Abstract
Microplastics and heavy metals are two different classes of pollutants that are often present in aquatic systems. However, the interaction between these two pollutants is poorly understood in freshwater systems. This research has examined the sorption of cadmium(II) ions onto polyethylene microplastic (PEMP) under freshwater conditions. The scanning electron microscope, X-ray diffraction, and Fourier transform infrared analyses confirmed the existence of different functional groups and the porous nature of the PEMP surface. The influences of physicochemical parameters such as the solution pH, contact time, and initial Cd(II) concentration have been examined. The Langmuir isotherm predicted the Cd(II) sorption capacity by PEMP at pH 5 as 1.37 mg/g. Several isotherm models were utilized, including the Freundlich, Langmuir, and Sips models. The results confirmed that the Sips model has been more appropriate for Cd(II)-PEMP isotherm based on percentage errors and correlation coefficient values. Furthermore, the pseudo-first kinetic model fitted Cd(II)-PEMP more accurately than the pseudo-second kinetic equation. Desorption experiments were conducted to release Cd(II) ions from Cd(II)-bearing PEMP using different chemical agents. The findings showed that using 0.01 M nitric acid resulted in a desorption efficiency exceeding 99.8%. This demonstrates that microplastics loaded with Cd(II) may release Cd(II) ions in highly acidic environments, potentially allowing for the uptake of Cd(II) ions by aquatic organisms in their digestive tracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics are small synthetic particles, ranging from 1 µm to 5 mm in size, that are either produced directly during manufacturing or as a result of the degradation of larger plastic items (Issac and Kandasubramanian 2021; Arpia et al. 2021). They have become a prevalent pollutant in both freshwater and marine environments (Nunes et al. 2023; Citterich et al. 2023; Das et al. 2023). Microplastics can be harmful to aquatic life, impacting the photosynthesis of marine and freshwater algae as well as the reproduction and hatching of several aquatic organisms, resulting in malnutrition, as well as the eventual demise of species (Banaee et al. 2019; Wang et al. 2023). Compared to larger plastic debris, microplastics have a larger surface area per unit of volume, are highly hydrophobic, and have a higher likelihood of interacting with microorganisms (Joo et al. 2021). These physical and chemical properties of microplastics result in an increased accumulation of pollutants, including heavy metals and persistent organic contaminants (Liu et al. 2022a; Fue et al. 2021). Moreover, plastic material itself constitutes toxic additives including plasticizers, flame retardants, and antibacterial agents, thereby contributing to additional contamination when released into water sources (Campanale et al. 2020). Microplastics can transport pollutants and deliver them globally, resulting in toxic effects on marine beings, accumulation, and transfer through the food chain (Brennecke et al. 2016). Therefore, they pose a significant risk to marine life and human health.
Many studies have investigated the interaction and adsorption of organic pollutants on microplastics in aquatic environments, including bisphenol (Liu et al. 2019), tetracycline hydrochloride (Chen et al. 2021a), phenanthrene (Bao et al. 2021), and triclosan (Chen et al. 2021b). Recently, there has been a growing focus on the role of microplastics in the accumulation of heavy metals (Kutralam-Muniasamy et al. 2021; Khalid et al. 2021). Wang et al. (2022) investigated the adsorption behavior of Cu(II) ions in simulated seawater onto polystyrene and polyethylene terephthalate microplastics. Due to the physisorption mechanism, both microplastics were able to adsorb Cu(II) ions. The authors established that microplastics act as vectors for metal ions in marine environments.
Industries such as electroplating, metal plating, textile, storage batteries, mining, and glass release heavy metals into waste, causing significant environmental harm. Heavy metal pollution is a significant issue due to its potential to harm human health even at low concentrations. Cadmium is one such important heavy metal, which is widely used in various industries. Cadmium is very toxic and known to cause renal disturbances, bone lesions, cancer, and hypertension in humans (Verougstraete et al. 2002; Vijayaraghavan et al. 2012). Due to its prevalent usage in various applications, cadmium is generally detected in urban stormwaters (Vijayaraghavan et al. 2010). The existence of microplastic particles increases the likelihood of Cd interacting with them, thereby altering its behavior, toxicological properties, environmental impact, as well as bioavailability. However, studies related to the behavior of Cd(II) and microplastics in freshwater environments are scarce. This research aimed to investigate the influences of various operational parameters (equilibrium pH, contact time, and initial solute concentrations) on the interaction between microplastics (polyethylene) and Cd(II) ions. To analyze the experimental data, different kinetics and isotherm models were used. The surface characterization techniques were also utilized to evaluate the adsorption removal mechanism.
