Abstract
Fungicidal effects of active component purified from Diospyros kaki roots and its analogues against Erysiphe graminis, Botrytis cinerea, Pyricularia grisea, Puccinia recondite, Rhizoctonia solani, and Phytophthora infestans were investigated using a whole-plant method. Active constituents isolated from the chloroform fraction of D. kaki roots were characterized as plumbagin, using various spectroscopic analyses. To establish the structure–activity relationships, the fungicidal effects of plumbagin and its structural analogues were bioassayed against phytopathogenic fungi. At 0.25, 0.125, and 0.0625 g/L, plumbagin had a greater fungicidal effect than p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, or menadione against P. grisea, B. cinerea, R. solani, and E. graminis. Our results suggest that the methyl and hydroxyl functional groups at the 5′- and 2′-positions of p-naphthoquinone play key roles in its fungicidal effect against six phytopathogenic fungi. Therefore, plumbagin and its analogues may be suitable as antifungal agents to control plant diseases.
Similar content being viewed by others
Introduction
Plant pathogens, such as bacteria, fungi, nematodes, and viruses, cause several diseases or damages in plants [1]. Among these plant pathogens, fungi are the major plant pathogen and cause many serious diseases of plants. Plant pathogenic fungi also cause pre- and post-harvest losses in economically important agricultural crops [2, 3]. For many years, synthetic chemicals have been used as fungicidal agents to control plant diseases [4]. Synthetic fungicides, such as benzimidazole and difenoconazole, are widely used to control plant diseases caused by phytopathogenic fungi [5, 6]. However, the intensive use of synthetic fungicides has adverse effects, including fungicide resistance and residual toxicity [7]. Thus, there is a need for the development of environmentally safe fungicides. One possible answer is to use plant-derived metabolites as sources for the development of fungicidal agents [8, 9].
Diospyros kaki L., in the Ebenaceae family, is traditionally used to treat burns, frostbite, hiccough, and paralysis [10]. Various biological activities, including antimicrobial, acaricidal, and fungicidal activities of this species, have been reported previously [11,12,13,14]. Although there are several studies of the antifungal effects of D. kaki against phytopathogenic fungi in vitro, to our knowledge the in vivo efficacy of D. kaki against phytopathogenic fungi has not been studied. The aim of our work was to study in vivo antifungal effects of the isolated component from D. kaki and its analogues in a greenhouse.
Materials and methods
Chemicals
2-Hydroxy-1,4-naphthalenedione (lawsone), 2-methyl-1,4-naphthalenedione (menadione), 2-methoxy-p-naphthoquinone, and 1,4-naphthalenedione (p-naphthoquinone) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dichlofluanid (C9H11Cl2FN2O2S2) was purchased from Dongbu Hitek Co.
Isolation and identification
D. kaki roots (1 kg) were dried at 35 °C and homogenized with a grinder. Afterward, D. kaki roots were extracted for 48 h with methanol; this extraction was repeated three times. The extract was filtered over Toyo filter paper No. 2 (Tokyo, Japan), and methanol was completely removed from the residual D. kaki roots by evaporation with a rotary vacuum evaporator. The methanol extract (18.4 g) was suspended in H2O and partitioned successively with hexane, chloroform, ethyl acetate, and butanol to obtain chloroform (3.94 g), hexane (5.41 g), ethyl acetate (2.96 g), and butanol (6.09 g), respectively.
The active component from the chloroform fraction derived from the D. kaki extract was purified by silica-gel chromatography (5.5 i.d. × 68 cm L, Merck 70-230 mash). Hexane and chloroform (30:70–0:100 v/v) were the elution solvents. The column chromatographic analysis yielded six fractions (K1–K6). The K1 showed the strongest fungicidal activity against six fungi. The K1 fraction was rechromatographed using a silica-gel column. Chloroform and hexane (70:30 v/v) were the elution solvents. From K1, six fractions (K11–K16) were obtained. The K11 was purified by prep HPLC (JAI Co., Tokyo, Japan) to separate the active component, and the eluates were tested. From K11, six fractions (K111–K116) were obtained using a Jaigel GS Series Column (21.4 mm i.d. × 500 mm L, JAI Co., Tokyo, Japan) with chloroform–hexane–isopropanol (70:30:2) at 4.8 mL/min (268 nm). The K114 was isolated using a Jaigel W Series Column (20.1 mm i.d. × 500 mm L, JAI Co., Tokyo, Japan) with chloroform–hexane–isopropanol (70:30:2) at 4.6 mL/min, resulting in four fractions (K1141–K1144). Finally, the K1144 was purified. The structure of K1144 was studied by spectroscopic analyses. 1H (600 MHz), 13C (150 MHz), and DEPT (100 MHz) NMR were studied using JNM-LA 400F7 (Jeol Ltd., Japan) in CDCl3. HMQC and 1H–1H COSY was measured to study the relationships between carbons and protons. Tetramethylsilane was used as the internal standard. EI-MS spectra were studied with a spectrometer (JEOL JMS-DX 30).
