Abstract
The removal of fruit cluster leaves was shown to be a valuable method for controlling fruit quality and producing high-grade apples. A chemical defoliant that functions as an activation switch was shown to turn on the genes necessary for fruit cluster leaf defoliation. Elucidating the mechanism involved in leaf defoliation is crucial to our understanding of the use of chemical defoliants in fruit trees. To gain insight into chemical defoliant-mediated leaf defoliation, we first confirmed the occurrence of ethylene production by applying a chemical defoliant on fruit cluster leaves. Then, we used RNA-seq analysis to obtain a series of transcriptome profiles for genes and proteins involved in leaf senescence induction. Within 2 days of applying the chemical defoliant to apple trees, 1-aminocyclopropane carboxylic acid (ACC) oxidase, ACC synthase, a chlorophyll binding protein, and polygalacturonase-related genes were up-regulated at least sixfold. An in vitro enzyme assay showed that lanolin oil activates ACC synthase activity, a key regulatory enzyme in the ethylene pathway. We also showed that chemical defoliant decreased the light saturation point and total chlorophyll content. Then, we used a polygalacturonase activity assay to confirm the effects of chemical defoliant on leaf senescence in vivo. Furthermore, treatment with chemical defoliant resulted in a significant increase in the chromaticity value of a*, whereas L* and b* decreased in the apple fruit. Taken together, we conclude that chemical defoliant could selectively affect fruit cluster leaves, which suggested that it can be used as a selective defoliant.
Similar content being viewed by others
Introduction
Apples are the most common fruit in Korea. Apple quality can be determined objectively by measuring the physical and/or chemical components that affect the fruit. Before the year 2000, the weight and size of an apple were two of the most important factors used by consumers when selecting apples, but after 2000, the selection of table apples and the determination fruit quality depended on other factors. These were, in the order color > shape > size.
As the antioxidant effect of red apples became known to consumers (Wolfe et al. 2003), preference based on fruit color increased. The anthocyanin content, the main factor involved in fruit color, can be enhanced by increased sugar content, temperature, nitrogen, and exposure to light (Vestrheim 1970; Westwood 1993). Therefore, people started to use certain cultivation practices, such as tree architecture, removing excess branches, intentionally defoliating leaves near growing fruit, turning fruit, and using reflectors, to increase anthocyanin accumulation. Of these cultivation practices, removing leaves near the fruit is considered to be the most efficient and direct way to improve fruit color quality (Choi et al. 2000).
In Korea, removing fruit cluster leaves has long been used as a management method for apple color improvement. It has been shown to control fruit quality and to increase the production of high-quality apple fruits (Choi et al. 2000). Most apple orchard managers use hand-stripping procedures to improve fruit color before harvesting, and it is well known that this cultivation practice significantly improves fruit color (Yim and Lee 1999). The hand-stripping of ‘Fuji’ apple trees is usually carried out 30–40 days before harvest. However, if there is too much defoliation, then the size and color of the fruit will decrease in future harvesting seasons and the flowering and early growth of apple trees will be negatively affected for several years (Choi et al. 2000). In addition, hand-stripping leaves causes direct physical damage and nutrient loss. Additionally, other problems are the high labor costs associated with hand-stripping and lack of people to undertake the task. Therefore, it is crucial to develop a spraying method by using chemical defoliants, so that the negative physical effects on apple trees and labor costs can be reduced.
Recently, many countries have used chemical defoliants. For example, 1,2,4-tributylphosphortrithioat (tribufos), N-phenyl-N′-1,2,3-triadiazol-5-yl urea (thidiazuron), 2,3-dihydro-5,6-dimethyl-1,4-dithiin 1,1,4,4-tetraoxide (dimethipin), and 2-chloroethylphosphonic acid (ethephon) have been used to improve cotton productivity and quality (Snipes and Cathey 1992). In Korea, some researchers (Kim and Yun 1971) carried out a chemical defoliant experiment where ‘Ralls Janet’ apple trees were sprayed with JON COLOR (2,3,5-triiodobenzoic acid as the main ingredient) and Pre-N. In addition, during the 1960s, a thinning experiment by using a chemical thinner was carried out using ‘Ralls Janet’ and ‘Jonathan’ cultivars in Japan (Yim et al. 2000). Previous research also reported the effect of natural abscisic acid (ABA) on defoliation and its influence on apple color (Yim and Lee 1999). Although 2,3,5-triiodobenzoic acid was shown to be effective in defoliation experiments, the chemical is not registered for use as a defoliant on fruit trees because of safety issues, and natural ABA is too expensive to use in most orchards.
