Abstract
Background:
Containing a certain proportion of mesenchymal stem cells, inflammatory dental tissue showed great tissue regeneration potential in recent years. However, whether it is applicable to promote tissue regeneration in vivo remains to be elucidated. Therefore, we evaluated the feasibility of stem cells from inflammatory dental pulp tissues (DPSCs-IPs) to reconstruct periodontal defects in miniature pigs.
Methods:
The autologous pig DPSCs-IPs were first cultured, appraised and loaded onto β-tricalcium phosphate (β-TCP). The compounds were then engrafted into an artificially-created periodontal defect. Three months later, the extent of periodontal regeneration was evaluated. Clinical examination, radiological examination and immunohistochemical staining were used to assess periodontal regeneration.
Results:
The data collectively showed that DPSCs-IPs from miniature pigs expressed moderate to high levels of STRO-1 and CD146 as well as low levels of CD34 and CD45. DPSCs-IPs have osteogentic, adipogenic and chondrogenic differentiation abilities. DPSCs-IPs were engrafted onto β-TCP and regenerated bone to repair periodontal defects by 3 months’ post-surgical reconstruction.
Conclusion:
Autologous DPSCs-IPs may be a feasible means of periodontal regeneration in miniature pigs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Periodontitis is the most common chronic oral disease worldwide, and it is characterized by the destruction of dental support tissues, ultimately leading to tooth loss [1, 2]. Periodontal tissues can be partially regenerated by the basic treatment and conventional surgical treatments to control plaque and eliminate periodontal inflammation [3,4,5]. However, periodontal tissue regeneration after destruction remains a challenge for clinical periodontics. Recently, the rapid development of tissue engineering for periodontal tissue repair and reconstruction has shown great potential [6, 7]. Odontogenic stem cells with biological materials have shown the ability to regenerate periodontal tissues [8,9,10]. Cultured human periodontal ligament stem cells (PDLSCs) can form periodontal ligament-like structure when implanted into surgically created periodontal defects in nude rats. And also, PDLSCs in combination with different biocompatible materials can promote bone regeneration in ovine model [8]. Stem cells from normal dental pulp tissues with biomaterials showed great bone regenerative potential in tissue engineering [11]. However, there are still some limitations. For example, availability becomes a problem when patients refuse to sacrifice their healthy dental tissues. Furthermore, inflammatory pulp tissues from patients with dental pulp diseases are discarded and ultimately become medical waste.
It has recently been found that inflammatory dental tissue retains tissue regeneration potential by containing a certain proportion of mesenchymal stem cells [12, 13]. However, whether the stem cell composition in the inflammatory pulp has a definite function similar to that of normal dental pulp stem cells remains unclear. In our previous study, two cases of periodontal-pulpal lesions were collected, and the stem cells were isolated from the inflammatory dental pulps. Autologous transplantation was used to treat the corresponding periodontal bone defects and a curative effect was achieved [14]. The aim of the present research was to establish a standardized periodontal defect model in large animal minipigs, isolate stem cells from the autologous inflammatory pulp, and observe the repair potential of DPSCs-IPs after excluding of external mitigating factors.
2 Materials and methods
2.1 Animals
Six inbred Guizhou minipigs (weighing 35–45 kg, male, 24 months old), which were obtained from the experimental animal breeding center (Chengdu, China), were placed under traditional conditions with free access to food and water. Animals in all of the experiments were treated in accordance with the guidelines and the approval by the Institutional Animal Care and Use Committee (IACUC) of Xi’an Jiaotong University College of Medicine (No. 2017036).
2.2 Generation of a model of periodontitis
An established protocols in Generation of a Model of Periodontitis was used in the present study [15]. By using the same general experimental design, three independent cohorts of animals were studied. The first molar and the third premolar were chosen as the experimental teeth; they were then randomly distributed into three groups (48 teeth were used). The details of the three groups are shown in Table 1. Experimental pigs were systematically anesthetized with a combination of xylazine (0.6 mg/kg) and ketamine chloride (6 mg/kg). A mucoperiosteal flap was raised and the alveolar bone was surgically drilled in the buccal region of the root furcation of the third premolars and first molars to create experimental periodontal defects after an initial clinical assessment. A 5 × 6 × 5 mm3 alveolar bone defect was created in the root surface. After the operation, a 4-0 silk was used to suture around the cervical portion of the third premolars and the first molars. Four weeks after the creation of the periodontal lesions, clinical assessments as well as X-ray evaluation of all 48 experimental teeth were performed.