Materials and methods
Microplastics and surface analyses
Microplastics (polyethylene) with an average particle diameter of 125 µm and a density of 0.94 g/cm3 were obtained from a local supplier. Cd(II) solution (stock) was made using Cd(NO3)2·4H2O (analytical grade, Sigma-Aldrich).
The surfaces of the polyethylene microplastics (PEMP) were analyzed using a Hitachi-S4800 scanning electron microscope (SEM) from Japan. The samples were placed on copper grids before being photographed. Fourier transform infrared spectroscopy (FTIR) was performed on the samples using a Bruker-ATR IR from Germany, with the KBr pellet procedure and a range of 600–4000 cm−1. The crystallographic structure of the samples was identified through X-ray diffraction (XRD) analysis using a Bruker D8 Advance from Germany.
Batch experiments
The study of Cd(II) adsorption onto PEMP in batch systems was conducted with 100 mL Cd(II) solution of desired initial concentrations and 0.1 g (1 g/L) of PEMP. The equilibrium pH of the Cd(II)-PEMP suspension was maintained in the range of 2.0–5.0 with the use of either 0.1 M HCl/0.1 M NaOH. The Cd(II)-PEMP suspension was placed in a shaker (MaxQ 8000, Thermo Scientific, USA) and agitated at 160 rpm, with temperature maintained at 30 ± 1 °C. After the Cd(II)-PEMP equilibrium was attained at 24 h of agitation, the samples were filtered with 0.45-mm polytetrafluoroethylene membrane, and Cd(II) in filtrate samples was examined using inductively coupled plasma optical emission spectroscopy (ICP-OES). For the detection and quantification of heavy metal ions in an aqueous solution, ICP-OES is generally used (Vijayaraghavan and Segovia 2013). The Cd(II)-PEMP isotherm experiments were conducted by altering the initial Cd(II) concentration (2–25 mg/L) at the optimum equilibrium solution pH. The kinetic experimental analyses were conducted similarly to that of isotherm trials, and it involves taking samples at predetermined time intervals to quantify the equilibrium time. The elution experiments were conducted using Cd(II)-loaded PEMP contacted with different eluants including acids (0.01 M HCl, 0.01 M H2SO4, and 0.01 M HNO3), deionized water, and base (0.01 M NaOH). The elution experiments were conducted for 1 h of agitation at 160 rpm.
Data modeling
The experimental Cd(II) isotherm data are described using the following models:
Langmuir model:
Freundlich model:
Sips model:
where Q is experimental Cd(II) uptake by PEMP (mg/g); Cf is the final Cd(II) concentration in solution (mg/L); Qmax is the maximum Cd(II) uptake by PEMP (mg/g); bL is the Langmuir model constant (L/mg); KF is the Freundlich model constant (mg/g) (L/mg)1/nF; nF is the Freundlich model exponent; KS is the Sips model isotherm constant (L/g)βS; aS is the Sips model constant (L/mg)βS; and βS is the Sips model exponent.
The experimental Cd(II) kinetics data are described using the following models,
Pseudo-first-order model:
Pseudo-second-order model:
where Qt (mg/g) corresponds to Cd(II) sorptional capacity at time t; Qe (mg/g) corresponds to equilibrium Cd(II) uptake by PEMP; k1 denotes pseudo-first-order model constant; and k2 denotes the pseudo-second-order model constant.
The models were evaluated through nonlinear regression in Sigma Plot software (version 11.0, USA). The experiments were duplicated, and error bars were included for all data.
