for 30 min every day for 10, 15, and 20 days, respectively. The chondrocyte-induced group was given chondrogenic media, whilst the control groups were given only complete medium to culture. Col-II and Aggrecan mRNA and protein expression levels were considerably greater in the low-frequency PEMFs or chondrocyte-induced groups than in the control group, according to the findings. In vitro, low-frequency PEMFs have been shown to help rat BM-MSCs differentiate into chondrogenic cells [42]. EMFs stimulation (50 Hz, 30 mT) and 5% platelet-rich plasma (PRP) stimulated MSCs chondrogenesis, according to Hesari et al. [43].
All in all, cartilaginous tissue comprises a collagen protein that is considered a piezoelectric substrate and is affected by electric fields, making explicit chondrogenic qualities an intriguing possibility for bio-electromagnetic studies [44]. The studies discussed above have been summarized in Table 2.
3.3 EMFs and neurogenic differentiation
The most widely employed elements in nerve tissue engineering are biochemical cues like growth factors, but due to the structure of nerve cells and their sensitivity to electromagnetic fields, EMFs has recently been explored as a distinguishing factor. Several studies utilizing BM-MSCs have been carried out to investigate the effects of electromagnetic field settings in the range of 1–5 mT at a frequency of 50 Hz on the neuronal differentiation of MSCs. After 12 days of exposure, Cho et al. discovered that low- frequency EMFs (50 Hz; 1mT) reduced the growth of hBM-MSCs. Their gene expression levels changed, with the expression of neural stem cell markers like nestin decreasing while the expression of neural cell markers like MAP2, NEUROD1, NF-L, and Tau increased. They also confirmed the expression of each protein of neural cells, as well as oligodendrocyte and astrocyte-related proteins including O4 and GFAP, after extremely low-frequency EMFs stimulation in an immunofluorescence analysis. According to their findings, EMFs can stimulate neuronal differentiation in BM-MSCs without the need of pharmacological agents [45]. Kim et al. conducted a similar study in which they looked into the link between extremely low-frequency EMF exposure and neural differentiation. During in vitro expansion, BM-MSCs were subjected to a 50 Hz EMFs reduced the rate of proliferation of BM-MSCs, which resulted in an increase in neural differentiation. Cells treated with extremely low-frequency EMF revealed higher levels of neuronal differentiation marker (MAP2), whereas the early neuronal marker (Nestin) was adversely regulated, similar to Cho et al. findings. [46]. Choi et al. then revealed that utilizing the same EMFs conditions (50 Hz; 1 mT), EMF is an effective means of differentiating into neural cells. PEG-phospholipid-encapsulated magnetite nanoparticles (Fe3O4) were employed in hBM-MSCs to increase intracellular absorption in this work. extremely low-frequency EMFs mixed with nanoparticles improved neuronal development by increasing the expression of NeuroD1, MAP2, DCX, NF-L, and MBP. Nanoparticles, on the other hand, can be cytotoxic, thus some considerations must be addressed [47]. Furthermore, Aikins et al. explored whether extremely low-frequency EMFs (50 Hz; 1mT) caused neuronal differentiation in hBM-MSCs. Cell proliferation was reduced and neural-like morphology developed after extremely low-frequency EMF stimulation, according to the researchers. At the mRNA level, neuronal markers such -tubulin3, pleiotrophin, and neurofilament-M were detected, as well as MAP2 at the protein level. Reduced expression of metal-response element-transcription factor 1 and MT3, as well as lower intracellular Zn content, were found to be associated with extremely low-frequency -EMF-induced neuronal differentiation. Additionally, upregulation of dihydropyrimidinase-related protein 2 was detected, although -enolase expression remained unchanged. These findings point to a potential MT3 regulation mechanism during neural differentiation [48]. Extremely low-frequency EMFs (50 Hz, 1mT) over 12 days influences the regulation of hBM-MSCs and stimulates astrocyte differentiation, according to Jeong et al. The astrocyte marker (GFAP) was upregulated in extremely low-frequency EMFs -treated cells, while the early neuronal marker (Nestin) and the stemness marker (OCT3/4) were downregulated. Furthermore, after exposure to extremely low-frequency EMF, the