Recently, we tested a chemical defoliant that can defoliate fruit cluster leaves. However, the mechanisms underlying leaf defoliation in plants are unclear. In this study, we hypothesized that JEOKYEOPSON (chemical defoliant; DaewonAgro, Gyeongbuk, Korea) functions as an ethylene biosynthesis, which is known to be involved in leaf senescence (Taiz and Zeiger 2002) and is a transcriptional activator of polygalacturonase (PG), which has been shown to be associated with leaf abscission; PG activity accompanies leaf abscission (Taylor et al. 1990; Gonzalez-Carranza et al. 2002). In this study, we provide experimental evidence to support these hypotheses.
Materials and methods
Plant materials and chemical defoliant treatment
‘Fuji’ apple fruit and leaf samples were collected from an experimental field at Kyungpook National University (Gunwi, Korea). The apple trees used in this study were 10 years old. Their rootstock was M9, their planting density was 1.5 m, and they displayed similar vigor, and produced about the same number of fruit. The cultivation practices were the same as those undertaken in commercial apple orchards. We used commercial chemical defoliant for the experiment. The chemical defoliant, JEOKYEOPSON (DaewonAgro, Korea), is composed with 0.05 % soluble boron, 0.0005 % soluble molybdenum, and lanolin oil (main ingredient) mixture. The chemical defoliant was first diluted by 1/500. Then, the diluted chemical defoliant was applied at a rate of 4 L per tree in mid-September, 2015 using an engine sprayer. The trees in the control treatment were only sprayed with water.
Measurement of the defoliation rate
We defined the fruit cluster leaves (FCL) as those that were located within 20 cm either side of the fruit petiole and the other leaves on a branch were defined as stem leaves (SL). The average length of the branches used in the experiment was 1.3 m, and the average number of leaves per branch was approximately 13 (FCL) and 287 (SL). The defoliation rate for FCL was defined as the number of defoliated FCL compared to the number of whole FCL on each branch; and for SL, it was defined as the number of defoliated SL compared to the number of whole SL on each branch. Thirty branches from five trees (six branches per tree) were used in the experiment, and the defoliation rate was evaluated 10 days after the chemical defoliant spray had been applied.
Ethylene production assay
We sampled FCL and SL treated with the chemical defoliant at 0, 1, and 2 days after treatment. These leaves were immediately put into glass test tubes, which were then capped with a rubber septum and incubated at 25 °C. The ethylene produced by the leaves was determined by withdrawing a 1 mL headspace gas sample and injecting it into a gas chromatograph (GC2010, Shimadzu, Kyoto, Japan) equipped with a Porapak Q column (80/100 2 m, Youngin Frontier, Seoul, Korea) and a flame ionization detector. The temperatures of the injector, oven, and detector were 100, 90, and 200 °C, respectively. The flow rates of the He carrier, H2, and air were 25, 40, and 400 mL per min, respectively (Yoo et al. 2015). A total of 60 leaves (20 leaves from three trees) were sampled and we used one tree as one biological replicate.
RNA extraction, sequencing, and analysis
We collected 60 leaves at 2nd day after treatment with water (control) or the chemical defoliant. Twelve leaves from five trees were sampled from the FCL and SL. Whole FCL and SL were used to extract total RNA with an RNeasy Plant Mini Kit (Qiagen, Düsseldorf, Germany). Libraries for Illumina sequencing were constructed using a TruSeq Stranded mRNA Sample Prep Kit (Illumina, San Diego, CA, USA) following the manufacturer’s protocol. RNA sequencing was performed on an Illumina HiSeq 2500 System using paired-end 100-bp sequencing. Four lanes of a flow-cell were used, one for each treatment. These yielded 54,374,906, 73,781,952, 69,454,932, and 83,635,398 total sequence reads for the control FCL, FCL treated with defoliant, control SL, and SL treated with defoliant, respectively. Sequence data for the reference genome (Malus domestica) were retrieved from the NCBI database. Quality-filtered reads were aligned to the reference-genome sequence using Bowtie2. Relative transcript abundance was measured in fragments per kilobase of transcript per million mapped reads. Visualization of the mapping results and the differentially expressed gene (DEG) analysis was carried out using the CLRNASeqTM program (ChunLab, Seoul, Korea). Two biological replicates were used in the experiment.