Results and discussion
Influence of equilibrium pH
The equilibrium pH is a critical characteristic that affects the chemical properties of metals and the surface characteristics of microplastic particles (Joo et al. 2021). To study this effect, the pH of the PEMP-Cd(II) mixture was varied by keeping the initial Cd(II) concentration fixed at 10 mg/L. As indicated in Fig. 1, as the pH of the PEMP-Cd(II) mixture increased from 2 to 5, the Cd(II) uptake capacity of PEMP increased with the highest sorption capacity reached at pH 5. This enhanced Cd(II) uptakes by PEMP as the equilibrium pH changes from 2.0 to 5.0 is due to various reasons. One reason for this is that when the pH is below 8, Cd exists in the form of Cd(II) in solutions (Vijayaraghavan et al. 2012). In highly acidic conditions, the surplus H+ binds to the microplastic surface instead of the divalent metal ion, leading to the protonation of the microplastic. This causes a decrease in the uptake of Cd(II) by the microplastic. As the pH amplifies, the availability of H+ ions declines, which allows additional Cd(II) ions to bind to the microplastic and increases the uptake of Cd2+. Experiments were not performed beyond pH 5 as there exists a possibility of metal ion precipitation, which may interfere with the sorption process. The results denoted that the Cd(II) adsorption capacity of PEMP increased from 0.34 at pH 2 to 1.21 mg/g at pH 5.
PEMP surface analyses
Figure 2 shows the SEM of raw PEMP and Cd(II)-bearing PEMP. The SEM technique is generally employed to analyze the surface morphology of microplastics (Dong et al. 2020; Wang et al. 2022). The raw PEMP surface was characterized by its irregularity and a high number of pores. Thus, the raw PEMP displayed characteristics of porous polymer, which make it more likely for pollutants to be adsorbed (Li et al. 2018). On the other hand, exposure of PEMP to Cd(II) results in a comparatively smooth surface. The XRD of both raw and Cd(II)-bearing PEMP is displayed in Fig. 3. The XRD spectra allow for the clear observation of the degree of crystallinity in polyethylene microplastics (Moura et al. 2023). Typically, microplastics with a high level of crystallinity display sharp diffraction peaks (Krasucka et al. 2022). The existence of sharp and clear peaks in the XRD analyses confirms that PEMP has an elevated crystalline nature (Fig. 3). On contact with Cd(II) ions, Cd(II)-bearing PEMP displayed decreased peaks at 21.5 and 23.4°.
FTIR spectra of both raw and Cd(II)-exposed PEMP samples are presented in Fig. 4. FTIR spectroscopy is frequently utilized to analyze the surface of microplastics (Liu et al. 2022b; Moura et al. 2023). The peaks observed at 2913, 2849, 1465, and 717 cm−1 in the FTIR spectra can be attributed to the carbon chains of polyethylene microplastics, specifically to CH2 symmetric stretching, CH2 symmetric stretching, bending deformation, and rocking deformation, respectively. As shown in Fig. 4, slight variations in peaks within the 600–1500 cm−1 range were observed in the PEMP sample exposed to Cd(II), indicating that electrostatic interaction between various functional groups on the surface of PEMP with Cd(II) ions caused by the existence of double bonds in PEMP.
Sorptional isotherms
The sorptional isotherms are crucial for determining the maximum adsorption capacity of a sorbent as well as the attraction of sorbate to the adsorbent (Vijayaraghavan and Yun 2008). The isotherm trials were performed at an equilibrium pH of 5, by altering the Cd(II) concentration from 2 to 25 mg/L. As indicated in Fig. 5, Cd(II) sorption isotherm by PEMP was favorable as indicated by a steep slope. This confirms that PEMP has a strong attraction to Cd(II) ions. The sorptional uptake of PEMP increased as the initial Cd(II) concentration enhanced and attained a maximum at 25 mg/L with the highest uptake of 1.36 mg/g.