number of reactive oxygen species (ROS) was found to be significantly raised, highlighting the modulatory involvement of sirtuin1 (SIRT1) and downstream SIRT1 molecules (TLE1, HES1, and MASH1) during astrocyte differentiation. These results suggest that extremelylow-frequencyEMFs induce astrocytic differentiation through activation of SIRT1 and SIRT1 downstream molecules [49]. In rats with BM-MSCs, Haghighat et al. investigated the effects of nitric oxide (NO) and physical factors (EMFs) on the expression of expression and neural differentiation markers. Cells exposed to high NO amounts in combination with EMFs began to differentiate [50]. Using in vitro and in vivo tests, Seo et al. evaluated the effects of low-frequency PEMF pretreatment on the proliferation and properties of BM-MSCs as well as the regeneration of the injured peripheral nerve. PEMFs increased not only the rate of BM-MSCs growth but also the expression of nerve growth factors in vitro. Additionally, when these treated PMSCs with PEMFs are introduced into a damaged mental nerve, they have a stronger influence on nerve regeneration than untreated BM-MSCs. This suggests that PEMFs pretreatment of BMSCs could be a more strategic tool in cell therapy for repairing damaged mental nerves [51]. In several investigations, graphene-based substrates were employed in conjunction with extremely low-frequency EMFs in nerve regeneration. So far, the findings of Lee et al. showed that the action of extremely low-frequency EMFs (50 Hz, 1 mT) and the graphene-coated substrate have a synergetic impact in increasing the biological efficacy of neuronal differentiation in hBM-MSCs. They claim that this increase in neurogenesis is due to a shift in the global gene expression profile, which up-regulated the gene expression profile, thereby up-regulating adhesion via intracellular calcium [52]. Moraveji et al. evaluated how extremely low-frequency EMFs (50 Hz, 1 mT) affected the expression of the MAP2 and Nestin genes in mesenchymal cells from the dermal papilla (DPCs). To see how chemical and electromagnetic elements affect gene expression, four experimental groups were created and treated for 5 days: chemical (cell exposure to chemical signals), EMFs (cell exposure to extremely low-frequency EMF), chemical-EMFs (cell exposure to chemical signals and extremely low-frequency EMF), and control (no treatment). Real-time PCR analysis proved that EMFs has a useful function in triggering neuronal differentiation. The expression of MAP2 was higher after 14 days than it was after 5 days. The effect of prolonging the treatment period on neuronal differentiation has also been demonstrated by decreased in cell proliferation after 5 to 20 days of EMFs influence [53]. The impact of the synergistic action of EMFs and other physical stimuli has been studied in certain studies. Choi et al. conducted an experiment to determine whether PEMFs (60 Hz) and sound waves (1 kHz and 81 dB) have a synergistic effect on the neurogenic differentiation of hBM-MSCs. These findings suggest that a combination of biophysical waves, PEMFs, and sound can help MSCs differentiate into neural cells [54]. Cruz et al. used a combination of flow-induced shear stress (FSS) and EMFs to boost neurogenesis for a brief period of time [55]. However, little is known about the molecular processes that regulate extremely low-frequency EMF-induced neuronal differentiation. Seong et al. used extremely low-frequency EMF (50 Hz frequency, 1 mT intensity) to stimulate neuronal differentiation in hBM-MSCs for 8 days and discovered that early growth response protein 1 (Egrl) is one of the main transcription factors in extremely low-frequency EMF-induced neuronal differentiation [56]. In addition, Park et al. employed hBM-MSCs treated with extremely low-frequency EMFs to investigate the signaling mechanism involved in neural differentiation (50 Hz, 1mT). EMFs exposure has been shown to influence cellular processes by increasing intracellular reactive oxygen species (ROS) levels. Researchers analyzed EMF-induced ROS production in BM-MSCs. Furthermore, pretreatment with N-acetylcystein, a ROS scavenger, and AG-1478, an EGFR inhibitor, inhibited phosphorylation of EGFR and downstream molecules. These findings imply that EMFs causes neuronal differentiation by activating EGFR signaling and causing a small amount of ROS [57]. The studies discussed above are summarized in Table 3.