2.3 Flow cytometry for characterization of DPSCs-IP
The inflammatory dental pulp tissues of miniature pigs were obtained by mechanically exposing the dental pulp using a fissure bur and leaving the 1 mm perforation uncovered for 1 day. The next day, the inflammatory dental pulp tissues were removed using a barbed broach, collected in the 10 mm dish with cold phosphate buffered saline (PBS) and immediately cultured. Anesthesia was used as mentioned above before removing the pulp. For cell culture, dental pulps were first digested in the collagenase IV (3 mg/ml) for 1 h at 37 °C, and the supernatant was centrifuged and suspended in Dulbecco’s Modified Eagle Media (DMEM) containing 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Culture media was changed every 3 days. Cells at passage 3 were used for flow cytometric analysis. The primary antibodies were mouse monoclonal anti-human STRO-1 and CD34 and rabbit monoclonal anti-human CD45 and CD146 (all from Abcam, Cambridge, MA, USA). Cells confirmed as DPSCs-IPs were loaded on the β-TCP scaffold.
2.4 Multi-directional differentiation of DPSCs-IP
Multi-directional differentiation was also performed to characterize DPSCs-IPs. Passage 3 DPSCs-IPs was seeded in the 12 well-plate. At the confluence of 90%, osteogenic, adipogenic and chondrogenic differentiation induction medium (Invitrogen, Carlsbad, CA, USA) was added. Induction medium was changed every 3 days. After 3 weeks, the induction was finished, and Alizarin red, oil-red O and toluidine blue were used for staining. ALP activity was also evaluated. Cells were fixed with 70% ethanol for 10 min, after washing with PBS, Alizarin red (pH 4.0), oil-red O (4.2 g of oil-red O in 1200 ml isopropanol) and toluidine (0.1%) blue were added for 15 min staining. Wash the stained cells with ddH2O.
2.5 Scanning electron microscope and transmission electron microscope
Cells at passage 3–5 were used for in vivo transplantation. 0.5 g β-TCPs in 3.5 mm diameter were distributed on the bottom of each 100 mm cell culture dish. After co-culture with the 107cell suspension per dish for 14 days, DPSCs-IP/β-TCP complex was isolated for scanning electron and transmission electron microscope observation. Briefly, for scanning electron microscope observation, cells were first put into 2.5% glutaraldehyde for 2 h, and further fixed with 1% Osmium tetroxide followed by dehydration with ethanol. Samples were ready to observe after displacement, desiccation and metal spraying. For transmission electron microscope observation, samples were loaded onto a carbon-coated grid followed by 3% phosphotungstic acid (pH 6.9) staining for 5 min. Samples were then ready to observe.
2.6 In vivo transplantation
8 weeks later after modeling, periodontal examinations were performed before in vivo treatment. For each experimental tooth, ligature of and the gutta gum was removed first, supragingival and subgingival scaling was performed. Bone defect was exposed following the flap surgery. The three treatment groups were as follows: (a) DPSCs-IPs group (16 defects): initial periodontal therapy, flap surgery, transplantation of DPSCs-IP/β-TCP. (b) β-TCP group (16 defects): initial periodontal therapy, flap surgery, transplantation of β-TCP scaffolds; and (c) Control group (16 defects): initial periodontal therapy alone without any other treatment. DPSCs-IPs/β-TCP and β-TCP were transplanted into the periodontal defects separately and carefully sutured.
2.7 Clinical and radiological evaluations
Plaque index (PLI), probing depth (PD), bleeding index (BI), gingival recession (GR), and tooth mobility were assessed on all teeth pre-transplantation and 12 weeks post-transplantation. X-ray was used to examine bone regeneration.
2.8 Statistical analysis
One-Way ANOVA was used to analyze the significance of differences among the three groups. A P value of less than 0.05 was considered significant.