Pathogens and cultures
Pyricularia grisea, Erysiphe graminis, Botrytis cinerea, Phytophthora infestans, Rhizoctonia solani, and Puccinia recondite were used (Table 1). Erysiphe graminis and P. recondita were maintained on barley and wheat, respectively. Rhizoctonia solani, P. grisea, P. infestans, and B. cinerea were grown on potato dextrose agar (PDA) slants and V-8 agar slants and kept at 4 °C.
In vivo bioassay for fungicidal effect
Fungicidal effects of samples against six phytopathogenic fungi using various concentrations (2.0, 1.0, 0.5, 0.25, 0.125, and 0.0625 g/L) were evaluated by a whole-plant method, as described by Kim et al. [15]. Each test sample was dissolved in DMSO (0.5 mL) and diluted in water (49.5 mL) containing of Tween 20 (0.25 mg/mL) to obtain a 2 g/L concentration. Samples (50 mL) were sprayed onto test plants at the same time. The treated plants were placed in a greenhouse for 24 h before inoculation with each pathogen. Negative controls were applied with the DMSO-Tween solution. Experiments were replicated three times. Test conditions of cucumber gray mold (CGM), rice sheath blight (RSB), rice blast (RCB), barley powdery mildew (BPM), wheat leaf rust (WLR), and tomato late blight (TLB) caused by B. cinerea, R. solani, P. grisea, E. graminis, P. recondita, and P. infestans, respectively, in a greenhouse are presented in Table 1.
Statistical analysis
The fungicidal effect of test materials on CGM, RSB, RCB, BPM, WLR, and TLB was measured by a control value (CV) calculated by the equation
where A and B represent the disease area on the untreated and treated plants, respectively. The plant disease area was studied with a Color Image Microscope System (Samsung MW-200B1). CV was transformed to arcsine square-root values for ANOVA. A Scheffe’s test was performed to compare and separate treatment means.
Result and discussion
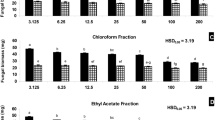
The in vivo fungicidal effects of the methanol extract and four fractions (hexane, chloroform, ethyl acetate, and butanol) derived from D. kaki roots against six phytopathogenic fungi when treated with 1.0 and 2.0 g/L are shown in Table 2. In a test with E. graminis, B. cinerea, P. grisea, and R. solani, strong fungicidal effects were produced by the methanol extract (86–100%) and chloroform fraction (88–100%) at 2.0 g/L. However, hexane, ethyl acetate, and butanol fractions had no fungicidal effect against six phytopathogenic fungi. A negative control containing DMSO-Tween 20 solution did not show fungicidal effects against six phytopathogenic fungi. However, the chloroform fraction partitioned from the methanol extract of D. kaki roots was selected to isolate the fungicidal compound of D. kaki roots.
To isolate the active compound of chloroform fraction, chromatographic analyses were performed. The active isolate (K1144) was identified by spectroscopic analyses (1H NMR, 13C NMR, 1H–1H COSY, EI-MS, DEPT, and 1H–13C HMQC). The fungicidal compound was identified as 5-hydroxy-2-methyl-1,4-naphthalenedione (plumbagin, C11H8O3, MW 188) based on the following evidence: 1H-NMR (600 MHz, CDCl3): δ 7.60–7.61 (1H, d), 7.56–7.59 (1H, d), 7.24–7.25 (1H, m), 6.78–6.79 (1H, d), 2.17–2.18 (3H, d). 13C-NMR (150 MHz, CDCl3): δ 190.0, 184.6, 161.0, 149.5, 135.9, 135. 3, 131.9, 124.0, 119.2, 115.0, 16.5. EI-MS (70 eV) m/z (% relative intensity): M+ 188 (100, base peak), 173 (18), 160 (19), 131 (16), 120 (12), 92 (9), 63 (4). The spectroscopic properties of plumbagin were compared with those of previous studies [11, 16].