4 Discussion/future prospects
Recent advancements in stem cell biology have opened the way for a new phase of tissue engineering and stem cell bio-engineering in the fast-growing field of regenerative medicine. Tissue engineers, on the other hand, face a challenge in accurately managing the timing and the result of this differentiation process. As a result, most strategies for regulating stem cell function have relied on chemical inducers, however, physical stimulation, such as electromagnetic fields, is found to be effective in inducing or boosting growth.
In the last 11 years, 40 studies examined the effect of EMFs on MSCs (Fig. 3) osteogenic [17,18,19, 23, 26, 27, 32,33,34,35,36], chondrogenic [38,39,40,41,42,43,44], and neurogenic [46,47,48,49,50,51,52,53,54,55,56,57,58] differentiation, and it has been shown that it may be able to improve preimplantation culture methods for seeding MSCs in biomaterials fabrication [52]. Due to the vast range of (frequency, magnetic flux density) and exposure lengths utilized by different research groups, the parameter of EMFs is a complex topic. Although much of the EMF’s research has focused on MSC differentiation to the bone, ASCs appear to be stimulated to commence chondrogenesis by the same 50 Hz frequency [24]. Furthermore, several studies have shown that exposing BM-MSCs to 50 Hz, 1 mT EMFs can successfully archive neurogenic differentiation and the use of EMFs to nerve regeneration [45,46,47,48,49, 52,53,54, 57, 58]. However, research on cartilage regeneration that has been conducted since 2010 shows the influence of EMFs on chondrogenesis with varied parameters, with the frequency of 15 Hz being the most frequently employed [38, 39, 41, 42]. Scaffolds have been approved as a platform for cell-biomaterial interactions, cell adhesion, proliferation, and differentiation. In vitro, it was given an extracellular microenvironment [1]. Because it matches the in vivo setting, some studies have found a synergistic effect of electromagnetic fields and 3D cell culture in stem cell differentiation [18, 28]. According to the culture condition, and the EMFs parameters, the effect on MSCs differentiation may vary (Fig. 4) and it is important to provide more appropriate reproducible studies with different EMF parameters in order to provide knowledge about its positive and negative effects on stem cell biology.
Many theories propose that EMFs have their principal effects on the plasma membrane due to their electrical characteristics. An EMF has been found to influence transmembrane signaling by modifying ion channels, ligand binding sites, and the density and distribution of receptors implanted in the cell membrane [47, 53]. Low-frequency EMF can considerably raise intracellular Ca2+ concentration, improving cell adhesion [53], as well as the modulator role of ferritin and thioredoxin-dependent peroxide reductase during neural differentiation [47]. In MSCs, electromagnetic stimulation increases Ca2+ flux and the expression/activity of Ca2+ binding proteins, including calmodulin, resulting in the activation of additional signaling pathways [9]. Considering the immunologic concerns raised by the use of bioactive molecules in tissue engineering, physical stimuli like ELF-EMFs may ensure no immune responses or, at the very least, fewer immune complications [59]. Additionally, it is less expensive, faster, and does not involve the use of expensive growth factors compared to other methods. However, it is very important to adjust the appropriate parameters of the electromagnetic field causing the therapeutic effect.
We were able to compare the effects of EMFs with different parameters on the osteogenic, chondrogenic, and neurogenic differentiation of stem cells in this analysis since we obtained groups of publications. Regardless of the parameters or biomaterials employed, studies have shown that EMFs and PEMFs increase lineage-specific gene expression. Standardization, on the other hand, is still faulty, and it should be further investigated to allow for more specific results regarding the EMFs protocols employed.
To define the best techniques, more research is needed to determine which types of EMFs and PEMFs stimuli (or their combinations with biomaterials) are most appropriate and when to initiate induction during culture. The responses found may improve the design of future EMFs and PEMFs systems.
In conclusion, EMFs and PEMFs are potential modulators of MSCs differentiation, and harnessing their effects may allow for improved pre-culture methods of MSCs in implantable constructs. Proper EMF parameters may provide faster and more effective mesenchymal stem cells differentiation and perhaps be of benefit to regeneration medicine.
