Abstract
Heavy metals are essential for the survival of all living organisms in trace amounts. Industrializations and urbanisation are the two major rationale behind the massive rise in the contamination of land and water bodies including marine and freshwater. The major sources of heavy metal are coal burning, smelting operations, tanneries, waste incineration, pesticides, fungicides, metallurgy, etc. Due to the toxicity of heavy metals when living beings encounter contaminated water of sediment laden with heavy metal endure health hazards. Heavy metals and metalloids such as chromium, lead, mercury, cadmium, nickel, and cobalt are poisonous and carcinogenic even in minute amounts, posing a major threat to human life. The most sustainable approach towards remediating these heavy metals is bioremediation. It involves bacterial bioremediation, fungal, biofilms and phytoremediation, which is not only sustainable but also efficient and cost effective. This review delivers a comprehensive overview of the recent trends in bioremediation of heavy metals, their sources, toxicity, and alternative approach of using marine microbes and their pottential for remediation of heavy metals.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the expansion of industry, there has been a significant rise in the discharge of industrial waste to the environment, mostly soil and water, resulting in the buildup of heavy metals, particularly in metropolitan areas. Heavy metals are slowly depleted by leaching, plant absorption, erosion, and deflation. Heavy metals are a class of metals recognized as widespread contaminants in terrestrial and aquatic ecosystems. They are comprehensively distinguished by their density exceeding 5 g/cm3 Mohseni et al. (2018). When present in excess of the critical values, HM such as Pb (II), Cd (II), As(III/V), Cr(VI), etc. are detrimental to the environment. Heavy metals are produced via weathering, volcanism, and erosion, among other natural processes. Heavy metals (HMs) are highly dangerous since they can increase biological activity, disrupt different trophic levels in food chains and webs, and are particularly difficult to biodegrade. In addition, Heavy metals (HMs) can accumulate in tissues. The long-lasting characteristics of heavy metals (HMs) cause bioaccumulation and biomagnification, which affect species at numerous levels and raise issues from local fish population declines to molecular modifications (Ahmed et al. 2021). Particularly impacted are coastal and estuary environments, which influence bottom-dwelling species' development and reproduction. Furthermore, eating seafood from metal-contaminated seas has an indirect negative impact on human health since heavy metals build up in biota and intensify their presence up the food chain (Ali et al. 2019). The problem statement discovered is the deterioration of terrestrial and aquatic environment and deleterious effects caused to human beings and other life forms on coming in contact with heavy metals released as a result of biproduct of industrial operations, coal combustion, mining, smelting, cloud seeding, etc. (Mahajan & Kaushal 2018; Al-Dhabi et al. 2019).
Traditional methods of heavy metal remediation involve solvent extraction, chemical oxidation, chemical precipitation (Al-Dhabi et al. 2019). The challenges faced by the current chemical and physical methods of remediation of heavy metal are costly and have adverse bi-products (Ahmed et al. 2021). Bioremediation is used as a tool to find a solution to this issue. The word "bioremediation" refers to the process of converting environmental pollutants into less harmful forms using microorganisms and plants (Upadhyay et al. 2016). It is considered as a viable solution for the issue since it aids in restoring the environment's natural condition after pollution. It is economical and provides long-term environmental advantages. (Dixit et al. 2015). Adapting bioremediation processes would be a far better approach as it is cost effective, sustainable and green. Another alternative approach would be the use of marine bacteria. Harnessing the ocean resources to remediate heavy metal will also push us towards the Sustainable development Goal 14; i.e., conversation and sustainable use of sea and marine resources towards sustainable development.
Studies on the variety of marine bacteria and their technological applications, many marine ecosystems and bacterial populations are still understudied and poorly understood (Smith et al. 2019). As a result, marine bacteria continue to hold promise as a source of untapped biotechnological capabilities that may be used to present global issues like pollution from heavy metals, oil, and plastic. This review examines developments in heavy metal bioremediation by compiling index from more than 50 publications that communicate current trends in bio-remediation in the context of adulterated environments it focuses on the sources, toxicity, health risks, and techniques and unmet gaps and how the use of marine microbes can prove to be an efficient attempt in bridging the gaps of alternative method of heavy metal remediation.
Heavy Metals Toxicity, sources and health effects
When present in excess of the critical values, HM such as Pb(II), Cd(II), As(III/V), Cr(VI), etc. are detrimental to the environment. Heavy metals are produced via weathering, volcanism, and erosion, among other natural processes. Additionally, HM are produced by anthropogenic activities such as mining, metal smelting, oil refining, agriculture, fertilisation, and drainage (Jiang et al. 2018) (Table 1).
Heavy metal pollution from industrial, agricultural, and home waste and by-products has led to the presence of heavy metals in the marine as well as aquatic environment. These metals such as Iron, Nickel, Tin, Chromium, Cobalt, Iodine, Selenium, Vanadium, Zinc, Molybdenum, Arsenic, Boron, Silicon, Fluorine, Copper, Boron, and Manganese, termed group II, which are found in some living organisms, but not in all. Most of them are necessary as co-factors for enzymes, for the prolongation of systemic coherence, facilitation in monitoring of the electrostatic interactions in the aqueous phase (Pande et al. 2022).