Measurement of the light saturation point
The light saturation point was determined according to Satou et al. (2014) with slight modifications. At 0 and 6 days after treatment with the chemical defoliant, the light saturation points of the FCL and SL were measured using a MINI-PAM Photosynthesis Yield Analyzer (Walz, Effeltrich, Germany) in lighting curve mode.
Total chlorophyll assay
The total chlorophyll (chlorophyll a and b) content of the ‘Fuji’ apple leaves was determined according to Arnon (1949) and Lichtenthaler (1987) with slight modifications. The leaves were ground into a fine powder in liquid nitrogen, homogenized with 80 % acetone, and then extracted twice with 80 % acetone. The supernatants were collected by centrifugation at 12,000×g for 15 min at 4 °C. The total chlorophyll content was calculated by measuring the absorbance at 663 and 645 nm.
Polygalacturonase (PG) activity and protein assay
The crude enzyme was extracted from FCL and SL at 0, 2, and 8 days after treatment with the chemical defoliant spray. The enzyme activity assay was performed according to Kuwar et al. (2015). Briefly, all steps were performed at 4 °C. Aliquots of the leaves were ground in liquid nitrogen and homogenized in 50 mM 2-(4-(2-hydroxyethyl) piperazin-1-yl) ethanesulfonic acid (HEPES) buffer (pH 7.5) containing 1 mM ethylenediaminetetraacetic acid, 5 mM dithiothreitol, 1 mM MgCl2, and 0.5 mM phenylmethanesulfonyl fluoride using a mortar and pestle at the ratio of 1:10 (w/v). The homogenate was centrifuged at 12,000×g for 15 min at 4 °C. The clear supernatant was used to determine enzyme activity and perform the protein assay. PG activity was assayed according to Deng et al. (2005) with modifications. The reaction mixture contained 200 mM Na-acetate buffer (pH 4.5) and a 1 % (w/v) solution of citrus pectin containing 0.6 % (w/v) sodium chloride and the crude enzyme in a total volume of 1 mL. The mixture was incubated at 37 °C for 1 h, followed by the addition of 3,5-dinitrosalicylic acid. Then the reaction was stopped by heating the sample in boiling water for 10 min. A blank was prepared for each sample by boiling the reaction mixture before the substrate was added. The concentration of the reducing groups was determined using d-galacturonic acid as a standard after measuring the absorbance at 540 nm. Protein content was assayed according to Bradford (1976).
1-Aminocyclopropane-1-carboxylate (ACC) synthase activity assay
All constructs used in this study were verified by DNA sequencing. Full-length ACC synthase (At4g08040) cDNA was amplified from Arabidopsis leaf cDNA by PCR using the primers 5′-CGCGGATCCATGTTGTCAAGCAAAGTTGTTG-3′ (forward) and 5′-GTGCTCGAGACGTTCTGATTCACAAGTAACAGA-3′ (reverse). The PCR product was cloned into a pET28a (+) vector. The vector construct was introduced into E. coli Rosetta-gami (DE3). ACC synthase activity was assayed according to Boller et al. (1979) and Kende and Hanson (1976) with slight modifications. The assay mixture contained recombinant ACC synthase, 600 mM HEPES buffer (pH 8.0), and 0.5 % (final concentration) lanolin oil (the main ingredient of JEOKYEOPSON) or water (control). The assay mixture was incubated at 30 °C for 3 h followed by the addition of 2 mM S-adenosyl-l-methionine (SAM) as a substrate. After the incubation period, the reaction was stopped by the addition of 100 mM sodium phosphate buffer (pH 11.5) and 1 M NaBH4 prepared in 100 mM sodium phosphate buffer (pH 11.5). Then, the mixture was incubated at 30 °C for 10 min followed by boiling water for 10 min. After cooling, 10 mM pyridoxal phosphate in 100 mM sodium phosphate buffer (pH 11.5), 20 mM MnCl2, and 500 mM H2O2 were added. The tube was thoroughly shaken, closed with a rubber septum, and placed on a horizontal shaker (250 cycle min−1) for 1 h at 25 °C. After 1 h, 1 mL samples were withdrawn from the gas phase for ethylene determination. For each enzyme assay, tests with blank samples (water) and samples containing the standards were performed in parallel.
