Abstract
Ginger (Zingiber officinale) essential oil (ZOE) possesses strong antibacterial and antifungal activities. In this study, the antifungal activity of ZOE against Aspergillus flavus was investigated, and a chemical analysis was carried out to identify compounds that control fungal growth. A total of 37 compounds were identified by gas chromatographic analysis with a mass detector, and the antifungal and antiaflatoxigenic properties of three constituents, γ-terpinene, isoborneol, and citral, against A. flavus were tested. All compounds exhibited strong antifungal activity at 1000 μg/mL, and the antifungal activity of γ-terpinene and citral remained until treatment with tenfold diluted solution. The decrease in aflatoxin production by the three compounds was observed until treatment with 10 μg/mL. To evaluate their antiaflatoxigenic activity, RT-qPCR was used to compare the expression of 11 genes involved in aflatoxin biosynthesis by A. flavus. Among the three compounds, γ-terpinene and citral markedly reduced the expression of most of the tested genes but a different pattern of downregulation of the expression was observed. γ-Terpinene did not downregulate aflR, aflS, and yap, whereas citral did not alter the expression of aflC and aflG. Therefore, γ-terpinene and citral may have the potential to control A. flavus growth and aflatoxin production in agricultural products, including at the storage stage.
Similar content being viewed by others
Introduction
The phytochemicals in ginger (Zingiber officinale) can control viruses, including Feline calicivirus, and bacteria [1,2,3]. The major constituents of fresh ginger are phenol derivatives such as gingerols, which are converted into shogaols during heat treatment [4]. These natural compounds exhibit strong pharmacological activities, such as lowering of lipid and glucose content in the blood [5], antiplatelet activity by decreasing production of platelet thromboxane-B2 [6], and anti-inflammatory effects [7].
In a previous study, Zingiber officinale essential oil (ZOE) was extracted by steam distillation and its constituents were identified by gas chromatography with a mass detector (GC–MS) [8]. ZOE showed antibacterial activity against eight pathogenic bacteria (125–500 μg/mL) and antifungal activities against two fungal strains (250 μg/mL) [9]. They identified sabinene, (E)-1-(3′,4′-dimethoxyphenyl)buta-1,3-diene, terpinen-4-ol, γ-terpinene, and β-phellandrene as the major constituents of ZOE among the 49 compounds determined [9]. In addition, ZOE exhibited antibacterial activity against bacteria isolated from fish and shellfish [10]. ZOE also inhibited reproduction of the cattle tick Rhipicephuls microplus [11].
Aspergillus flavus produces cancer-stimulating compounds, such as aflatoxins. Birds are particularly susceptible to these natural compounds [12, 13]. Selenium and zinc can neutralize aflatoxin toxicity in poultry through dietary supplementation [13]. Interestingly, many essential oils have been used as reducing agents against aflatoxin contamination by A. flavus or A. parasiticus in agricultural products [14, 15]. The direct inhibition of aflatoxin biosynthesis in A. flavus or indirect inhibition pathways that reduce the respiration ability in aflatoxin-producing fungi are responsible for the antiaflatoxigenic properties [16].
Recently, we identified natural compounds containing a methylenedioxy moiety that exhibit antifungal and antiaflatoxigenic activities against A. flavus by direct inhibition of aflatoxin production through the suppression of certain major genes involved in the biosynthesis [17, 18]. 4-Hydroxy-7-methyl-3-phenylcoumarin also possessed similar inhibitory properties against A. flavus by strongly suppressing aflK-expressing versicolorin B synthase [19].
In the present study, the antifungal activity of ZOE against A. flavus was investigated, and a chemical analysis was performed using GC–MS. The antifungal and antiaflatoxigenic activities of the three compounds, which are easily available in the market, against A. flavus were evaluated. Finally, the modes of antiaflatoxin action of the three compounds were evaluated and confirmed by RT-qPCR by comparing the gene expression after treatment.
Materials and methods
Chemicals and microorganisms
ZOE was purchased from Lhasa Karnak Herb Company (Berkeley, CA, USA). Tested γ-terpinene, isoborneol, and citral were also purchased from Sigma-Aldrich Co. (St. Louise, MO, USA). Aspergillus flavus ATCC 22546 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA).