Additionally, metal speciation can either lessen or increase toxicity. Likewise, elemental and organic forms of mercury are less fatal than organic forms, known to be crucial for the survival of some bacterial species is Cr(III), but Cr(VI) and elemental mercury are massively toxic. Heavy metals with various origins, toxicities, and environmental implications include chromium, lead, arsenic, mercury, silver, cadmium, and vanadium. Chromium pollution from industrial operations endangers human health and contaminates soil and water. Lead, which is found in paint and petrol, has a negative effect on the neurological system, and soil and water poisoning endangers both wildlife and humans. Arsenic from natural deposits and human activity is linked to cancer and has a negative influence on aquatic ecosystems and human populations. Mercury bioaccumulates in fish as a result of industrial operations, impacting human health and damaging aquatic ecosystems. The presence of silver in industrial effluent disturbs aquatic microbial populations. Cadmium from mining causes threats to kidneys and bones, as well as having a negative influence on soils, water, and human health. Vanadium derived from combustion processes is less hazardous, but its potential environmental influence on aquatic ecosystems warrants additional examination. Overall, these heavy metals highlight the importance of comprehensive research and mitigation techniques to protect both ecosystems and human health (Jeyakumar et al. 2022) (Table 2).
Various studies have proposed copious points of origination of heavy metals. Natural sources of heavy metals are found in the surroundings. Pollution brought on by seasonal changes, geological activity, and weathering has less of an impact than pollution caused by anthropogenic or man-made activities, which are ongoing and permanent. (Mohapatra et al. 2017). Industrialization, urbanisation, and agriculture are the three main drivers of anthropogenic pollution. (Mohapatra et al. 2018). The two main pathways through which heavy metals are delivered into ecosystems are through natural resources and diverse human activities (also known as anthropogenic activities). Natural causes of heavy metal re-release include volcanic eruptions, degradation of the soil (such as surface erosion), and dissolving rock Jadaa and Mohammed 2023) (Table 3).
Based on their capacity to trigger carcinogenesis, heavy metals have been classified by the International Agency for Research on Cancer (IARC). While Cu, Zn, Fe, Ni, Mn, and Co have been classified as non-carcinogenic, elements like Cd, Cr, Pb, and As have been classified as carcinogenic. Obayeni stated that due to heavy metal contamination health of fish consumers may be at danger if they consume it in excess over an extended period. Therefore, consuming fish might expose consumers to a long-term risk of developing cancer (Obayemi et al. 2023) (Table 4).
The Implication of Heavy Metal Pollution on Aquatic Biota
Although some heavy metals, known as essential heavy metals, are crucial to biological systems, depending on the amount and length of exposure, they are typically hazardous to living things. Distinct metal species are generated as they transit from freshwater to marine due to modifications in pH and salinity. Biogeochemical processes control whether heavy metals are present in the water column as dissolved or particulate matter. Sediments, which are the main sink for these metals, may receive a fraction of the metals. Since they can adjust more quickly to changes in the environment and degradation, microorganisms are crucial to the maintenance and sustainability of any ecosystem (Singh and Kalamdhad 2011). Metals are ingested by aquatic biota through the ocean or the food chain, which could leave detrimental effects by changing their range, abundance, and physiology. Due to its increased toxicity, nonbiodegradability, and propensity to bioaccumulate and bio magnify in the tissues of living creatures, heavy metal pollution is a severe problem. To determine how hazardous these metals are to various marine organisms, including fish, phytoplankton, and zooplankton, it is crucial to evaluate their effects on these categories of marine creatures. The potential negative consequences of heavy metals might have on human health and the environment, heavy metal pollution in aquatic ecosystems recedes as a global problem (Fulke et al. 2020).
Bioaccumulation of Heavy Metals in Fish
Heavy metals found to be present in the liver of the fish are Fe, As, Cd, Zn and Pb (in descending order) whereas, those found in kidney are Zn, Fe, As, Cd and Pb (in descending order) (Sarah et al. 2019); as a result, their presence in aquatic habitats is extremely harmful to fish and shellfish. The disturbing rise in heavy metal concentrations in fish has compelled researchers to examine the risks brought on by heavy metal accumulation and bioaccumulation of living things. Since heavy metals do not decompose, they become significantly concentrated in a niche or medium like air, water, or soil, that niche or medium becomes hazardous and accumulates in the ecosystem. As stated by Chatta, Pb, followed by Cr and Cd, had the largest accumulation of heavy metals in farmed carps. Mean Pb content remained over the WHO-permitted limit for fish (0.123 μg/1), ranging from 0.1842 ± 0.0733 to 0.3316 ± 0.0143 μg/1, whereas Cr values ranged from 0.0262 ± 0.0015 μg/l to 0.4880 0.103 μg/l (Sfakianakis et al. 2015).
These heavy, poisonous metals can have detrimental effects on life and aquatic biomes, fish life, variety, and conservation if they are persistently accumulated (Agbugui et al. 2019). Humans ingest heavy metals when they consume contaminated fish and other aquatic foods from such ecosystems. The living organisms when they interact with aquatic ecosystems that are contaminated by organic and inorganic heavy metals (HM). (Baharom and Ishak 2015).Since heavy metals are potent neurotoxins in fish, their interaction with chemical cues may prevent fish from communicating with their surroundings. Fish abnormalities have been linked to heavy metals in both laboratory and wild populations. Such malformations often have a detrimental impact on fish populations because they impair fish survival, growth rates, wellbeing, and appearance. These fish abnormalities can be very good indicators of heavy metal contamination in the environment.