Measurement of fruit color
The fruit color intensity was measured using a CR-400 Chroma Meter (Konica Minolta, Inc., Japan). Forty ‘Fuji’ apples from each treatment (water control and chemical defoliant) were harvested on October 26, 2015, and the fruit color intensity was the average of three measurements taken from the equatorial region of the fruit. Differences were considered significant at p < 0.05.
Results
Effect of the chemical defoliant on fruit cluster leaves
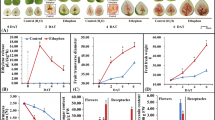
The functional mechanism by which the chemical defoliant triggers FCL defoliation was investigated in mid-September, 2015 in Gunwi, Korea. A chemical defoliation test was carried out on FCL and SL from 30 branches of five, 10-year-old ‘Fuji’ apple trees (six branches per tree). Within 8 d of the chemical defoliant spray, FCL had begun to yellow, but SL remained green (Fig. 1). The defoliation rate for FCL was 42.1 %, whereas it was 16.3 % for SL (Fig. 2). The control (water only) test did not show any FCL and SL defoliation (Fig. 1A). These results indicated that the defoliation was caused by the chemical defoliant spray. We then performed a time course GC analysis to examine changes in ethylene production. In FCL, ethylene production was induced as early as 2nd day after the chemical defoliant spray, but ethylene production was lower in SL (Fig. 3). These results indicated that chemical defoliant-induced FCL defoliation may be related to ethylene production.
Defoliation rates of FCL and SL in a ‘Fuji’ apple trees treated with the chemical defoliant. The defoliation rate was determined 10 days after the chemical defoliant spray. A total of 30 branches from five trees (six branches per tree) were used in the experiment. The average number of leaves per branch was approximately 13 (FCL) and 287 (SL). One tree represented one biological replicate. Error bars represent the standard deviation of five biological replicates
Changes in ethylene production of FCL and SL in ‘Fuji’ apple trees. The chemical defoliant treatment was carried out during mid-September, 2015, and all the foliar applications were made at the same time. A total of 60 leaves (20 leaves from three trees), were sampled. Ethylene production dramatically increased in a time-dependent manner following the chemical defoliant spray. Error bars represent the standard deviation of three biological replicates
Transcriptome analysis using RNA-seq identifies genes that are up- and down-regulated during fruit cluster leaf senescence
We performed whole transcriptome analysis using RNA-seq to identify genes differentially expressed during leaf senescence caused by the chemical defoliant spray. Based on the results of the ethylene production analysis (Fig. 3), we isolated FCL and SL total RNA from ‘Fuji’ apple leaves. The RNA was collected 2nd day after they had been sprayed with the chemical defoliant and/or water (control). A total of 33,242 transcripts were differentially expressed in response to the chemical defoliant spray (Fig. 4). In FCL treated with the chemical defoliant, DESeq 2 screening showed that 496 genes were up- or down-regulated sixfold compared to that of water control. Of these, 400 genes were up-regulated and 96 genes were down-regulated in FCL receiving the chemical defoliant spray (Fig. 5A). Many of the differentially expressed genes in FCL were implicated in ethylene biosynthesis and photosynthesis (Table 1). The list in Table 1 includes ACC oxidase-related genes, ACC synthase-related genes, chlorophyll a-b binding protein-related genes, and a polygalacturonase-related gene. For example, the ACC synthase-related gene (LOC103411726) was up-regulated more than 30.1-fold, whereas a chlorophyll a-b binding protein-related gene (LOC103450616) was down-regulated up to 12.7-fold. In SL, after treatment with the chemical defoliant spray, the DESeq 2 screening showed that 356 genes were up- or down-regulated threefold compared to that of water control. Of these, 334 genes were up-regulated and 22 genes were down-regulated (Fig. 5B), but only the ACC oxidase homolog gene was involved in the ethylene biosynthesis pathway (Table 1). Our transcript expression analysis, which compared FCL and SL 2nd day after treatment, provides insights into gene expression profiles and leaf senescence, and suggests that JEOKYEOPSON targets FCL more efficiently than SL.