References
Li S, L’Heureux N, Elisseeff JH. Stem cell and tissue engineering. Singapore: World Scientific; 2011.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep. 2015;35:e00191.
Cruciani S, Santaniello S, Montella A, Ventura C, Maioli M. Orchestrating stem cell fate: novel tools for regenerative medicine. World J Stem Cells. 2019;11:464–75.
Cruciani S, Garroni G, Ventura C, Danani A, Nečas A, Maioli M. Stem cells and physical energies: Can we really drive stem cell fate? Physiol Res. 2019;68:S375–84.
Massari L, Benazzo F, Falez F, Perugia D, Pietrogrande L, Setti S, et al. Biophysical stimulation of bone and cartilage: state of the art and future perspectives. Int Orthop. 2019;43:539–51.
Marino C, Galloni P, Merla C. Biological effect of electromagnetic fields: reference module in materials science and materials engineering. London: Oxford Elsevier; 2016.
Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15:96–108.
Funk RH, Monsees T, Özkucur N. Electromagnetic effects–from cell biology to medicine. Prog Histochem Cytochem. 2009;43:177–264.
Leone L, Podda MV, Grassi C. Impact of electromagnetic fields on stem cells: common mechanisms at the crossroad between adult neurogenesis and osteogenesis. Front Cell Neurosci. 2015;19:228.
Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T, et al. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther. 2016;7:54.
Pesce M, Patruno A, Speranza L, Reale M. Extremely low frequency electromagnetic field and wound healing: implication of cytokines as biological mediators. Eur Cytokine Netw. 2013;24:1–10.
Santini MT, Rainaldi G, Indovina PL. Cellular effects of extremely low frequency (ELF) electromagnetic fields. Int J Radiat Biol. 2009;85:294–313.
Levin M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–56.
Tamrin SH, Majedi FS, Tondar M, Sanati-Nezhad A, Hasani-Sadrabadi MM. Electromagnetic fields and stem cell fate: when physics meets biology. Rev Physiol Biochem Pharmacol. 2016;171:63–97.
Mathews J, Levin M. The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr Opin Biotechnol. 2018;52:134–44.
McLaughlin KA, Levin M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev Biol. 2018;433:177–89.
Aldebs AI, Zohora FT, Nosoudi N, Singh SP, Ramirez-Vick JE. Effect of pulsed electromagnetic fields on human mesenchymal stem cells using 3D magnetic scaffolds. Bioelectromagnetics. 2020;41:175–87.
Wang H, Tang X, Li W, Chen J, Li H, Yan J, et al. Enhanced osteogenesis of bone marrow stem cells cultured on hydroxyapatite/collagen I scaffold in the presence of low-frequency magnetic field. J Mater Sci Mater Med. 2019;30:89.
Ehnert S, Van Griensven M, Unger M, Scheffler H, Falldorf K, Fentz AK, et al. Co-culture with human osteoblasts and exposure to extremely low frequency pulsed electromagnetic fields improve osteogenic differentiation of human adipose-derived mesenchymal stem cells. Int J Mol Sci. 2018;19:994.
Arjmand M, Ardeshirylajimi A, Maghsoudi H, Azadian E. Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J Cell Physiol. 2018;233:1061–70.
Poh PS, Seeliger C, Unger M, Falldorf K, Balmayor ER, Van Griensven M, et al. Osteogenic effect and cell signaling activation of extremely low-frequency pulsed electromagnetic fields in adipose-derived mesenchymal stromal cells. Stem Cells Int. 2018;2018:5402853.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–69.
Wu S, Yu Q, Sun Y, Tian J. Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis. Am J Transl Res. 2018;10:1431–43.
Ferroni L, Tocco I, De Pieri A, Menarin M, Fermi E, Piattelli A, et al. Pulsed magnetic therapy increases osteogenic differentiation of mesenchymal stem cells only if they are pre-committed. Life Sci. 2016;152:44–51.
Lim K, Hexiu J, Kim J, Seonwoo H, Cho WJ, Choung PH, et al. Effects of electromagnetic fields on osteogenesis of human alveolar bone-derived mesenchymal stem cells. Biomed Res Int. 2013;2013:296019.