Bioremediation
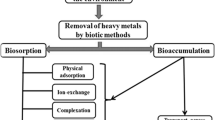
Bioremediation is an environmentally friendly and cutting-edge approach that uses natural biological processes to entirely eradicate hazardous substances. It might be any procedure that employs microbes, fungus, green plants, or their enzymes to restore the natural environment that has been contaminated. Heavy metal bioremediation is accomplished via a variety of methods, including but not limited to biosorption, bioaccumulation, bioreduction, bioprecipitation, biovolatilization, bioleaching, composting, land farming, bioreactors, biopiles, and biosparging. (Voica et al. 2016). To clean up and bio-detoxify such places in a cost-effective and sustainable fashion, bioremediation utilising metal-resistant bacteria has been explored as a feasible solution. (Voica et al. 2016).To ensure the long-term sustainability of the biotic and abiotic environment, the remediation strategy for heavy metals should be carefully assessed. All remediation procedures require treating large amounts of sludge, which not only costs a lot of money but also damages natural ecosystems Microorganisms are regarded as the most globally distributed organisms because of their extraordinary metabolic capabilities, they can survive in a broad variety of environmental circumstances. Additionally, they have a very diverse nutritional intake, which makes them a very helpful tool for cleaning up the nearby area. (Voica et al. 2016)However, the use of microorganisms with a demonstrated capacity for remediation and tolerance of high toxicity is essential for the success of the bioremediation process. Because they have a few methods to experience metal toxicity, microorganisms are crucial in the clean-up of metals tainted from habitats (Monga et al. 2022a, b).
There are primarily two techniques for removing and transporting wastes for treatment. In situ bioremediation technologies remediate pollution on the site. The use of these particular techniques based on a numerous variable, inclusive of the extent of contamination, characteristics of the chemicals, concentration of the pollutants, and the amount of time needed to accomplish it. Because it costs less and needs less material handling, this approach is typically suggested. Intrinsic bioremediation and engineered in situ bioremediation are two subcategories of in situ bioremediation. As illustrated in Fig. 1 primarily by bioventing, disparaging, bio stimulation, bioaugmentation, and phytoremediation (Sayakal and Ahmed, 2021(Ahmed et al. 2021).The process of treating the sediment sample before reintroducing it to its initial state is stated as ex situ bio-remediation. The region can be decontaminated more quickly if the contaminated material is removed and processed on or off site. Solid phase and slurry phase systems of ex situ bioremediation are different types of systems. As illustrated in Fig. 1 the most crucial methods include farming on land, composting, biopiles, and bioreactors.
Algal Bioremediation Mechanism
While some microalgae exhibit toxicity towards heavy metals, such as Cd, Pb, As, Hg, and Cr, whereas others, including Mo, Mn, Br, Cu, Zn, and Fe are needed by microalgae as trace elements to support enzymic responses and metabolic processes. Detrimental heavy metal present in rudimentary dose promote the development and metabolism of microalgae (Sun et al. 2015). Several Cyanophyceae species, including, Phormidium, Oscillatoria, Spirogyra, and Anabaena naturally flourish in heavy metal laden water bodies due to their resistance against heavy metal disquiet (W. Sun et al. 2021). In wastewater, Cyanophyceae exhibit affinity towards reactive groups for active binding sites. This results in flocculation, which lowers the load of total suspended and dissolved solids (Balaji et al. 2015).
The main components of the microalgae cell wall are polysaccharides (cellulose and alginate), lipids, and organic proteins. These components also contain a variety of functional groups that can bind heavy metals, including amino, carboxyl, hydroxyl, imidazole, phosphate, sulfonate, thiol, and others (Priatni et al. 2018). Additionally, they include a large number of deprotonated sulphates, monomeric alcohols, and carboxyl groups and laminarin, all of which are attracted to both cationic and anionic HM species. These many functional groups and EPS found in biomass help to effectively remove heavy metals from the environment (Pradhan et al. 2019).
Fungal Bioremediation mechanisms
The widespread occurrence of fungi in nature and their frequent usage in industrial applications are well recognised (Abdi & Kazemi 2015). In terms of their ability to withstand challenging environmental factors including high moisture, nutritional conditions, and pH, the fungal strains are also strong in nature. Mycoremediation is the process of using fungi to purge toxins from various environmental components, either alive or dead ( (Adenipekun & Lawal 2012). Mycoremediation is an inexpensive process that doesn't create any hazardous waste. Because all of the pollutants in nature have been completely mineralized, it offers a full remedy (Kapahi & Sachdeva 2019). Since heavy metals may induce cell lysis and death in fungus, several studies ushered towards the ascertain of the high HM dosage of fungal biomass and can vary prominently at genus level. Aspergillus species, Penicillium species, and Fusarium species are some of the most resilient strains (Priyadarshini et al. 2021).
Pytoremediation
Phytoremediation uses plants and their accompanying rhizosphere microbes to clean up heavy metal-contaminated sediments. Heavy metal accumulation in plant systems involves a variety of stages that include heavy metal mobilisation for efficient adsorption, absorption by root systems, xylem loading, root to shoot transfer, compartmentation, sequestration, and detoxification (An et al. 2020). Heavy metal ions are mobilised in the rhizosphere via exudates synthesised by plants and microbiomes. Heavy metal ions are taken up by plant roots via active and passive transport channels, where they form complexes with organic acids such as carbonates, phosphate, and sulphates and are immobilised in cellular walls or intracellular vacuoles. Heavy metal ion complexes contained in vacuoles are transferred into the xylem and shoots (An et al. 2020). Based on the primary processes in the plant metal system, phytoremediation of heavy metals may be categorised into four categories: phytostabilization, phytoextraction, phytofiltration, and phytotransformation. Among these processes, phytoextraction has been widely used as a remediation technology, with three steps generally involved: cultivation of suitable metal accumulating plants, harvest of metal-enriched plant biomass, and post-harvest treatment of plant biomass for added market value (e.g., energy recovery from thermal treatment).