3 Results
3.1 Generation of periodontitis in miniature swine
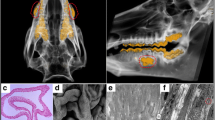
To identify whether DPSCs-IPs-mediated treatment could regenerate new bones to treat periodontitis-associated bone defects, we generated a stable periodontitis model in miniature pigs. Because they share many features of human periodontitis, miniature pigs were chosen as the animal model. Clinical indices, including PLI, BI, PD, GR, AL and tooth mobility were used to assess the status of the periodontal tissues. Moreover, histopathology was used to evaluate periodontal inflammation. The alveolar bone defect that was created was shown (Fig. 1). Four weeks later, the inflammation was obvious with gingiva bleeding after lightly probing the edge (Fig. 2A). By analyzing X-ray images, we were able to compare the alveolar bone before and after modeling (Fig. 2B). The infiltration of inflammatory cells was also seen histologically (Fig. 2C). PLI, BI and PD were significantly increased along with GR and tooth mobility compared with those in the pre-operation group (Table 2).
Evaluation of periodontitis model in miniature pigs. Typical clinical findings of periodontitis were apparent in this model. Four weeks after modeling, periodontal inflammation was observed. A Bleeding after probing was obvious 4 weeks after modeling. Scale bar: 10 mm. B X-ray image showing that the mineral density of the alveolar bone was decreased at the regions of the root furcation. Scale bar: 10 mm. C Infiltration of inflammatory cells can be seen in the bone defeat area of the inflammatory periodontal tissue (arrow). Scale bars = 100 µm
3.2 Characterization of stem cells from inflammatory dental pulp tissue of miniature pigs
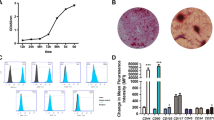
There are several markers that are routinely used to identify MSCs. We selected four of these to characterize cells isolated from inflamed dental pulps. DPSCs-NPs were used as a comparison. As shown, STRO-1 and CD146 were positive on both cell types tested. In contrast, the hematopoietic markers CD34 and CD45 were negatively expressed on both cell types tested (Fig. 3). Moreover, DPSCs-IPs were found to be positively stained with Alizarin red, oil-red O and toluidine blue, and with a high ALP activity 7 days after osteogenic differentiation, which indicated that DPSCs-IPs have muti-differentiation potential (Fig. 4).
Immunophenotype analysis of stem cells from inflammatory dental pulp and normal dental pulp by flow cytometry. A DPSCs-IPs at passage 3 were incubated with STRO-1, CD146, CD34 and CD45, with secondary antibodies following. The blue area indicates positive signals. DPSC: Dental pulp stem cell; IP: Inflamed pulp; NP: Normal pulp. B Statistical analysis of mean fluorescence intensity of both groups. ***p < 0.001. (Color figure online)
Muti-differentiation potential of DPSCs-IPs. A DPSCs-IPs were positively stained with Alizarin red, oil-red O and toluidine blue. B ALP activity was evaluated 7 days after osteogenic differentiation (left). Statistical analysis of Alizarin red stainding (middle) and oil-red O staining (right). ***p < 0.001. (Color figure online)
3.3 DPSCs-IP-mediated periodontal tissue regeneration
In our study, DPSCs-IPs-mediated treatment was hypothesized to provide an effective and applicable approach for functional tissue regeneration after periodontitis. Thus, transplantation of DPSCs-IPs combined with β-TCP to the bone defect in experimental periodontitis was used to test the theory. First, the complex of DPSCs-IPs/β-TCP was examined by scanning electron microscope and transmission electron microscope. As shown in Fig. 5A, DPSCs-IPs are densely packed on β-TCP, stretched sufficiently, with good growth state. From transmission electron microscope observation, integrity organelle was seen, and also connections between cells obvious (Fig. 5B). Procedure of DPSCs-IPs/β-TCP transplantation was shown in Fig. 6. Firstly, mechanically open the dental pulp cavity (Fig. 6A), expose the cavity for 1 