To measure the fungicidal activity of plumbagin isolated from D. kaki roots, its fungicidal activities against six phytopathogenic fungi at 0.5, 0.25, 0.125, and 0.0625 g/L were measured in vivo and compared with that of dichlofluanid, which served as a positive control (Table 3). At 0.5 and 0.25 m/L, plumbagin exhibited strong (80–100%) and moderate (60–80%) fungicidal effects against E. graminis B. cinerea, P. grisea, and R. solani, but not against P. infestans and P. recondite. At 0.125 and 0.0625 g/L, plumbagin showed moderate and weak (< 60%) fungicidal effects against E. graminis B. cinerea, P. grisea, and R. solani. Strong fungicidal effects were observed with dichlofluanid against B. cinerea at 0.05 g/L, whereas no fungicidal effect was produced by dichlofluanid against E. graminis, P. grisea, P. infestans, P. recondite, or R. solani. The in vivo trials in this study showed that plumbagin exhibited consistent fungicidal effects against E. graminis, B. cinerea, P. grisea, and R. solani. Our results agreed well with those reported in previous studies. Indeed, Shin et al. [16] found that the methanol extract of Nepenthes ventricosa leaves inhibited the growth of eight plant pathogenic fungi (Aspergillus niger, Alternaria alternate, Fusarium oxysporum, Sclerotinia sclerotiorum, Bipolaris oryzae, Rhizoctonia solani, Phytophthora capsici, and Rhizopus stolonifera). According to these authors, the antifungal effect of N. ventricosa extract resulted from the presence of plumbagin. Furthermore, Dzoyem et al. [17] studied the antifungal effect of plumbagin against five yeast fungi (Candida tropicalis, Candida glabrata, Candida albicans, Candida neoformans, and Candida krusei) and seven filamentous fungi (Aspergillus flavus, Aspergillus niger, Cladosporium sp., Alternaria sp., Fusarium sp., Penicillium sp., and Geotrichum candidum). These authors suggested that plumbagin might be a better fungicidal agent than ketoconazole, which was used as the standard fungicidal agent.
To establish the structure–activity relationships between plumbagin analogues and fungicidal effects against phytopathogenic fungi, p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, and menadione were selected as plumbagin analogues for testing (Fig. 1). The fungicidal effects of analogues structurally related to plumbagin, and how toxicity varies with structure was examined (Table 3). In a test with E. graminis, strong fungicidal effects (100, 90%, respectively) were produced by menadione at 0.5 and 0.25 g/L. Moderate or weak fungicidal effect was obtained from menadione at 0.125 and 0.0625 g/L. However, no activity was observed for p-naphthalenedione, juglone, lawsone, or 2-methoxy-p-naphthoquinone when treated at 0.5, 0.25, 0.125, and 0.0625 g/L. With B. cinerea, control values over 80% were obtained with p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, and menadione at 0.5 g/L. Moderate or weak fungicidal effect was obtained in p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, and menadione at 0.25, 0.125, and 0.0625 g/L. In a test with P. grisea, juglone and lawsone revealed a strong fungicidal effect (95, 90%, respectively) at 0.5 g/L, whereas a moderate or weak fungicidal effect was observed with juglone and lawsone at 0.25, 0.125, and 0.0625 g/L. The other analogues showed no antifungal effects. With R. solani, control values over 85% were obtained with p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, and menadione at 0.5 g/L. A moderate or weak fungicidal effect was obtained in p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, and menadione at 0.25, 0.125, and 0.0625 g/L. In a test with P. infestans, 2-methoxy-p-naphthoquinone showed a weak effect at 0.5 g/L, and the other plumbagin analogues showed no fungicidal effect. In a test with P. recondita, no fungicidal effect was obtained with p-naphthoquinone, juglone, lawsone, 2-methoxy-p-naphthoquinone, or menadione at 0.5, 0.25, 0.125, and 0.0625%.
Structures of plumbagin and its analogues. (A) 1,4-Naphthalenedione (p-naphthoquinone); (B) 5-Hydroxy-1,4-naphthalenedione (juglone); (C) 2-Hydroxy-1,4-naphthalenedione (lawsone); (D) 2-Methoxy-1,4-naphthalenedione (2-methoxy-p-naphthoquinone); (E) 2-Methyl-1,4-naphthalenedione (menadione); (F) 5-Hydroxy-2-methyl-1,4-naphthalenedione (plumbagin)
In our study, the fungicidal effects of plumbagin and its analogues against phytopathogenic fungi were affected by the position and number of functional groups as well as by the various types of functional groups in the chemical structure. Jeon et al. [18] suggested that the insecticidal effects of plumbagin that contains the hydroxyl and methyl functional groups on p-naphthoquinone were greater against two species of planthoppers than that of menadione containing the methyl functional group on p-naphthoquinone, Nilaparvata lugens, and Laodelphax striatellus. Furthermore, Park et al. [19] reported that the antimicrobial effects of 2,6-dimethoxy-1,4-benzoquinone analogues against the Bacillus cereus, Salmonella enterica, and S. typhimurium are related to the different functional groups on the 1,4-benzoquinone. That the plumbagin analogues lack fungicidal effects may be connected to the lack of hydroxyl or methyl functional groups. In this regard, plumbagin containing hydroxyl and methyl functional groups at positions 5′- and 2′- exhibited broad fungicidal effects against phytopathogenic fungi. In conclusion, plumbagin and its analogues may be used as natural fungicides to control phytopathogenic fungi that cause plant disease.