Bacterial Bioremediation
To tolerate higher levels of heavy metals, bacteria have evolved a number of mechanisms, including extracellular sequestration of the metal by exopolysaccharides, enzymatic detoxification, cell surface biosorption by negatively charged groups and intracellular sequestration of the metal by protein binding (Dash et al. 2012). In recent years, a number of bacterial species have been discovered that are capable of bioremediation (Banat et al. 2014). As stated by Campos, Chromate reductases may be involved in normal metabolism since both chromate-resistant and chromate-sensitive bacterial isolates were able to reduce Cr (VI).). Only a small number of bacterial species have been for chromate reductase out of the many that have been reported. These include Pseudomonas and Enterobacter species. As illustrated in Fig. 2 reduction to trivalent chromium take place in the presence of chromate reductase.
Genetically Engineered Microbes in Bioremediation
Four main approaches are being taken into consideration when developing genetically engineered microbes (GEM) for bioremediation. It is series of biochemical reactions which include, end point analysis, chemical sensing, bioprocess creation, monitoring and control, affinity, enzyme specificity enhancement, toxicity reduction using bio affinity bioreporter sensors, and route construction and regulation. A vital issue to consider is the choice of cellular factories. Between fungus, bacteria, and algae strains, bacterial systems have been shown to have enormous potentials due to several inherent characteristics, including a faster rate of development, confinement, and simplicity of genetic modification (Jeyakumar et al. 2022).The following criteria asks if biosorption or bioaccumulation is preferable. The decision between biosorption and bioaugmentation is largely influenced by the heavy metal to be remedied, its concentration, the source of remediation, environmental factors, additional nutritional factors, the existence of other interfering molecules/ions, etc. Despite the fact that biosorption and bioaccumulation are the main methods for removing heavy metals from the environment, techniques are being developed to increase the amount of heavy metal that may be adsorped and increase their capacity to collect HM by using chauffeurs (Diep et al. 2018; Jeyakumar et al. 2022).For instance, several bacteria integrate and overexpress the import system s for ion which comprise of secondary carriers, channels, etc., to increase the intake of certain heavy metals. On the other side, after bioaccumulation, heavy metals are extensively targeted for further reduction or cleanup. Proteins and enzymes that particularly lower the levels of heavy metal complexes are created and added to various species to improve their capacity for remediation (Jeyakumar et al. 2022).
Marine microbes in bioremediation
Marine microbes offer unparalleled advantages in bioremediation, surpassing traditional methods. Marine bacteria can adapt swiftly to rapidly changing, noxious settings and might potentially be used to remedy the problem by remediating poisonous elements. This approach minimizes ecological disruption and mitigates unintended consequences. Marine biomass contains a wide range of microbes, including primary producers such as green algae, brown algae, and red algae; grazers; archaea; photoheterotrophs such as Gram-positive and -negative bacteria; nitrogen-fixer bacteria such as Azotobacter; nitrifiers; and various types of viruses, among others. (Husain et al. 2022).The many marine microorganisms play important functions in the marine environment. Additionally, the inherent resilience of marine ecosystems facilitates sustainable remediation. Marine bacteria such as Pseudomonas fluorescens, P. putida, Dechloromonas aromatica, Alcanivorax borkumensis, Methylibium petroleiphilum, Bacillus subtilis, and Phanerochaete chrysosporium play an important part in heavy metal cleanup by ensuring sustainability. The widespread involvement of such bacteria to changing heavy metal concentrations in soil and water and assisting plants to deal with heavy metal concentrations with ecological advantages was observed (Zhou et al. 2023)The majority of bacterial populations in the Indian Ocean have been classified into six primary taxonomic groups: proteobacteria, actinobacteria, bacilli, and flavobacteria. The cost-effectiveness and reduced environmental footprint of marine microbial bioremediation underscore its potential as a potent and environmentally conscientious solution for addressing contamination in marine environments. Despite the fact that numerous research have been undertaken and a huge number of marine microbial entities have been found, the microbial diversity from diverse maritime environments has yet to be investigated (Zhang et al., 2023).
Mechanism of bioremediation
Despite the fact that heavy metals are normally harmful, organisms have evolved unique resistance mechanisms and intricate intracellular pathways to use and detoxify heavy metals for cellular reproduction. Surface adsorption owing to physical interaction (electrostatic or Van der Waals interaction), chemical interaction (ion exchange displacement of connected metal cations), complexation, diffusion, or precipitation are among the methods. The cellular surface normally has a negative charge that attracts heavy metals aggressively, but in other cases, the cellular surface may have a mucus or polysaccharide layer that significantly adsorbs heavy metals through physical interactions. Heavy metals are passively adsorbed on the surface, requiring no energy expenditure until equilibrium is established (Bala et al. 2022). A few mechanisms have been discussed in brief:
Bioprecipitation
Cellular metabolism may or may not be required for precipitation. As a kind of defence, bacteria create certain compounds that aid in the precipitation process in response to the harmful metal present in the area around them. The bio-chemical interaction of the ions of the specified metal and the cell wall bacteria may result in precipitation in the absence of biological metabolism (Mohapatra et al 2017).