day. Then isolate cells from inflammatory dental pulp tissue for cell culture (Fig. 6B). Complex was formed by cells and biomaterials (Fig. 6C). Implant the complex into corresponding alveolar bone defect (Fig. 6D), and suture (Fig. 6E). Clinical assessment, X-ray and Immunohistochemical staining were used to evaluate the effect of DPSCs-IPs treatment (Fig. 7). At 3 months’ post-transplantation, the PLI were 2.20 ± 0.13 in the DPSCs-NPs/β-TCP group 2.40 ± 0.22 in the DPSCs-IPs/β-TCP group, 2.93 ± 0.17 in the β-TCP group, and 3.07 ± 0.15 in the control group. The BI was 1.05 ± 0.11 in the DPSCs-NPs/β-TCP group,1.40 ± 0.19 in the DPSCs-IPs/β-TCP group, 1.40 ± 0.12 in the β-TCP group and 2.13 ± 0.43 in the control group. The PD were 2.45 ± 0.15 mm in the DPSCs-NPs/β-TCP group, 3.00 ± 0.18 mm in the DPSCs-IPs/β-TCP group, 3.66 ± 0.24 mm in the β-TCP group, and 4.66 ± 0.14 mm in the control group. The GR were 2.65 ± 0.24 mm in the DPSCs-NPs/β-TCP group 3.40 ± 0.17 mm in the DPSCs-IPs/β-TCP group, 3.73 ± 0.32 mm in the β-TCP group, and 4.4 ± 0.19 mm in the control group (Fig. 7A). Statistical analysis of the clinical assessment indicated that DPSCs-IPs treatment significantly improved periodontal tissue regeneration.
DPSCs-IPs/β-TCP complex evaluation by scanning electron microscope and transmission electron microscope A, B Scanning electron microscope evaluation of DPSCs-IPs/β-TCP complex. Scale bars = 10 µm. (red circle) C, D Transmission electron microscope evaluation of DPSCs-IPs/β-TCP complex. Scale bars = 1 µm
Procedure of DPSCs-IPs/β-TCP transplantation. A Mechanically exposing the dental pulp by odontotrypy. Scale bar: 10 mm. B Isolation and culture of DPSCs-IPs. Scale bar: 50 μm. C The biomaterial complex used for grafts: DPSCs-IPs/β-TCP. Scale bar: 25 mm. D Transplantation of the biomaterial into the area of the bone defect. Scale bar: 10 mm. E Suture of the operation site. Scale bar: 10 mm
Clinical assessment of periodontal condition. A Plaque index (PLI), bleeding index (BI), probing depth (PD) and gingival recession (GR) 3 months after transplantation. B X-ray images 3 months after transplantation. Scale bar: 10 mm. C Immunohistochemical staining of osteoprotegerin. Scale bars = 200 µm
Bone regeneration could be observed on X-ray images (Fig. 7B) and osteoprotegerin was stained to evaluate the suppressive effect on osteoclastogenesis (Fig. 7C). In both DPSCs-IPs and DPSCs-NPs group, new bone was regenerated in the periodontal defect area; the height was greater than that in the β-TCP group and the untreated control group, but still lower than the normal level. Osteoprotegerin expressed at a higher level in both DPSCs-IPs and DPSCs-NPs group, and DPSCs-NPs showed a stronger osteoprotegerin expression level compared with DPSCs-IPs group. Although the effect of DPSCs-IPs in bone regeneration was not as good as DPSCs-NPs, DPSCs-IPs still can be one of the candidates in treating periodontal bone defeats.
4 Discussion
In the present study, we reveal for the first time that stem cells from inflammatory dental pulp tissue have the ability to regenerate tissue in periodontal bone defects of miniature pigs, thus expanding the availability of seed cells for tissue engineering. We first selected two most representative stem cell markers to characterize our isolated cells from inflammatory dental pulp tissues. Stro-1 was considered the best known marker of mesenchymal stem cells, particularly in dental tissues [16, 17]. CD146 is notably emerged as an attractive candidate for MSCs [18]. More importantly, it was known to be one of the first markers explained for dental pulp stem cells [19]. The cells isolated from inflammatory dental pulp tissues of miniature swine were strongly positive for these markers which indicated their stem cell properties. In addition, we also selected CD34 and CD45 for exclusion of hematopoietic stem cells origin of the isolated cells [20, 21]. The negative expression of these markers revealed their non-hematopoietic background.