Preparation of spore solution
For the preparation of the isolate, A. flavus was grown on malt extract agar (MEA: Difco Laboratories, Sparks, MD, USA) and this isolate generated aflatoxin B1 and B2, but not G1 and G2 [19]. It was grown on MEA medium at 30 °C for 5 days until fungal spores formed. After spore formation, they were collected from slants by shaking with 0.05% (v/v) of Tween 80 and stored at − 70 °C in 20% glycerol solution (v/v).
Chemical analysis of ZEO using GC–MS
Chemical analysis for ZOE was undertaken using a HP-6890 (Agilent Technologies, Wilmington, DE, USA) gas chromatograph equipped with a DB-5 fused silica capillary column (60 m × 0.25 mm, 0.25 μm film thickness) interfaced with a 59,731 V (Agilent) mass detector. The GC–MS was connected to a computer equipped with Wiley 7 spectra library. The condition of GC–MS was column temperature ranged from 60 to 250 °C with an elevation of 5 °C. Injector temperature was set at 250 °C, and injection volume was 1.0 μl with a split ratio of 1:50. The determined mass range was 50–600 amu. Compounds were identified by comparing their mass spectra with those in Wiley 7 mass spectral database for GC–MS (Table S1).
Aflatoxin analysis using HPLC-fluorescence detector
Fungal spore suspension adjusted to 106 density was inoculated into the liquid culture media comprising potato dextrose broth (25 mL) (Difco Laboratories). All the test compounds were spiked into the corresponding liquid media serially, and the culture was incubated at 25 °C for 5 days under shaking conditions. All experiments were performed in triplicate for each concentration of the tested compounds.
Following cultivation in liquid medium for 5 days, the fungal growth was measured using a filter paper to weigh the mycelial and sclerotial residues with overnight drying in an oven. Separately, the mycelia from each treatment were subjected to the extraction procedure using an ultrasonic cleaner, and aflatoxin B (AFB) and G were analyzed using an HPLC-fluorescence detector [19]. The average of the three replicates was calculated for each experiment. One-way ANOVA was used for comparisons between two groups, and statistical significance was set at p < 0.05 [20].
Real-time qPCR after isolation of total RNA
To understand the inhibition of fungal growth and aflatoxin production, real-time qPCR was employed. Fungal mycelia in liquid media were carefully collected and total RNA was extracted using the QIAzol Lysis reagent, supplied by QIAGEN Inc. (Dusseldorf, Germany), after grinding to a fine powder under addition of liquid nitrogen. Total RNA extracted from the treated fungi were quantified with μDrop™ Plate (Thermo Fisher Scientific Inc., Waltham, MA, USA), and the extracted RNAs were quantified using 1% of agarose gel with ethidium bromide. Complementary DNA (cDNA) for extracted RNAs (2 μg) was synthesized using Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
RT-qPCR was performed using Rotor-Gene SYBR Green PCR Kit (QIAGEN Inc.) with an appropriate amount of cDNA (100 ng). Primers for genes, such as yap, aflR, aflS, aflK, aflD, aflQ, and 18S rRNA, were synthesized by Genotech (Daejeon, Korea) (Table S2) and used to understand the relationship between aflatoxin biosynthesis and the active compound [18]. Forty cycles of thermal cycling were performed for amplification (denaturation at 95 °C for 30 s, annealing at 60 °C for 20 s, and elongation at 72 °C for 30 s, followed by an additional step at 95 °C for 5 min). RT-qPCR was performed in triplicate for each treatment. Significant differences in gene expression were calculated using delta/delta Ct methods [21]. Data were standardized with 18S rRNA, and the gene expression between the treatment group and controls was compared.
Results
The components of ZEO were identified by GC–MS analysis (Table S1). After the analysis, γ-terpinene, isoborneol, and citral were chosen to investigate their inhibitory effects on the growth of A. flavus and aflatoxin production with the assessment of their molecular modes of inhibitory action.
The three major components of ZEO showed a strong inhibitory effect against A. flavus growth at 1000 μg/mL. This inhibition by isoborneol decreased approximately twofold when the fungi were treated with 250 μg/mL of ZEO and disappeared at 100 μg/mL. γ-Terpinene exhibited 50% inhibitory effect on the fungal growth at 100 μg/mL, but this effect was not observed at 10 μg/mL. Citral exhibited a similar inhibitory pattern as the other two monoterpenes (Table 1).