Yan J, Dong L, Zhang B, Qi N. Effects of extremely low-frequency magnetic field on growth and differentiation of human mesenchymal stem cells. Electromagn Biol Med. 2010;29:165–76.
Mirzaei A, Saburi E, Enderami SE, Barati Bagherabad M, Enderami SE, Chokami M, et al. Synergistic effects of polyaniline and pulsed electromagnetic field to stem cells osteogenic differentiation on polyvinylidene fluoride scaffold. Artif Cells Nanomed Biotechnol. 2019;47:3058–66.
Fathi E, Farahzadi R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/β-catenin signaling pathways. PLoS One. 2017;12:e0173877.
Yong Y, Ming ZD, Feng L, Chun ZW, Hua W. Electromagnetic fields promote osteogenesis of rat mesenchymal stem cells through the PKA and ERK1/2 pathways. J Tissue Eng Regen Med. 2016;10:E537–45.
Çakmak AS, Çakmak S, White JD, Raja WK, Kim K, Yiğit S, et al. Synergistic effect of exogeneous and endogeneous electrostimulation on osteogenic differentiation of human mesenchymal stem cells seeded on silk scaffolds. J Orthop Res. 2016;34:581–90.
Kang KS, Hong JM, Kang JA, Rhie JW, Jeong YH, Cho DW. Regulation of osteogenic differentiation of human adipose-derived stem cells by controlling electromagnetic field conditions. Exp Mol Med. 2013;45:e6.
Habib M, Horne DA, Hussein K, Coughlin D, Waldorff EI, Zhang N, et al. Magnetic nanoparticles synergize with pulsed magnetic fields to stimulate osteogenesis in vitro. Tissue Eng Part A. 2021;27:402–12.
Jazayeri M, Shokrgozar MA, Haghighipour N, Bolouri B, Mirahmadi F, Farokhi M. Effects of electromagnetic stimulation on gene expression of mesenchymal stem cells and repair of bone lesions. Cell J. 2017;19:34–44.
Meshkini A, Sistanipour E, Izadi A. Mg.ATP-decorated ultrafine magnetic nanofibers: a bone scaffold with high osteogenic and antibacterial properties in the presence of an electromagnetic field. Colloids Surf B Biointerfaces. 2022;210:112256.
Ye M, Liu W, Yan L, Cheng S, Li X, Qiao S. 3D-printed Ti6Al4V scaffolds combined with pulse electromagnetic fields enhance osseointegration in osteoporosis. Mol Med Rep.2021;23:410.
Faust HJ, Guo Q, Elisseeff JH. Cartilage tissue engineering. In: Principles of regenerative medicine. Academic press; 2019. pp. 937–952
Parate D, Franco-Obregón A, Fröhlich J, Beyer C, Abbas AA, Kamarul T, et al. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci Rep. 2017;7:9421.
Mayer-Wagner S, Hammerschmid F, Blum H, Krebs S, Redeker JI, Holzapfel BM, et al. Effects of single and combined low frequency electromagnetic fields and simulated microgravity on gene expression of human mesenchymal stem cells during chondrogenesis. Arch Med Sci. 2018;14:608–16.
Kavand H, Haghighipour N, Zeynali B, Seyedjafari E, Abdemami B. Extremely low frequency electromagnetic field in mesenchymal stem cells gene regulation: chondrogenic markers evaluation. Artif Organs. 2016;40:929–37.
Yoo A, Go G, Nguyen KT, Lee K, Min HK, Kang B, et al. Magnetoresponsive stem cell spheroid-based cartilage recovery platform utilizing electromagnetic fields. Sens Actuators B Chem. 2020;307:127569.
Mayer-Wagner S, Passberger A, Sievers B, Aigner J, Summer B, Schiergens TS, et al. Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells. Bioelectromagnetics. 2011;32:283–90.
Qiu F, He X, Yao X, Li K, Kuang W, Wu W, et al. Low frequence pulsed electromagnetic fields induce chondrocyte-like cells differentiation of rat bone marrow-derived mesenchymal stem cells in vitro. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012;29:501–7.
Hesari R, Keshvarinia M, Kabiri M, Rad I, Parivar K, Hoseinpoor H, et al. Combination of low intensity electromagnetic field with chondrogenic agent induces chondrogenesis in mesenchymal stem cells with minimal hypertrophic side effects. Electromagn Biol Med. 2020;39:154–65.
Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit Rev Biomed Eng. 2007;35:363–411.
Cho H, Seo YK, Yoon HH, Kim SC, Kim SM, Song KY, et al. Neural stimulation on human bone marrow-derived mesenchymal stem cells by extremely low frequency electromagnetic fields. Biotechnol Prog. 2012;28:1329–35.
Kim HJ, Jung J, Park JH, Kim JH, Ko KN, Kim CW. Extremely low-frequency electromagnetic fields induce neural differentiation in bone marrow derived mesenchymal stem cells. Exp Biol Med (Maywood). 2013;238:923–31.
Choi YK, Lee DH, Seo YK, Jung H, Park JK, Cho H. Stimulation of neural differentiation in human bone marrow mesenchymal stem cells by extremely low-frequency electromagnetic fields incorporated with MNPs. Appl Biochem Biotechnol. 2014;174:1233–45.
Aikins AR, Hong SW, Kim HJ, Yoon CH, Chung JH, Kim M, et al. Extremely low-frequency electromagnetic field induces neural differentiation of hBM-MSCs through regulation of (Zn)-metallothionein-3. Bioelectromagnetics. 2017;38:364–73.
Jeong WY, Kim JB, Kim HJ, Kim CW. Extremely low-frequency electromagnetic field promotes astrocytic differentiation of human bone marrow mesenchymal stem cells by modulating SIRT1 expression. Biosci Biotechnol Biochem. 2017;81:1356–62.
Haghighat N, Abdolmaleki P, Parnian J, Behmanesh M. The expression of pluripotency and neuronal differentiation markers under the influence of electromagnetic field and nitric oxide. Mol Cell Neurosci. 2017;85:19–28.
Seo N, Lee SH, Ju KW, Woo J, Kim B, Kim S, et al. Low-frequency pulsed electromagnetic field pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve. Neural Regen Res. 2018;13:145–53.
Lee YJ, Jang W, Im H, Sung JS. Extremely low frequency electromagnetic fields enhance neuronal differentiation of human mesenchymal stem cells on graphene-based substrates. Curr Appl Phys. 2015;15:S95–102.
Moraveji M, Haghighipour N, Keshvari H, Nourizadeh Abbariki T, Shokrgozar MA, Amanzadeh A. Effect of extremely low frequency electromagnetic field on MAP2 and Nestin gene expression of hair follicle dermal papilla cells. Int J Artif Organs. 2016;39:294–9.
Choi YK, Urnukhsaikhan E, Yoon HH, Seo YK, Cho H, Jeong JS, et al. Combined effect of pulsed electromagnetic field and sound wave on In vitro and In vivo neural differentiation of human mesenchymal stem cells. Biotechnol Prog. 2017;33:201–11.
Mascotte-Cruz JU, Ríos A, Escalante B. Combined effects of flow-induced shear stress and electromagnetic field on neural differentiation of mesenchymal stem cells. Electromagn Biol Med. 2016;35:161–6.
Seong Y, Moon J, Kim J. Egr1 mediated the neuronal differentiation induced by extremely low-frequency electromagnetic fields. Life Sci. 2014;102:16–27.
Park JE, Seo YK, Yoon HH, Kim CW, Park JK, Jeon S. Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem Int. 2013;62:418–24.
Brady MA, Waldman SD, Ethier CR. The application of multiple biophysical cues to engineer functional neocartilage for treatment of osteoarthritis. Part I: cellular response. Tissue Eng Part B Rev. 2015;21:1–19.
Azadian E, Arjmand B, Khodaii Z, Ardeshirylajimi A. A comprehensive overview on utilizing electromagnetic fields in bone regenerative medicine. Electromagn Biol Med. 2019;38:1–20.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safavi, A.S., Sendera, A., Haghighipour, N. et al. The Role of Low-Frequency Electromagnetic Fields on Mesenchymal Stem Cells Differentiation: A Systematic Review. Tissue Eng Regen Med 19, 1147–1160 (2022). https://doi.org/10.1007/s13770-022-00473-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00473-1