Odontogenic mesenchymal stem cells have been widely used in tissue engineering, but for a long time, few sources of seed cells have been available. The inflamed dental pulp tissue, which is always disposed of as medical waste, is reported to retain the potential to isolated stem cells to regenerate tissues [22, 23]. We reported that dental pulp stem cells from inflamed dental pulp tissues have the ability to repair periodontal bone defects, which greatly enriched the source of odontogenic mesenchymal stem cells and make good use of the clinical production of medical waste. DPSCs are the most commonly used stem cells for tissue regeneration and have been isolated from dental tissue in recent years [24, 25]. However, because of clinical implications, the common method of harvesting DPSCs is by the extraction of healthy teeth. As a new source of DPSCs, stem cells isolated from inflamed dental tissues broaden the availability of these seed cells for tissue regeneration. This will provide great convenience for clinical applications if they can be used to treat periodontal bone defects or even other oral and maxillofacial defects.
However, to determine whether this type of newly identified stem cells was suitable for use in tissue engineering, it was necessary to test them in animal models. By creating alveolar bone defects with silk suture in minipigs, we established an animal model of periodontitis in this study. We found that typical periodontitis featured by probing bleeding, significant bone loss, a deep periodontal pocket, loss of clinical attachment and histological inflammation can be created in miniature pigs. Large animal models have been used because they are superior to small animals for assessing the efficacy and safety of certain treatments [26]. Miniature pigs have a similar oral–maxillofacial region to humans [27]. When an osteotome with a silk suture was used to create periodontal bone defects, serious periodontitis was generated in the miniature pig after 1 month [28]. Histological inflammation was shown by HE staining. In our current study, transplantation of autologous DPSCs-IPs directly into periodontal defects could contribute to periodontal tissue repair in an experimental model of periodontitis in miniature pigs. The bone defect area was restored to some extent 3 months after transplantation of DPSCs-IPs/β-TCP. The clinical bleeding, probing depth, and attachment loss were all obviously improved after treatment, and the improvements were greatest in the DPSCs-IPs/β-TCP group. Compared with the control group, the loss of adhesion in the β-TCP group was not significantly different, but the difference in the probing depth between the two groups was significant. Further studies will be required to identify the reason for this discrepancy.
Although the gums and buccal alveolar bone level of the autologous DPSCs-IPs/β-TCP group did not reach normal levels, the formation of new adhesive tissue was observed, and new bone regeneration was seen in the root furcation. There may be a variety of factors that play roles in this process. The horizontal absorption of alveolar bone may influence the fixation of the implant. As the gums retreat, conditions are unfavorable for the protection and fixation of the materials. The attached gingiva of miniature pigs is shorter than in humans, so it is possible that there was not enough buccal attached gingiva to completely cover the implanted materials and the root bifurcation in order to protect the implants. On the other hand, the oral environment of the miniature pig is complex, making it difficult to position the protective periodontal pack on the buccal side, in addition to difficulty maintaining good oral hygiene. In an attempt to solve this problem, oral health maintenance was performed every 2 weeks to prevent the occurrence of periodontal inflammation and to thus promote periodontal tissue regeneration.
Histological observations showed that there were normal periodontal ligament fibers and alveolar bone morphology before modeling. After the modeling process, the root bifurcation area showed a large empty area with many inflammatory cells as well as bone resorption. After 3 months of treatment, there was a large amount of epithelium and fibrous tissue formation in the root bifurcation area of only the blank control group. The β-TCP group also had gingival epithelium, but it had a small amount of alveolar bone formation. Although there were small amounts of gingival epithelium and fibrous tissue formation, new bone formation was visible on the alveolar area, and periodontal ligament collagen fibers were deposited into the new bone.
In this study, the DPSCs-IPs/β-TCP complex group did not achieve preoperative gum and buccal alveolar bone levels, but there was significant formation of new attachment. It is possible that the horizontal absorption of the alveolar bone after modeling is not conducive to the fixation of the buccal root surface of the implanted material, and the associated gingival retraction is not conducive to the protection and fixation of the material. To solve this problem, future studies should retain the teeth attached to the cheek side of the gingival, parallel to the return valve flap, and completely cover the implanted material and root bifurcation to protect the implants to prevent their prolapse. On the other hand, the oral environment of the miniature pig is more complicated, and it is difficult to maintain a good oral hygiene environment. It is necessary to perform oral hygiene maintenance to prevent the occurrence of periodontal inflammation.