Aflatoxin production after treatment with the three compounds was determined (Table 2), and the three compounds completely inhibited aflatoxin biosynthesis at 100 μg/mL. At 10 μg/mL, γ-terpinene and citral showed some antiaflatoxigenic activities.
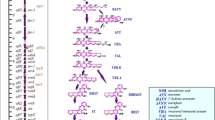
To understand the inhibitory effect of γ-terpinene on aflatoxin production, the expression of 11 genes, which are involved in aflatoxin biosynthesis, was analyzed by RT-qPCR (Fig. 1). The expression of aflC, aflD, aflE, aflK, aflO, and aflQ genes was inhibited by approximately 80% compared with that of the control at 100 μg/mL. However, aflR, aflS, and yap were not affected by the treatment (Fig. 1). At tenfold dilution, γ-terpinene downregulated only five genes by approximately 50% and upregulated aflG gene approximately threefold (Fig. 1).
Expression of genes involved in aflatoxin biosynthesis in Aspergillus flavus after the treatment of γ-terpinene. (A), aflC; (B), aflD; (C), aflE; (D), aflG; (E), aflK; (F), aflL; (G), aflO; (H), aflQ; (I), aflR; (J), aflS; (K), yap. 18S rRNA was used for the standardization of gene expression. Different letters indicate significantly different from the control group (p < 0.05)
Citral downregulated the expression of most of the tested genes, except aflC and aflG, at 100 μg/mL (Fig. 2). However, the inhibition was higher at 10 μg/mL than at 100 μg/mL. In addition, aflR was the only gene to be more strongly inhibited by citral at 100 μg/mL.
Expression of genes involved in aflatoxin biosynthesis in Aspergillus flavus after the treatment of citral. (A), aflC; (B), aflD; (C), aflE; (D), aflG; (E), aflK; (F), aflL; (G), aflO; (H), aflQ; (I), aflR; (J), aflS; (K), yap. 18S rRNA was used for the standardization of gene expression. Different letters indicate significantly different from the control group (p < 0.05)
Discussion
In a previous study, ZEO exhibited antifungal activity against A. flavus and antiaflatoxigenic activity [22]; the mycelial growth of A. flavus was completely inhibited at 150 μg/mL. The major components in ZEO are monoterpenes such as β-phellandrene (0.95%), camphene (0.61%), and β-pinene (0.61%) as well as sesquiterpenes such as zingiberene (29.54%), β-sesquiphellandrene (18.42%), and germacrene D (3.58%) (Onyenekwe and Hashimoto, 1999). However, higher amounts of monoterpenes such as camphene (3.0%), β-phellanfrene (1.4%), 1,8-cineole (1.9%), borneol (2.1%), neral (7.4%), and geranial (25.9%) and lower amounts of sesquiterpenes such as zingiberene (9.5%), β-sesquiphellandrene (5.1%), and germacrene B (0.3%) in ZEO have also been reported (Singh et al., 2008). In our study, γ-terpinene (1.9%), campene (17.2%), shisool (19.86%), citral (3.41%), γ-curcumene (3.03%), and ar-curcumene (6.16%) were the main volatile components extracted from ZEO (Table S1). ZEO contained 0.22% zingiberene, and its proportion was much lower than that reported earlier [23, 24].
Compositional difference in essential oils extracted from the same plant sources may be due to the extraction methods for essential oils [25]. They used two different extraction methods, hydrodistillation and supercritical fluid extraction (SCFE), to obtain essential oils from cumin. Then, the authors analyzed these two essential oils by GC–MS [25]. Cumin essential oil obtained by hydrodistillation had a higher proportion of cuminaldehyde (52.6%) than that in the essential oil (37.3%) obtained by SCFE extraction method. In the case of cuminic alcohol, the essential oil obtained by SCFE contained higher portions than that by hydrodistillation [25]. In addition, the essential oil composition may differ with weather condition during cultivation, e.g., guava cultivated during the dry season primarily comprises nerolidol [26]. Finally, one of primary parameters for the difference in composition may be related to the plant chemo-type [27]. Therefore, essential oils, including ZEO, need to be standardized before use in the agricultural or other industries [28, 29].