Bioleaching
It is a method of extracting metals from low-grade ores and mineral concentrates. Metal recovery from sulphide minerals is based on the action of chemolithotrophic bacteria, primarily Thiobacillus ferrooxidans and T. thiooxidans, which convert insoluble metal sulphides to soluble metal sulphates. Heterotrophic bacteria and fungi can be used to treat non-sulfide ores and minerals. In these circumstances, metal extraction is caused by the excretion of organic acids and chelating and complexing chemicals into the environment. Currently, bioleaching is mostly utilised for the recovery of copper, uranium, and gold, and the primary processes used are heap, dump, and in situ leaching. Tank leaching is used in the treatment of refractory gold ores. Bioleaching offers some promise for metal recovery and decontamination of heavy contaminated waste from industries products, sewage sludge, and soil (Dong et al. 2023).
Biomineralization
Mining activities are a significant source of heavy metal pollution in the environment. Several studies have found higher metal levels around metalliferous mines and industrial areas. Among the present environmental issues are the consequent pollution of surrounding agricultural lands and waterways. Because of phytotoxicity, metalliferous soils provide relatively restricted habitats for plants, resulting in significant selection pressures. Heavy metal plant communities are genetically changed ecotypes with specialised tolerance to heavy metals such as cadmium, copper, lead, nickel, zinc, and arsenic that have evolved through microevolutionary mechanisms (Rajendran et al. 2022). Biomineralization is a natural process that produces complex structured inorganic minerals that serve important purposes in living systems. Scientists have been inspired by the diverse morphologies of biominerals to mimic these materials using the underlying chemistry of biomineralization, which has enabled the replication of biominerals' outstanding mechanical and optical properties as well as their unique biological functions such as navigation, storage, and homeostasis (Pande et al. 2022).
Adsorption and ion exchange
Vander Waals forces of attraction cause the adsorption of metal ions. By using electrostatic interactions between ions of the metal and components cell wall of bacteria present in aqueous medium, dead bacterial biomass can biosorb metals (; Mohapatra et al 2017).Bacterial cell walls demonstrates the interaction of bivalent metal ions and polysaccharides, due to the interplay of counter ions present in the aqueous medium (Mohapatra et al. 2018). The fundamental cellular ions Ca2+, Mg2+, K+ and Na+, are exchanged for the proliferating ions Pb2+,Cd2+, As3+, Cu2+, CO2+nd Cr6+, etc., resulting in biosorptive metal absorption. (, Mohapatra et al. 2018).
Biosorption and bioaugmentation
Several microbial species have been observed to create extracellular biopolymers that facilitate flocculation. Bioflocculation or biosorption is the process of any substance being absorbed by biological materials through metabolically independent or dependent absorption processes (Pande et al. 2022).A type of amphipathic molecules known as surfactants, commonly referred to as flocculants, can eliminate HM from adulterated samples of sediments while also being environmentally beneficial ((Banat et al. 2014). For high bio flocculant output and efficiency and cost effective production employing bioprospecting for microbial strains with bio flocculant-producing capabilities is necessary (Pande et al. 2022).
Biosurfactants are hence "green" alternatives to manufactured chemical surfactants. Unfortunately, due to several technological and financial considerations, biosurfactants have not yet been widely employed in the industry. The biosorptive capacities of various microbial biomass vary, and these variations are also significant within each category. Each biosorbent's potential for biosorption, however, is influenced by its prehistory, pre-treatment, experimental circumstances, and more. The biosorbent need to be affordable, efficient, and simple to cultivate and harvest. The organism should also be adaptable to changes in physical and chemical conditions, as well as the architecture of the bioreactor, to facilitate biosorption (Rajendran et al. 2022).
Siderophores
A crucial micronutrient for bacterial cells is iron (Fe). Fe tends to generate oxyhydroxides and insoluble hydroxides in aerobic circumstances (Rajkumar et al. 2010 and Fulke et al. 2020). It is known that Fe(III)-sequestering siderophores are produced by bacteria to protect them from their environment. Siderophores work as solubilizing agents to make Fe accessible to living organisms. Fe3+ is reduced to Fe2+ upon contact with siderophores and is used by the cell (Fulke et al. 2020). In addition to attaching to Fe, siderophores may also bind to metals that are deemed non-essential including Al(III), Ga(III) and Cr(III) as well as essential elements like Ca, Mn, and Mg (Fulke et al. 2020). This suggests a role for bacteria that produce siderophores in the bioremediation of heavy metals. The remediation of Cr-contaminated tannery effluent has been carried out using marine microorganisms that produce siderophores. (Vijayaraj et al. 2019 and Fulke et al. 2020).
Factors affecting bacterial biosorption of heavy metals
Operational factors are biomass content, temperature, starting metal ion concentration, interfering co-ions, contact time, and pH, significant HM on the surface of the cell (Mohapatra et al 2017). The pH is a crucial factor that has an impact on the surface functional groups on the bacterial cell wall as well as the solution chemistry of metal ions (Rajendran et al. 2022).pH significantly alters the biosorption potential of the cell as there will more negative binding sites that are exposed on the surface of biomass. (Mohapatra et al 2017).Since the solute's surface motion and kinetic energy increase with temperature, biosorption of heavy metals often progresses more rapidly. However, elevated temperatures have the potential to destroy certain binding sites for metal ions (Rajendran et al. 2022).Whether metal ions interact with binding sites exothermically or endothermically determines the capacity of bacteria to sorb metal ions. However, most of the research found between 20 and 35 °C to be the ideal temperature for heavy metal sorption (Mohapatra et al 2017). The concentration of biomass utilised as the sorption medium affects the biosorption of heavy metals. Since there are more binding sites on the surface of the biosorbent, an increase in biomass content often leads to an increase in biosorption efficiency. It was shown that the sorption efficiency rose as the concentration of biomass increased, but that the effects of biomass concentrations above 1.0 & 2.0 g/l on the sorption efficiency were less significant (Mohapatra et al 2017). Uptake capacity is nearly constant at concentrations above the optimal biomass concentration, which may be caused by active site interference and between potential binding sites According to certain research, the ability of heavy metals to be absorbed diminishes with increasing biomass content due to severe restrictions on ionic species mobility in the biosorption medium, which results in fewer metal ion binding sites(Aryal & Liakopoulou-Kyriakides 2014).