To evaluate the sorption process and the affinity of PEMP toward Cd(II) ions, several isotherm model equations were used. This includes the Freundlich, Langmuir, and Sips models. The constants of isotherm models, the respective correlation coefficient, and percentage errors are provided in Table 1. The Langmuir equation is a popular sorption isotherm for describing the sorption isotherm (Langmuir 1918). The model comprises of two constants, with the first constant (Qmax) representing the highest possible sorption capacity and the second constant (bL) characterizing the affinity of the adsorbate toward the sorbent. In the case of Cd(II)-PEMP, the bL, and Qmax were recorded as 4.93 L/mg and 1.37 mg/g, respectively (Table 1). Additionally, the Langmuir equation exhibited the potential to predict the Cd(II) isotherm accurately, as proved by the low percentage errors and high correlation coefficients. The Freundlich equation is empirical and was often utilized to assess adsorbent surfaces with different degrees of attraction (Freundlich 1907). The model involves two constants, with the first constant (KF), representing the adsorbent binding strength and the second constant (nF) representing the attraction between PEMP and Cd(II) ions. The KF was observed to be 0.920, and the nF was recorded as 6.32. Nevertheless, the Freundlich model was unable to accurately represent the experimental Cd(II)-PEMP isotherm as evidenced by the significantly low correlation coefficient and high percentage errors (Table 1). It is important to note that the model is only applicable at the low-to-intermediate concentration levels. Additionally, as signified in Fig. 5, the Cd(II)-PEMP isotherm as predicted by the Freundlich model does not plateau as the Cd(II) concentration increases. The Sips model (Sips 1948) is a three-parameter equation that integrates the characteristics of Langmuir and Freundlich equations. The Sips model behaves similarly to the Freundlich equation at low adsorbate concentration. However, the model reduces to the Langmuir equation at a high adsorbate concentration. The Sips model, which includes an additional parameter, was able to accurately predict the adsorption of Cd(II) by PEMP, as demonstrated by the high correlation coefficient and low percentage error values (Table 1). The model predicted sorptional uptake agreed well with the experimental Cd(II) sorptional uptake. The exponent (βS) of the Sips model was determined to be closer to unity, indicating that Cd(II)-PEMP isotherm was consistent with the Langmuir form. Figure 5 presents isotherm curves calculated from all three isotherm equations.
Kinetics and modeling
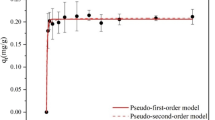
Figure 6 illustrates time versus Cd(II) sorptional capacity plots for PEMP at various initial Cd(II) concentrations (10 and 20 mg/L). Complete knowledge of the adsorption kinetic profile is essential for understanding the sorption mechanism, interaction pathway, and estimation of contact time required to attain sorbent–sorbate equilibrium (Azizian 2004). As clear from Fig. 6, rapid Cd(II) sorptional rate by PEMP was observed when the Cd(II) concentration was at its lowest (10 mg/L). The results indicated that PEMP recorded 85% total Cd(II) removal in 12 h. After this initial quick sorption rate, the Cd(II) sorption rate decelerated, and equilibrium was reached after 22 h. This dual-stage behavior is frequently visualized in adsorption systems due to the abundance of binding groups that can be exchanged on the PEMP surface at the start of the adsorption (Vijayaraghavan and Ashokkumar 2019). As the Cd(II)-PEMP sorption continues, the binding sites were occupied, which leads to a decrease in the rate and eventually attaining equilibrium. As seen in Fig. 6, the sorption rate declined as the Cd(II) concentrations enhanced.
The pseudo-first- and second-order models were employed to evaluate Cd(II)-PEMP kinetic data (Ho and McKay 1999; McKay et al. 1999). The results, including predicted uptakes, rate constants, R2 values, and percentage error, are shown in Table 2. The pseudo-first-order equation was observed to be more accurate in describing the Cd(II) kinetics, as it had a low percentage error and high R2 values. Additionally, the predicted Cd(II) equilibrium uptake was in good agreement with the experimentally predicted values. Furthermore, as the initial Cd(II) concentrations enhanced, the rate constant (k1) increased. While the pseudo-second-order kinetics gave good results with high correlation coefficient values and low percentage errors as indicated in Table 2, it was found to over-evaluate the equilibrium sorption capacity. Furthermore, a comparison of the percentage error and correlation coefficient values (Table 2) indicated that the pseudo-first model equation better predicted the experimental Cd(II)-PEMP kinetics. This can also be observed in Fig. 6, which displays the experimental Cd(II) kinetics data alongside the model predictions.