DEG (differentially expressed gene) analysis of whole transcripts of ‘Fuji’ tree leaves treated with the chemical defoliant. (A), X-axis, FCL control; Y-axis, FCL treatment. (B), X-axis, SL control; Y-axis, SL treatment. The DEG analysis was carried out using DESeq 2 in CLRNAseq™ (ChunLab, Seoul, Korea). The results were considered significant at p < 0.05
Measurement of photosynthesis
We measured the light saturation point in SL treated with JEOKYEOPSON and compared it to the light saturation point for FCL. The comparison showed that SL had a much higher light saturation point at 6th day after treatment than FCL (Fig. 6A). Furthermore, the SL light saturation point was similar to FCL treated with the water control (Fig. 6B). We also investigated the effects of the chemical defoliant on the chlorophyll content of ‘Fuji’ apple leaves. Total chlorophyll content had dropped by more than 51 % in FCL at 8th day after treatment. In contrast, there was only a minimal decrease in SL total chlorophyll content (Fig. 7). These results indicate that JEOKYEOPSON can be used as a selective defoliant.
The photosynthetic capacities of FCL and SL from ‘Fuji’ apple trees at 6th day after sprayed with the chemical defoliant (A), and changes in the photosynthetic capacities of FCL and SL treated with the water control (B). The photosynthetic capacity was measured 6 days after the chemical defoliant had been sprayed. 20 leaves were measured in the experiment. Error bars represent the standard deviation of three biological replicates. Asterisk photosynthetically active radiation, µmol m−2 s−1. Two asterisk electron transport rate, µmol m−2 s−1
Polygalacturonase (PG) is up-regulated by chemical defoliants
The expression of PG-related genes increased in response to JEOKYEOPSON (Table 1). In this study, we hypothesized that the chemical defoliant may up-regulate the expression of the PG-related gene. To test this hypothesis, we measured the activity of PG in ‘Fuji’ apple leaves treated with JEOKYEOPSON. After the chemical defoliant spray, total proteins were extracted from FCL and SL. The enzyme activity assay showed that PG activity levels increased dramatically in FCL treated with JEOKYEOPSON, which indicated that the chemical defoliant could activate PG activity (Fig. 8). Interestingly, PG activity levels in SL at 0 day after the JEOKYEOPSON treatment were the highest of all. According to Mishra et al. (2008), PG activity is higher in petiole tissue than in the leaves, stems, and roots. We used whole leaves, including the petioles, and furthermore, SL have thicker petioles than do FCL. Therefore, these results may reflect the increased levels of petiole tissue in the SL samples. Nevertheless, when SL were exposed to JEOKYEOPSON, significant decreases in PG activities were observed as time progressed (Fig. 8). These results indicate that chemical defoliant may play a role in polygalacturonase-regulated leaf abscission processes that specifically occur in FCL. This suggestion is supported by the finding that polygalacturonase-like gene expression levels were up-regulated when FCL was treated with the chemical defoliant (Table 1).
Lanolin oil activates ACC synthase in vitro
We assayed ACC synthase activity using recombinant ACC synthase to ascertain whether lanolin oil triggers the ethylene biosynthesis pathway. Recombinant ACC synthase could convert SAM to ACC and produce ethylene, whereas the boiled recombinant ACC synthase did not convert SAM to ACC, which demonstrated that the recombinant protein displays ACC synthase activity in vitro (Fig. 9A). These results suggest that recombinant ACC synthase can be used for the enzyme activity assays to examine the effect of lanolin oil on ACC synthase. As expected, lanolin oil activated ACC synthase to a greater extent than the control treatment (without an activator). This finding indicated that the FCL senescence mechanism involved the activation of ACC synthase by the chemical defoliant (Fig. 9B).
In vitro activation of the recombinant ACC synthase using the chemical defoliant. (A) Recombinant ACC synthase shows ACC synthase activity in vitro; C1, Denatured recombinant ACC synthase; and C2, Recombinant ACC synthase. (B) Lanolin oil (The main ingredient in the chemical defoliant) promotes ACC synthase activity. Error bars represent the standard deviation of three biological replicates
Measurement of fruit color
We examined changes in the fruit color of apples from trees treated with the chemical defoliant or the control. The chromaticity value of a* (red vs. green, where a positive number indicates red) increased from 17.21 for the untreated leaves to 24.03 for the treated leaves, which represents a 39.6 % increase. L* (light vs. dark, where a low number indicates dark) decreased from 56.15 for the untreated leaves to 48.68 for the treated leaves, which represents a 13.3 % decrease; and b* (yellow vs. blue, where a positive number indicates yellow) decreased from 26.80 for the untreated leaves to 20.87 for the treated leaves, which represents a 22.1 % decrease. The chromaticity values of L* and b* in the chemical defoliant spray treatments were lower than that of the control. The a* chromaticity value after the chemical defoliant spray treatment was higher than the control. Color differences (∆E * ab) between the chemical defoliant spray treatment and the control were much more significant (11.70 %) (Table 2).