The exposure period is a further vital component in the process of metal biosorption. The sorption–desorption processes that take place after metal ions have saturated the surface of the biomass are indicated by the contact time (equilibrium time). Following the equilibrium period, equilibrium capacities remain nearly constant, comprehending an equilibrium against the sorption process (Aryal & Liakopoulou-Kyriakides 2014). A critical variable in the biosorption process is the initial metal ion concentration. Since the initial metal ion concentration provides the necessary driving force to overcome the resistance to the mass transfer of metal ions between aqueous and solid phases, but decreases the sorption percentage, the amount of metal ions per unit mass of biomass increases as metal ion concentrations rise (Aryal and Liakopoulou-Kyriakides 2014).The entire concoction of ions interferes the process of biosorption when treating wastewater that contains numerous metals. Metal cations and anions, among others, may interfere with biosorption processes and create competitive conditions in wastewater (Aryal and Liakopoulou-Kyriakides 2014).
Limitations, challenges and future aspects
The limitations of bioremediation are related to its slowness and time consumption; also, the products of biodegradation are frequently more harmful than the original chemical. The crucial bottle neck in bioremediation is the irregularity and incompleteness. Moreover, there is no acceptable endpoint, evaluating the efficacy of bioremediation may be challenging. new research is needed to improve bioremediation methods and identify new biological solutions for bioremediation of heavy metal pollution from various environmental systems. Abiotic (temperature, pH, moisture, accessible electron acceptors or electron donors, etc.) and biotic (competition, predation, etc.) factors can all limit bioremediation. Recent research has found that strains with high pollutant biodegradation rates in laboratory may be less efficient and survive poorly in field-scale bioremediation (Bodor et al. 2020).
Furthermore, the challenges faced in treating inorganic pollutants and determining whether toxins have been eliminated or not. Heavily chlorinated materials biodegrade slowly, resulting in the formation of more hazardous or carcinogenic by-products. (Sayqal and Ahmed 2021). Given the existing scarcity of research on biomass use following heavy metal-contaminated environment cleanup, more investigation into post-treatment concerns is critical. The post-treatment biomass, which contains residual heavy metals and their intermediate derivatives, is regarded dangerous, needing a thorough study of its composition and potential environmental repercussions Lee et al., 2019.
The role of phytoremediation in reducing environmental pollution can also be studied. The phytoremediation process has a number of advantages over other remediation strategies, including lower costs, greater public acceptance, and increased pollution degradation capacity. On the contrary, the complex metabolic pathways and photosynthetic activities of cyanobacteria and microalgae, which resemble the plant kingdom, are what contribute to their sustainability and economic viability. Establishing sufficient tactics to regulate environmental circumstances, highlight genetic components, and optimise metabolic pathways will be beneficial in increasing the bioremediation capability of marine bacteria. Marine microbe genetic engineering and modification may improve bioremediation effectiveness even more (Zhang et al., 2023). As a result, finding effective isolating and culturing procedures for uncultured marine microorganisms may lead to the discovery of novel microbial resources for marine pollution remediation (Bodor et al. 2020).
Conclusion
The global requirement for a solution to this problem comprises multiple remediation components, however bioremediation is one step ahead of all of them due to its numerous benefits over other kinds of remediation procedures. The selection of an appropriate bio-sorbent in terms of efficiency and cost is a major challenge. Because microorganisms perform redox reactions, metal mobilization/immobilization has an impact on bioremediation processes. Metal-microbe interactions impact microbial activities such as proliferation, colonisation, and the formation of microbial biofilms for remediation. The use of biomass from bacteria, fungus, algae, and plants has been proven to be successful for heavy metal transformation. The comprehensive study of different techniques and bio-mechanism in the cells suggested in this review contributes to a better understanding of heavy metal remediation. This can aid in making existing chromium cleanup systems more efficient. Marine bacteria have a wide range of metabolic activities, and their potential for bioremediation is currently underutilised. Simultaneously, there is an urgent need to pursue, deploy, and popularise recent biotechnology developments in bioremediation. There is a broader scope for scientific innovations, with an emphasis on the cost-effectiveness, suitability, and sustainability of techniques to mitigate the impact of environmental change, contamination of food products and biological systems, impact of anthropogenic activities on the environment, and exploration of the aforementioned opportunities, as well as new initiatives for environmental restoration. To summarise Bacterial systems have demonstrated significant potential due to several intrinsic properties, including a higher rate of growth, containment, and ease of genetic manipulation. These techniques, which include biosorption, bioaccumulation, and rhizoremediation, are thought to be environmentally beneficial, efficient, and cost-effective. This review will be insightful in the development of microbial-based heavy metal removal technologies, which will considerably contribute to the preservation of human and environmental health.