The antifungal activity and antiaflatoxigenic activities of three monoterpenes, γ-terpinene, isoborneol, and citral, against A. flavus were evaluated in this study. γ-Terpinene is a major component of several essential oils that are known to inhibit Aspergillus spp. growth and aflatoxin production [22, 30, 31]. However, its antifungal and antiaflatoxigenic activities against A. flavus have not been evaluated yet. Isoborneol has not been reported to exhibit antifungal and antiaflatoxigenic activities against A. flavus. In our study, γ-terpinene and isoborneol strongly inhibited A. flavus growth at 1000 μg/mL and aflatoxin production at 100 μg/mL. However, their mode of action in terms of the downregulation pattern of aflatoxin biosynthesis genes was different. γ-Terpinene downregulated 7 genes at 100 μg/mL, while isoborneol did not downregulate genes at the same concentration. γ-Terpinene showed a similar pattern of gene downregulation as eugenol and cinnamaldehyde, which inhibited aflatoxin production by modifying the major genes for aflatoxin biosynthesis in A. flavus [32]. Another aflatoxin-producing fungi, A. parasiticus, has been used to study the inhibitory effect of eugenol on its growth and aflatoxin production [33]. Eugenol downregulated aflatoxin biosynthetic genes ver-1, nor-1, pks A, omt A, and aflR at 62.5–125 μg/mL [33]. ver-1 is equivalent to aflM in this study, nor-1 to aflD, pksA to aflC, and omtA to aflP [34]. In our study, the expression of aflC was only 20% in the presence of 100 μg/mL of γ-terpinene. aflD was downregulated approximately twofold and fivefold in the presence of 10 and 100 μg/mL of γ-terpinene, respectively. However, the expression of aflR was not affected. Therefore, the inhibition of major genes involved in the biosynthesis of aflatoxins in A. flavus contributes toward the inhibitory action of γ-terpinene.
In the case of isoborneol, aflK, aflL, and aflQ were downregulated approximately twofold at 10 μg/mL. It is likely that isoborneol possesses a different mode of inhibitory action on aflatoxin production, suggested by the penetration of H2O2 generated after treatment with isoborneol [35].
In a previous study, citral showed antifungal and antiaflatoxigenic activities against A. flavus growth and aflatoxin biosynthesis, respectively [32]. They demonstrated that at 2.80 mmol/L, citral completely inhibited A. flavus growth and aflatoxin production [32]. It also downregulated aflT completely and inhibited the expression of aflM, aflP, aflL, aflR, and aflD 257-, 29-, 3.5-, and 2.5-fold compared with the control group. In our study, citral treatment downregulated aflC, aflD, aflE, aflG, aflK, aflL, aflO, aflQ, aflR, aflS, and Yap in A. flavus. All the tested genes were affected by the addition of 10 μg/mL citral (Fig. 3). We did not use aflT in this study. However, our results were similar to those reported [32] that aflL, aflR, and aflD were downregulated following treatment with citral. This indicates that citral inhibits aflatoxin production in A. flavus by interfering with the expression of aflatoxin biosynthesis genes. In addition to the downregulation of the expression of aflatoxin-producing genes, another inhibitory mechanism has been suggested—the secretion of H2O2 after treatment with citral [35]. With these monoterpenoids, other major constituents such as turmerone from turmeric essential oil may also need to determine their antifungal and antiaflatoxigenic activities to A. flavus to develop ecofriendly used fungicides in agricultural fields [36].
Expression of genes involved in aflatoxin biosynthesis in Aspergillus flavus after the treatment of isoborneol. (A), aflC; (B), aflD; (C), aflE; (D), aflG; (E), aflK; (F), aflL; (G), aflO; (H), aflQ; (I), aflR; (J), aflS; (K), yap. 18S rRNA was used for the standardization of gene expression. Different letters indicate significantly different from the control group (p < 0.05)
Conclusively, three natural compounds (isoborneol, γ-terpinene, and citral) in ZOE showed antifungal and antiaflatoxigenic activities against A. flavus. Aflatoxin production by A. flauvs was strongly inhibited at 100 μg/mL of γ-terpinene and citral. These two compounds downregulated the primary genes involved in aflatoxin biosynthesis in A. flavus at 10 μg/mL. However, their inhibitory action on gene expression was different, and γ-terpinene suppressed aflC, aflD, aflE, aflK, aflO, and aflQ genes, which express polyketide synthase, reductase, NOR-reductase, VERB synthase, O-methyltransferase B, and oxidoreductase, respectively. Citral downregulated nine genes among the tested genes, except aflC and aflG, which express polyketide synthase and P450 monooxygenase, respectively. Isoborneol did not suppress any of the tested genes. The different inhibitory patterns of gene expression in A. flavus warrant further studies.