Desorption
The goal of the desorption trials is to determine how effectively metal ions, such as Cd(II), are transported from Cd-loaded microplastics to aquatic environments when various acidic/alkaline environments prevail. The pH of the aquatic freshwater environment is generally favorable for the sorption of metal ions onto microplastics. However, if the microplastics are transported to environments such as the digestive tract of aquatic animals where acidic (low pH) conditions exist, the adsorbed metal ions may be released from the microplastics (Prinz and Korez 2020). To investigate how Cd(II)-loaded PEMP behaves in acidic environments, three different acidic agents were tested, namely 0.01 M nitric acid, sulfuric acid, and hydrochloric acid. These desorbents were effective at releasing Cd(II) from PEMP, with desorption efficiencies of 99.1, 99.8, and 99.2% respectively, observed for 0.01 M hydrochloric, nitric acid, and sulfuric acids. These findings align with the results of the pH trials (Fig. 1), which demonstrate that acidic pH resulted in inferior Cd(II) sorption uptake due to the protonation of the microplastics. In contrast, control experimental trials with distilled water as desorbent showed that PEMP exhibited a high affinity toward Cd(II), with a low desorption efficiency of 2.5%. Similarly, the desorption was not as effective in the case of alkaline desorbent, as 0.01 M NaOH exhibited 6.4% elution efficiency. Therefore, the elution experimental trials demonstrate that microplastics can act as carriers for metal ions, such as Cd(II), and eventually release them into acidic environments and biological tissues.
Conclusion
Microplastics and heavy metals often pollute freshwater ecosystems. The combination of these pollutants can pose a significant threat to aquatic environments. The present research studied the adsorption potential of PEMP toward Cd(II) ions as well as desorption under different pH conditions. The XRD, SEM, and FTIR characterization of Cd(II)-PEMP samples provided evidence on the presence of different functional sites and the porous nature of the PEMP surface. Under the conditions examined, pH edge experiments indicated that maximum Cd(II) adsorption by PEMP occurred at pH 5. The experimental sorption isotherm was well-described by the Sips model, followed by the Langmuir and Freundlich models. Kinetic experiments signified that the adsorption of Cd(II) was slow and attainment of equilibrium required 22 h. The pseudo-first-order equation predicted the Cd(II) kinetics well with a high correlation coefficient and low percentage error values. Elution experiments indicate that it is possible to completely desorb Cd(II) ions from Cd(II)-loaded PEMP with acidic eluants. These results confirmed that microplastics containing Cd(II) may release Cd(II) ions in acidic conditions, potentially leading to the ingestion of Cd(II) ions by aquatic animals. Thus, the present study provided evidence that polyethylene microplastics can act an adsorbent for Cd(II) ions under examined optimum conditions and can leach the bounded Cd(II) ions in acidic environments.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arpia AA, Chen WH, Ubando AT, Naqvi SR, Culaba AB (2021) Microplastic degradation as a sustainable concurrent approach for producing biofuel and obliterating hazardous environmental effects: a state-of-the-art review. J Hazard Mater 418:126381
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47–52
Banaee M, Soltanian S, Sureda A, Gholamhosseini A, Haghi BN, Akhlaghi M, Derikvandy A (2019) Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236:124335
Bao ZZ, Chen ZF, Zhong Y, Wang G, Qi Z, Cai Z (2021) Adsorption of phenanthrene and its monohydroxy derivatives on polyvinyl chloride microplastics in aqueous solution: model fitting and mechanism analysis. Sci Total Environ 764:142889
Brennecke D, Paiva DB, F, Caçador I Canning-Clode J, (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195
Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF (2020) A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health 17(4):1212
Chen X, Gu X, Bao L, Ma S, Mu Y (2021a) Comparison of adsorption and desorption of triclosan between microplastics and soil particles. Chemosphere 263:127947
Chen Y, Li J, Wang F, Yang H, Liu L (2021b) Adsorption of tetracyclines onto polyethylene microplastics: a combined study of experiment and molecular dynamics simulation. Chemosphere 265:129133
Citterich F, Giudice AL, Azzaro M (2023) A plastic world: a review of microplastic pollution in the freshwaters of the Earth’s poles. Sci Total Environ 869:161847