Discussion
It has been shown that removing FCL improves apple fruit quality. However, the manpower required for the hand-stripping method is very expensive. Thus, any means of stimulating FCL defoliation in mid-September would be important in apple orchards. To better employ the chemical defoliant, it is critical to understand the functional roles of leaf defoliation. Recently, many kinds of chemical defoliants have been used. However, little information is available regarding chemically mediated leaf defoliation. In this study, we described the functional roles of JEOKYEOPSON, a chemical defoliant. In the RNA-seq transcriptome analysis, we identified candidate genes whose expression coincided with the production of ethylene (Table 1). The chlorophyll content analysis, followed by a light saturation point assay, confirmed that chemical defoliation occurred in vivo (Figs. 6, 7). Furthermore, treatment with the chemical defoliant up-regulated the polygalacturonase-like gene (Table 1), resulting in a substantial increase (up to twofold) in polygalacturonase activity in FCL (Fig. 8).
Mishra et al. (2008) demonstrated that when plant leaves were exposed to ethylene, abscission zone tissue showed a significant increase in PG activity. These results were consistent with our results, which also showed that the leaves sprayed with JEOKYEOPSON produced large amounts of ethylene and that this caused a significant PG activity increase in abscission zone tissue. Furthermore, PGs have been widely studied in various plants and their roles during leaf abscission have been reported in the previous studies (Brummel et al. 1999; Gonzalez-Carranza et al. 2002). We also used a recombinant ACC synthase enzyme activity assay to confirm that lanolin oil, the main ingredient of JEOKYEOPSON, activated ethylene production (Fig. 9B). ACC synthase is a key enzyme in the ethylene biosynthesis pathway in plants and it is interesting to note that lanolin oil activated ACC synthase. Since lanolin oil can also activate ACC synthase activity (Fig. 9B), it was expected that the chemical defoliant spray would also cause ‘Fuji’ apple leaf senescence. If SL also senesced, then the photosynthesis rate would decrease, which would lead to poorer quality fruit. There was a minimal decrease in SL total chlorophyll content after the SL had been treated with the chemical defoliant (Fig. 7). These results indicated that JEOKYEOPSON can be used as a selective defoliant. Finally, we examined changes in the fruit color of apples from trees treated with the chemical defoliant. The color of the apples sprayed with the chemical defoliant improved compared to the control apples (Table 2).
In summary, we have described a strategy that could be used to identify the biological mechanism underlying chemical defoliation by using whole transcriptome profiling, plant photosynthesis measurements, and an enzyme activity assay. Our results show that the leaf defoliation mechanism identified here most likely includes increased ethylene production in FCL compared to SL. According to Iqbal et al. (2012), the response of plants to defoliation could be used to manipulate source–sink relation by removing senescing leaves to obtain greatest photosynthetic capacity under stressful environments. Therefore, we suggest that FCL defoliation in apple trees is one of the defense mechanisms.
In this study, further information related to the chemical defoliation mechanism was obtained. Leaf abscission is multifaceted and multilayered; therefore, the exact functional roles played by chemical defoliants during leaf defoliation need further research. The fact that multiple mechanisms are involved is consistent with the hypothesis that defoliation is a multifaceted process. Further studies on chemical defoliation may provide valuable insights into the mechanism. Taken together our results suggest that JEOKYEOPSON can be used as a selective defoliant to improve ‘Fuji’ fruit quality. Also, this chemical defoliant can be applied as the defoliant to other fruit crops.