References
Montesinos E (2003) Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol 6:245–252
Fletcher J, Bender C, Budowle B, Cobb WT, Gold SE, Ishimaru CA, Luster D, Melcher U, Murch R, Scherm H, Seem RC, Sherwood JL, Sobral BW, Tolin SA (2006) Plant pathogen forensics: capabilities, needs, and recommendations. Microbiol Mol Biol Rev 70:450–471
Chang HT, Cheng YH, Wu CL, Chang ST, Chang TT, Su YC (2008) Antifungal activity of essential oil and its constituents from Calocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi. Bioresour Technol 99:6266–6270
Choi H, Kim JH (2018) Risk and exposure assessment for agricultural workers during treatment of cucumber with the fungicide fenarimol in greenhouses. Appl Biol Chem 61:1–6
Davidse LC (1986) Benzimidazole fungicides: mechanism of action and biological impact. Annu Rev Phytopathol 24:43–65
Bajpai V, Shin SY, Kim MJ, Kim HR, Kang SC (2004) Anti-fungal activity of bioconverted oil extract of linoleic acid and fractionated dilutions against phytopathogens Rhizoctonia solani and Botrytis cinerea. Agric Chem Biotechnol 47:199–204
Aguín O, Mansilla JP, Sainz MJ (2006) In vitro selection of an effective fungicide against Armillaria mellea and control of white root rot of grapevine in the field. Pest Manag Sci 62:223–228
Sanyacharernkul S, Nantapap S, Sangrueng K, Nuntasaen N, Pompimon W, Meepowpan P (2016) Antifungal of modified neolignans from Mitrephora wangii Hu. Appl Biol Chem 59:385–389
Choi H, Lee BH, Moon YS, Kim K, Lee HS, Lee SE (2017) Antifungal and antiaflatoxigenic effects of a fumigant, ethanedinitrile, on Aspergillus flavus. Appl Biol Chem 60:473–476
Matsuo T, Ito S (1978) The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki L.). Agric Biol Chem 42:1637–1643
Lee CH, Lee HS (2008) Acaricidal activity and function of mite indicator using plumbagin and its derivatives isolated from Diospyros kaki Thunb. roots (Ebenaceae). J Microbiol Biotechnol 18:314–321
Jeong EY, Jeon JH, Lee CH, Lee HS (2009) Antimicrobial activity of catechol isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food Chem 115:1006–1010
Sun L, Zhang J, Lu X, Zhang L, Zhang Y (2011) Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem Toxicol 49:2689–2696
Zhang J, Kopparapu NK, Yan Q, Yang S, Jiang Z (2013) Purification and characterisation of a novel chitinase from persimmon (Diospyros kaki) with antifungal activity. Food Chem 138:1225–1232
Kim MK, Choi GJ, Lee HS (2003) Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J Agric Food Chem 51:1578–1581
Shin KS, Lee SK, Cha BJ (2007) Antifungal activity of plumbagin purified from leaves of Nepenthes ventricosa x maxima against phytopathogenic fungi. Plant Pathol J 23:113–115
Dzoyem JP, Tangmouo JG, Lontsi D, Etoa FX, Lohoue PJ (2007) In vitro antifungal activity of extract and plumbagin from the stem bark of Diospyros crassiflora Hiern (Ebenaceae). Phytother Res 21:671–674
Jeon JH, Kim YK, Lee SG, Lee GH, Lee HS (2011) Insecticidal activities of a Diospyros kaki root-isolated constituent and its derivatives against Nilaparvata lugens and Laodelphax striatellus. J Asia Pac Entomol 14:449–453
Park JH, Shim KS, Lee HS (2014) Antimicrobial activities of 2,6-dimethoxy-1,4-benzoquinone and its structurally related analogues against seven food-borne bacteria. J Korean Soc Appl Biol Chem 57:699–701
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2016R1A2A2A05918651).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, JH., Lee, HS. In vivo fungicidal properties of Diospyros kaki-isolated compound and its analogues. Appl Biol Chem 61, 383–388 (2018). https://doi.org/10.1007/s13765-018-0369-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-018-0369-1