Das P, Halder G, Bal M (2023) A critical review on remediation of microplastics using microalgae from aqueous system. Sci Total Environ 898:166425
Dong Y, Gao M, Song Z, Qiu W (2020) As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 239:124792
Freundlich H (1907) Ueber die adsorption in loesungen. Z Phys Chem 57:385–470
Fu L, Li J, Wang G, Luan Y, Dai W (2021) Adsorption behavior of organic pollutants on microplastics. Ecotoxicol Environ Safety 217:112207
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Issac MN, Kandasubramanian B (2021) Effect of microplastics in water and aquatic systems. Environ Sci Pollut Res 28:19544–19562
Joo SH, Liang Y, Kim M, Byun J, Choi H (2021) Microplastics with adsorbed contaminants: mechanisms and treatment. Environ Chall 3:100042
Khalid N, Aqeel M, Noman A, Khan SM, Akhter N (2021) Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ Poll 290:118104
Krasucka P, Bogusz A, Baranowska-Wójcik E, Czech B, Szwajgier D, Rek M, Ok YS, Oleszczuk P (2022) Digestion of plastics using in vitro human gastrointestinal tract and their potential to adsorb emerging organic pollutants. Sci Total Environ 843:157108
Kutralam-Muniasamy G, Pérez-Guevara F, Martínez IE, Shruti VC (2021) Overview of microplastics pollution with heavy metals: analytical methods, occurrence, transfer risks and call for standardization. J Hazard Mater 415:125755
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Poll 237:460–467
Liu X, Shi H, Xie B, Dionysiou DD, Zhao Y (2019) Microplastics as both a sink and a source of bisphenol a in the marine environment. Environ Sci Technol 53(17):10188–10196
Liu Q, Wu H, Chen J, Guo B, Zhao X, Lin H, Li W, Zhao X, Lv S, Huang C (2022a) Adsorption mechanism of trace heavy metals on microplastics and simulating their effect on microalgae in river. Environ Res 214:113777
Liu S, Huang JH, Zhang W, Shi LX, Yi KX, Yu HB, Zhang CY, Li SZ, Li JN (2022b) Microplastics as a vehicle of heavy metals in aquatic environments: a review of adsorption factors, mechanisms, and biological effects. J Environ Manage 302:113995
McKay G, Ho YS, Ng JCP (1999) Biosorption of copper from waste waters: a review. Sep Purif Methods 28:87–125
Moura DS, Pestana CJ, Moffat CF, Hui J, Irvine JTS, Lawton LA (2023) Characterisation of microplastics is key for reliable data interpretation. Chemosphere 331:138691
Nunes BZ, Huang Y, Ribeiro VV, Wu S, Holbech H, Moreira LB, Xu EG, Castro IB (2023) Microplastic contamination in seawater across global marine protected areas boundaries. Environ Poll 316(1):120692
Prinz N, Korez Š (2020). Understanding how microplastics affect marine biota on the cellular level is important for assessing ecosystem function: a review. In: Jungblut, S., Liebich, V., Bode-Dalby, M. (eds) YOUMARES 9—The Oceans: Our Research, Our Future. Springer, Cham. https://doi.org/10.1007/978-3-030-20389-4_6
Sips R (1948) On the structure of a catalyst surface. J Chem Phys 16:490–495
Verougstraete V, Lison D, Hotz P (2002) A systematic review of cytogenetic studies conducted in human populations exposed to cadmium compounds. Mutation Res 511:15–43
Vijayaraghavan K, Ashokkumar T (2019) Characterization and evaluation of reactive dye adsorption onto biochar derived from Turbinaria conoides biomass. Environ Prog Sustain Energy 38:13143
Vijayaraghavan K, Segovia E (2013) Development of bench-scale bio-packed column for wastewater treatment from optical emission spectrometry. Clean: Soil, Air, Water 41:1093–1099
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Vijayaraghavan K, Joshi UM, Balasubramanian R (2010) Removal of metal ions from storm-water runoff by low-cost sorbents: batch and column studies. J Environ Eng 136:1113–1118
Vijayaraghavan K, Gupta S, Joshi UM (2012) Comparative assessment of Al(III) and Cd(II) biosorption onto Turbinaria conoides in single and binary systems. Water Air Soil Pollut 223:2923–2931
Wang X, Zhang R, Li Z, Yan B (2022) Adsorption properties and influencing factors of Cu(II) on polystyrene and polyethylene terephthalate microplastics in seawater. Sci Total Environ 812:152573
Wang Y, Wu Y, Pu Q, Sun P, Zhao W, Liu M, Li Y (2023) Aquatic toxicity of tire microplastics on marine and freshwater organisms: an in silico approach. Chemosphere 313:137523
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reddy Prasad, D.M., Naveen Prasad, B.S., Senthilkumar, R. et al. Interactive behavior of cadmium ions onto polyethylene microplastics in aquatic system. Int. J. Environ. Sci. Technol. 21, 7915–7922 (2024). https://doi.org/10.1007/s13762-024-05508-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-024-05508-9