There are also some limitations to this study. First, pig tissues may differ from those of humans, so future studies are needed in humans. Second, complete evaluation of a definite curative effect will also require a larger amount of experimental observations. Our findings indicate that a new cellular/bio-material treatment may offer a readily available therapeutic approach for periodontal tissue regeneration.
References
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20.
Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–78.
Elangovan S, Srinivasan S, Ayilavarapu S. Novel regenerative strategies to enhance periodontal therapy outcome. Expert Opin Biol Ther. 2009;9:399–410.
Needleman IG, Worthington HV, Giedrys-Leeper E, Tucker RJ. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006;19:CD001724.
Venezia E, Goldstein M, Boyan BD, Schwartz Z. The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta-analysis. Crit Rev Oral Biol Med. 2004;15:382–402.
Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010;16:219–55.
Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–927.
Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006;40:164–72.
Khorsand A, Eslaminejad MB, Arabsolghar M, Paknejad M, Ghaedi B, Rokn AR, et al. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implantol. 2013;39:433–43.
Padial-Molina M, Rios HF. Stem cells, scaffolds and gene therapy for periodontal engineering. Curr Oral Health Rep. 2014;1:16–25.
Graziano A, d’Aquino R, Laino G, Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21–6.
Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5:617–31.
Attar A, Eslaminejad MB, Tavangar MS, Karamzadeh R, Dehghani-Nazhvani A, Ghahramani Y, et al. Dental pulp polyps contain stem cells comparable to the normal dental pulps. J Clin Exp Dent. 2014;6:e53–9.
Li Y, Zhao S, Nan X, Wei H, Shi J, Li A, et al. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res Ther. 2016;7:141.
Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–73.
Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204.
Dehghani Nazhvani A, Hosseini SM, Tahoori B, Tavangar MS, Attar A. Identification of mesenchymal stem cell marker STRO-1 in oral reactive lesions by immunofluorescence method. J Dent (Shiraz). 2015;16:246–50.
Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–19.
Tavangar MS, Hosseini SM, Dehghani-Nazhvani A, Monabati A. Role of CD146 enrichment in purification of stem cells derived from dental pulp polyp. Iran Endod J. 2017;12:92–7.
Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–9.
Nakano A, Harada T, Morikawa S, Kato Y. Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines. Acta Pathol Jpn. 1990;40:107–15.
Yazid FB, Gnanasegaran N, Kunasekaran W, Govindasamy V, Musa S. Comparison of immunodulatory properties of dental pulp stem cells derived from healthy and inflamed teeth. Clin Oral Investig. 2014;18:2103–12.
Sun HH, Chen B, Zhu QL, Kong H, Li QH, Gao LN, et al. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials. 2014;35:9459–72.
Di Benedetto A, Carbone C, Mori G. Dental pulp stem cells isolation and osteogenic differentiation: a good promise for tissue engineering. Methods Mol Biol. 2014;1210:117–30.
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, et al. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015;33:627–38.
Wang S, Liu Y, Fang D, Shi S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007;13:530–7.
Weaver ME, Jump EB, McKean CF. The eruption pattern of permanent teeth in miniture swine. Arch Oral Biol. 1969;14:323–31.
Singh G, O’Neal RB, Brennan WA, Strong SL, Horner JA, Van Dyke TE. Surgical treatment of induced peri-implantitis in the micro pig: clinical and histological analysis. J Periodontol. 1993;64:984–9.
Acknowledgements
This research was funded by Shaanxi Provincial Science and Technology Innovation Project co-ordination of Resources Oriented Industries of Key Technologies Project (Grant No. 2011KTCL03-24 for A.L.) and Scientific and Technological Innovation Project in Xi’an (Grant No. SF1421 for T.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study has been independently reviewed and approved by the ethical board of Xi’an Jiaotong University College of Medicine for Experimental Animals (No. 2017036).
Rights and permissions
About this article
Cite this article
Li, Y., Nan, X., Zhong, TY. et al. Treatment of Periodontal Bone Defects with Stem Cells from Inflammatory Dental Pulp Tissues in Miniature Swine. Tissue Eng Regen Med 16, 191–200 (2019). https://doi.org/10.1007/s13770-018-00175-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-00175-7