References
Abdi O, Kazemi M (2015) A review study of biosorption of heavy metals and comparison between different biosorbents. ResearchGate. https://www.researchgate.net/publication/281951859_A_review_study_of_biosorption_of_heavy_metals_and_comparison_between_different_biosorbents
Adenipekun CO, Lawal R (2012) Uses of mushrooms in bioremediation: a review. Biotechnol Mole Biol Rev. https://doi.org/10.5897/bmbr12.006
Agbugui M (2022) Heavy metals in fish: bioaccumulation and health, pp 47–66. https://doi.org/10.37745/bjesr.2013
Ahmed SF, Mofijur M, Nuzhat S, Chowdhury AT, Rafa N, Uddin MA, Inayat A, Mahlia T, Ong HC, Chia WY, Show PL (2021) Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.125912
Al-Dhabi NA, Esmail GA, Ghilan AM, Arasu MV (2019) Optimizing the management of cadmium bioremediation capacity of metal-resistant Pseudomonas sp. strain al-dhabi-126 isolated from the industrial city of Saudi Arabian environment. Int J Environ Res Public Health 16(23):4788. https://doi.org/10.3390/ijerph16234788
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 2019:1–14. https://doi.org/10.1155/2019/6730305
An Y, Wang Y, Tan SN, Yusof MLM, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00359
Aryal M, Liakopoulou-Kyriakides M (2014) Bioremoval of heavy metals by bacterial biomass. Environ Monit Assess. https://doi.org/10.1007/s10661-014-4173-z
ATSDR (1999) Toxicological profile for mercury. http://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
Baharom ZS, Ishak MY (2015) Determination of heavy metal accumulation in fish species in Galas River, Kelantan and Beranang Mining Pool Selangor. Proc Environ Sci. 10:1–12. https://doi.org/10.1016/j.proenv.2015.10.057
Bala S, Garg D, Thirumalesh BV, Sharma M, Sridhar K, Inbaraj BS, Tripathi M (2022) Recent strategies for bioremediation of emerging pollutants: a review for a green and sustainable environment. Toxics 10(8):484. https://doi.org/10.3390/toxics10080484
Balaji S, Kalaivani T, Shalini M, Gopalakrishnan M, Muhammad MR, Rajasekaran C (2015) Sorption sites of microalgae possess metal binding ability towards Cr(VI) from tannery effluents—a kinetic and characterization study. Desalind Water Treatment 57(31):14518–14529. https://doi.org/10.1080/19443994.2015.1064032
Banat I, Satpute S, Cameotra S, Patil R, Nyayanit N (2014) Cost effective technologies and renewable substrates for biosurfactants’ production. Front Microbiol 5:697. https://doi.org/10.3389/fmicb.2014.00697
Bodor A, Bounedjoum N, Vincze G, Kis GE, Laczi K, Bende G, Szilágyi R, Kovács T, Perei K, Rákhely G (2020) Challenges of unculturable bacteria: environmental perspectives. Rev Environ Sci Bio/technol. https://doi.org/10.1007/s11157-020-09522-4
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Chai WS, Cheun JY, Kumar P, Mubashir M, Majeed Z, Banat F, Ho S, Show PL (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.126Chan,S.S.,Khoo,K.S
CPCB|Central Pollution Control Board. (n.d.). CPCB. https://cpcb.nic.in/who-guidelines-for-drinking-water-quality/
Dash H, Kungwani N, Chakraborty J, Kumari S, Das S (2012) Marine bacteria: potential candidates for enhanced bioremediation. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-012-4584-0
Diep P, Mahadevan R, Yakunin AF (2018) Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2018.00157
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustain for 7:2189–2212
Dong Y, Zan J, Lin H (2023) Bioleaching of heavy metals from metal tailings utilizing bacteria and fungi: mechanisms, strengthen measures, and development prospect. J Environ Manage. https://doi.org/10.1016/j.jenvman.2023.118511
Fulke AB, Kotian A, Giripunje MD (2020) Marine microbial response to heavy metals: mechanism, implications and future prospect. Bull Environ Contam Toxicol 105(2):182–197. https://doi.org/10.1007/s00128-020-02923-9
Garg S, Gauns M (2022) Marine environmental chemistry and ecotoxicology of heavy metals. https://doi.org/10.1016/B978-0-323-95919-3.00011-2.