References
Hasan S, Danishuddin M, Khan AU (2015) Inhibitory effect of Zinziber officinale towards Streptococcus mutans virulence and caries development: in vitro and in vivo studies. BMC Microbiol 15:1
Aboubakr HA, Nauertz A, Luong NT, Agrawal S, El-Sohaimy SA, Youssef MM, Goyal SM (2016) In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J Food Prot 79:1001–1012
Chitra K, Manikandan A, Antony SA (2016) Effects of poloxamer of Zingiber officinale extracted green synthesis and antibacterial studies of silver nanoparticle. J Nanosci Nanotechnol 16:758–764
Wohlmuth H, Leach DN, Smith MK, Myers SP (2005) Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe). J Agric Food Chem 53:5772–5778
Kadnur SV, Goyal RK (2005) Beneficial effects of Zingiber officinale Roscoe on fructose induced hyperlipidemia and hyperinsulinemia in rats. Indian J Exp Biol 43:1161–1164
Thomson M, Al-Qattan KK, Al-Sawan SM, Alnaqeeb MA, Khan I, Ali M (2002) The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids 67:475–478
Grzanna R, Lindmark L, Frondoza CG (2005) Ginger–a herbal medicinal product with broad anti-inflammatory actions. J Med Food 8:125–132
Gupta S, Pandotra P, Ram G, Anad R, Gupta AP, Husain K, Bedi YS, Mallavarapu GR (2011) Composition of a monoterpenoid-rich essential oil from the rhizome of Zingiber officinale from north western Himalayas. Nat Prod Commun 6:93–96
Verma RS, Joshi N, Padalia RC, Singh VR, Goswami P, Verma SK, Iqbal H, Chanda D, Verma RK, Darokar MP, Chauhan A, Kandwal MK (2017) Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. J Sci Food Agric 98:321–327
Snuossi M, Trabelsi N, Ben Taleb S, Dehmeni A, Flamini G, De Feo V (2016) Laurus nobilis, Zingiber officinale and Anethum graveolens essential oils: composition, antioxidant and antibacterial activities against bacteria isolated from fish and shellfish. Molecules 21:1414
Pazinato R, Volpato A, Baldissera MD, Santos RC, Baretta D, Vaucher RA, Giongo JL, Boligon AA, Stefani LM (2016) In vitro effect of seven essential oils on the reproduction of the cattle tick Rhipicephalus microplus. J Adv Res 7:1029–1034
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG (2017) Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol 7:2170
Mughal MJ, Peng X, Kamboh AA, Zhou Y, Fang J (2017) Aflatoxin B1 induced systemic toxicity in poultry and rescue effects of selenium and zinc. Biol Trace Elem Res 178:292–300
Hu Y, Zhang J, Kong W, Zhao G, Yang M (2017) Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem 220:1–8
Kedia A, Dwivedy AK, Pandey AK, Kumar RR, Regmi P, Dubey NK (2015) Efficacy of chemically characterized Foeniculum vulgare Mill seed essential oil in protection of raw tobacco leaves during storage against fungal and aflatoxin contamination. J Appl Microbiol 119:991–998
Sakuda S, Yoshinari T, Furukawa T, Jermnak U, Takagi K, Limura K, Yamamoto T, Suzuki M, Nagasawa H (2015) Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci Biotechnol Biochem 80:43–54
Moon YS, Choi WS, Park ES, Bae IK, Choi SD, Paek O, Kim SH, Chun HS, Lee SE (2016) Antifungal and antiaflatoxigenic methylenedioxy-containing compounds and piperine-like synthetic compounds. Toxins 8:E240
Choi H, Lee BH, Moon YS, Kim K, Lee HS, Lee SE (2017) Antifungal and antiaflatoxigenic effects of a fumigant, ethanedinitrile, on Aspergillus flavus. Appl Biol Chem 60:631–636
Moon YS, Kim L, Chun HS, Lee SE (2017) 4-hydroxy-7-methyl-3-phenylcoumarin suppresses aflatoxin biosynthesis via downregulation of alfK expressing versicolorin B synthase in Aspergillus flavus. Molecules 22:E712
SAS (2001) SAS User’s Guide, 4th edn. SAS Institute, Cary, NC