References
Arnon DI (1949) Copper enzyme in isolated chloroplasts. Polyphenoloxides in Beta Vulgaris. Plant Physiol 24:1–15
Boller T, Herner RC, Kende H (1979) Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta 145:293–303
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brummel DA, Hall BD, Bennett AB (1999) Antisense suppression of tamato endo-1,4-beta-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol Biol 40:615–622
Choi SW, Kim JO, Kim KR (2000) Effects of defoliation treatments during maturation on fruit quality of ‘Fuji’ apples. J Kor Soc Hort Sci 41:383–386
Deng Y, Wu Y, Li Y (2005) Changes in firmness, cell wall composition and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Res Int 38:769–776
Gonzalez-Carranza ZH, Whitelaw CA, Swarup R, Roberts JA (2002) Temporal and spatial expression of a polygalacturonase during leaf and flower abscission in oilseed rape and arabidopsis. Plant Physiol 128:534–543
Iqbal N, Masood A, Khan NA (2012) Analyzing the significance of defoliation in growth, photosynthetic compensation and source-sink relations. Photosynthetica 50:161–170
Kende H, Hanson AD (1976) Relationship between ethylene evolution and senescence in morning-glory flower tissue. Plant Physiol 57:523–527
Kim WC, Yun CJ (1971) Effects of defoliation with chemical spray before harvest on improvement of fruit color in apples. Ann Rep Hort Expt Sta 25:200–207
Kuwar SS, Pauchet Y, Vogel H, Heckel DG (2015) Adaptive regulation of digestive serine proteases in the larval midgut of Helicoverpa armigera in response to a plant protease inhibitor. Insect Biochem Mol Biol 59:18–29
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Plant Cell Membr 148:350–382
Mishra A, Khare S, Trivedi PK, Nath P (2008) Effect of ethylene, 1-MCP, ABA and IAA on break strength, cellulase and polygalacturonase activities during cotton leaf abscission. S Afr J Bot 73:282–287
Satou M, Enoki H, Oikawa A, Ohta D, Saito K, Hachiya T, Sakakibara H, Kusano M, Fukushima A, Saito K, Kobayashi M, Nagata N, Myouga F, Shinozaki K, Motohashi R (2014) Integrated analysis of transcriptome and metabolome of Arabidopsis albino or pale green mutants with disrupted nuclear-encoded chloroplast proteins. Plant Mol Biol 85:411–428
Snipes CE, Cathey GW (1992) Evaluation of defoliant mixtures in cotton. Field Crop Res 28:327–334
Taiz L, Zeiger E (2002) Plant physiology. Ethylene: the gaseous hormone, 3rd edn. Sinauer Associates Inc, Sunderland, pp 530–531
Taylor JE, Tucker GA, Lasslett Y, Smith CJS, Arnold CM, Watson CF, Schuch W, Grierson D, Roberts JA (1990) Polygalacturonase expression during leaf abscission of normal and trangenic tomato plants. Planta 183:133–138
Vestrheim S (1970) Effects of chemical compound on anthocyanin formation in ‘Mcintosh’ apple skin. J Am Soc Hortic Sci 95:712–715
Westwood MN (1993) Temperate zone pomology, physiology and crop culture, 3rd edn. Timber press, Portland, pp 310–316
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614
Yim YJ, Lee HC (1999) Effect of pre-harvest defoliation on fruit color and tree physiology in apples. J Kor Soc Hort Sci 40:209–212
Yim YJ, Jang JY, Lee HC (2000) Effect of optically active ABA and its synthetic intermediate STC4771 on defoliation and fruit color in ‘Fuji’ apple trees. J Kor Soc Hort Sci 41:53–55
Yoo JG, Kang BK, Lee JW, Kim DH, Lee DH, Jung HY, Choi DG, Choung MG, Choi IM, Kang IK (2015) Effect of preharvest and postharvest 1-methylcyclopropene (1-MCP) treatments on fruit quality attributes in cold-stored ‘Fuji’ apples. Kor J Hort Sci Technol 33:542–549
Author information
Authors and Affiliations
Corresponding authors
Additional information
Won-Chan Kim and Sang-Jae Kang contributed equally and are considered co-corresponding authors
Rights and permissions
About this article
Cite this article
Lee, CH., Seo, SH., Kwon, OJ. et al. Functional characterization of a chemical defoliant that activates fruit cluster Leaf defoliation in ‘Fuji’ apple trees. Appl Biol Chem 59, 711–720 (2016). https://doi.org/10.1007/s13765-016-0218-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-016-0218-z