Historical Water Quality Criteria Documents|US EPA. (2023). US EPA. https://www.epa.gov/wqc/historical-water-quality-criteria-documents
Husain R, Vikram N, Yadav G, Kumar D, Pandey S, Patel M, Khan N, Hussain T (2022) Microbial bioremediation of heavy metals by Marine bacteria. Elsevier eBooks. https://doi.org/10.1016/b978-0-323-85839-7.00014-1
Jeyakumar P, Debnath C, Vijayaraghavan R, Muthuraj M (2022) Trends in bioremediation of heavy metal contaminations. Environ Eng Res 28(4):220631. https://doi.org/10.4491/eer.2021.631
Kapahi M, Sachdeva S (2019) Bioremediation options for heavy metal pollution. J Health Pollut 9(24):191203. https://doi.org/10.5696/2156-9614-9.24.191203
Kaparwan AG (2023) Hexavalent chromium induced toxicity in nature and living beings. ResearchGate. https://www.researchgate.net/publication/368642358_HEXAVALENT_CHROMIUM_INDUCED_TOXICITY_IN_NATURE_AND_LIVING_BEINGS
Lee S, Yun J, Lee J, Hong G, Kim J, Kim D, Han J (2021) The remediation characteristics of heavy metals (Copper and lead) on applying recycled food waste ash and electrokinetic remediation techniques. Appl Sci 11(16):7437. https://doi.org/10.3390/app11167437
Mahajan P, Kaushal J (2018) Role of phytoremediation in reducing cadmium toxicity in soil and water. J Toxicol 2018:1–16. https://doi.org/10.1155/2018/4864365
Mohapatra R, Pandey S, Thatoi H, Panda C (2017) Reduction of chromium(VI) by marine bacterium brevibacillus laterosporus under varying saline and pH conditions. Environ Eng Sci 34:617–626. https://doi.org/10.1089/ees.2016.0627
Mohapatra RK, Parhi PK, Patra JK, Panda CR, Thatoi HN (2018) Biodetoxification of toxic heavy metals by marine metal resistant bacteria- a novel approach for bioremediation of the polluted saline environment. Microb Biotechnol. https://doi.org/10.1007/978-981-10-6847-8_15
Monga A, Fulke A, Dasgupta D (2022a) Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J Hazardous Mater Adv 7:100113. https://doi.org/10.1016/j.hazadv.2022.100113
Monga A, Fulke A, Gaud A, Sharma A, Ram A, Dasgupta D (2022b) Isolation and identification of novel chromium tolerant bacterial strains from a heavy metal polluted urban creek: an assessment of bioremediation efficiency and flocculant production. Thalassas Int J Marine Sci. https://doi.org/10.1007/s41208-022-00458-w
Obayemi OE, Ayoade MA, Komolafe OO (2023) Health risk assessment of heavy metals in Coptodon zillii and Parachanna obscura from a tropical reservoir. Heliyon. https://doi.org/10.1016/j.heliyon.2023.e16609
Pande V, Pandey SC, Sati D, Bhatt P, Samant M (2022) Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front Microbiol. https://doi.org/10.3389/fmicb.2022.824084
Pradhan D, Sukla LB, Mishra B (2019) Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J Clean Prod 209:617–629. https://doi.org/10.1016/j.jclepro.2018.10.288
Priatni S, Ratnaningrum D, Warya S, Audina E (2018) Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. IOP Conf Ser Earth Environ Sci 160:012006. https://doi.org/10.1088/1755-1315/160/1/012006
Priyadarshini E, Priyadarshini SBB, Cousins BG, Pradhan N (2021) Metal-fungus interaction: review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 274:129976
Rai S, Singh VK (2023) Mycoremediation of arsenic: an overview. In Environmental science and engineering, pp 301–315. https://doi.org/10.1007/978-3-031-37561-3_15
Rajendran S, Priya A, Kumar PS, Hoang TK, Karthikeyan S, Chong KY, Khoo KS, Ng HS, Show PL (2022) A critical and recent developments on adsorption technique for removal of heavy metals from wastewater—a review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.135146
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149. https://doi.org/10.1016/j.tibtech.2009.12.002
Sarah R, Tabassum B, Idrees N, Kumar A (2019) Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga (UP) India. Saudi J Biol Sci 26(5):979–984. https://doi.org/10.1016/j.sjbs.2019.02.009
Sayqal A, Ahmed O (2021) Advances in heavy metal bioremediation: an overview. Appl Bio Biomech 2021:1–8. https://doi.org/10.1155/2021/1609149
Sfakianakis DG, Renieri E, Kentouri M, Tsatsakis AM (2015) Effect of heavy metals on fish larvae de-formities: a review. Environ Res 137:246–255
Singh J, Kalamdhad AS (2011) Effects of heavy metals on soil, plants, human health and aquatic life. Int J Res Chem Environ 1(2):15–21
Sun J, Cheng J, Yang Z, Li K, Zhou J, Cen K (2015) Microstructures and functional groups of Nannochloropsis sp. cells with arsenic adsorption and lipid accumulation. Biores Technol 194:305–311. https://doi.org/10.1016/j.biortech.2015.07.041
Sun W, Cheng K, Sun KJ, Ma X (2021) Microbially mediated remediation of contaminated sediments by heavy metals: a critical review. Curr Pollut Rep 7(2):201–212. https://doi.org/10.1007/s40726-021-00175-7
Sutar H, Kumar D (2012) A review on: bioremediation. Int J Res Chem Environ 2:13–21
Vijayaraj AS, Mohandass C, Joshi D (2019) Microremediation of tannery wastewater by siderophore producing marine bacteria. Environ Technol 41(27):3619–3632. https://doi.org/10.1080/09593330.2019.1615995
Voica DM, Bartha L, Banciu HL, Oren A (2016) Heavy metal resistance in halophilic bacteria and archaea. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnw146
WHO (1995). Lead. Environmental Health Criteria, Vol. 165.Geneva, Switzerland: World Health Organization
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Ecology. https://doi.org/10.5402/2011/402647
Yadav K, Gupta N, Kumar V, Singh J (2017) Bioremediation of heavy metals from contaminated sites using potential species: a review. Indian J Environ Prot 37:65–84
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359. https://doi.org/10.3389/fpls.2020.00359
Zhou B, Zhang T, Wang F (2023) Microbial-based heavy metal bioremediation: toxicity and eco-friendly approaches to heavy metal decontamination. Appl Sci 13(14):8439. https://doi.org/10.3390/app13148439
Acknowledgements
Authors are grateful to Director, CSIR-National Institute of Oceanography (CSIR-NIO), Goa, India and Scientist-in-Charge, CSIR-NIO, Regional Centre, Mumbai and Academy of Scientific and Innovative Research (AcSIR) for their encouragement and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sonker, S., Fulke, A.B. & Monga, A. Recent trends on bioremediation of heavy metals; an insight with reference to the potential of marine microbes. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05673-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05673-x