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinform Biomath 3:71–85
Nerilo SB, Rocha GHO, Tomoike C, Mossini SA, Grespan R, Mikcha JMG, Machinski M Jr (2016) Antifungal properties and inhibitory effects upon aflatoxin production by Zingiber officinale essential oil in Aspergillus flavus. Int J Food Sci Technol 51:286–292
Onyenekwe PC, Hashimoto S (1999) The composition of the essential oil of dried Nigerian ginger (Zingiber officinale Roscoe). Eur Food Res Technol 209:407–410
Singh G, Kapoor IPS, Singh P, de Heluani CS, de Lampasona MP, Catalan CAN (2008) Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem Toxicol 46:3295–3302
Saha S, Walia S, Kundu A, Sharma K, Singh J, Tripathi B, Raina A (2016) Compositional and functional difference in cumin (Cuminum cyminum) essential oil extracted by hydrodistillation and SCFE. Cogent Food Agric 2:1143166
Shaabana HAE, El-Ghoraba AH, Shibamoto T (2012) Bioactivity of essential oils and their volatile aroma components: review. J Essent Oil Res 24:203–212
Tucker AO, Maciarello MJ, Landrum LR (1995) Volatile leaf oils of American Myrtaceae III. Psidium cattleianum Sabine, P. friedrichsthalianum (Berg) Niedenzu, P. guajava L., Psidium guineense Sw., and Psidium sartorianum (Berg) Niedenzu. J Essent Oil Res 7:187–190
Rantzsch U, Vacca G, Duck R, Gillissen A (2009) Anti-inflammatory effects of Myrtol standardized and other essential oils on alveolar macrophages from patients with chronic obstructive pulmonary diseases. Eur J Med Res 14(Suppl 4):205–209
Papamaroupa M, Gillissen A (2016) Is Myrtol® standardized a new alternative toward antibiotics? Pharmacogn Rev 10:143–146
Tian J, Ban X, Zeng H, Huang B, Wang Y (2011) Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int J Food Microbiol 145:464–470
Kohiyama CY, Ribeiro MMY, Mossini SAG, da Silva Bomfim N, Nerilo SB, Rocha GHO, Grespan R, Mikcha JMG, Macinski M Jr (2015) Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L by Aspergillus flavus Link. Food Chem 173:1006–1010
Liang D, Xing F, Selvaraj JN, Liu X, Hua H, Zhou L, Zhao Y, Wang Y, Liu Y (2015) Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin biosynthetic gene expression and aflatoxin B1 biosynthesis in Aspergillus flavus. J Food Sci 80:M2917–M2924
Janhanshiri Z, Shams-Ghahfarokhi M, Allameh A, Razzaghi-Abyaneh M (2015) Inhibitory effect of eugenol on aflatoxin B1 production in Aspergillus parasiticus by downregulating the expression of major genes in the toxin biosynthetic pathway. World J Microbiol Biotechnol 31:1071–1078
Yu J, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW (2004) Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol 70:1253–1262
Li J, Li J, Lu Z, Liu Y, Li CM (2015) Transient transmembrane secretion of H2O2: a mechanism for the citral-caused inhibition of aflatoxin production from Aspergillus flavus. Chem Commun 51:17424–17427
Hwang KW, Son D, Jo HW, Kim CH, Seong KC, Moon JK (2016) Levels of curcuminoid and essential oil compositions in tumerics (Curcuma longa L) grown in Korea. Appl Biol Chem 59:209–215
Acknowledgments
This research was supported by Kyungpook National University Bokhyeon Research Fund (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Young-Sun Moon, Hoi-Seon Lee and Sung-Eun Lee authors have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moon, YS., Lee, HS. & Lee, SE. Inhibitory effects of three monoterpenes from ginger essential oil on growth and aflatoxin production of Aspergillus flavus and their gene regulation in aflatoxin biosynthesis. Appl Biol Chem 61, 243–250 (2018). https://doi.org/10.1007/s13765-018-0352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-018-0352-x