Abstract
With the development of the mining and metallurgy industries, heavy metal emissions are rising and increasingly polluting the soil. Heavy metals cause soil degradation, reductions in crop yield and quality, and the sustainability of land resources, and threaten regional biodiversity and human health. Accordingly, soil heavy metal pollution and remediation are attracting increasing global attention. Biochar is an excellent fixation agent that has been widely used in the remediation of heavy metal-contaminated soils. In this study, the feasibility of biochar remediation was explored by adding various doses of it to contaminated soil. Five treatments were explored: contaminated soil (controls), and contaminated soil with biochar doses of 1%, 2%, 4% and 10%. The influences of biochar on the forms and contents of soil heavy metals and microbial activity were determined. Biochar was found to passivate heavy metals, reduce the contents of acid-soluble and reducible Cd, Pb, Cu, and Zn, increase the contents of oxidizable and residual Cd, Pb, Cu, and Zn, increase soil basal respiration and microbial carbon, reduce microbial respiration entropy, reduce fluorescein diacetate (FDA) hydrolase activity, and increase the activities of dehydrogenase, catalase, and urease. Biochar clearly affected the forms and availability of heavy metals in red soil and soil microbial activity. Biochar is an ideal conditioner for the remediation of heavy metal-contaminated red soil in mining areas, for which the present study provides a theoretical basis and practical guidance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid growth in industrial and agricultural production, humans are increasingly exploiting the earth's resources and our living environment (water, soil, gas, natural resources, etc.) is constantly deteriorating (Li and Yang 2021). Soil is essential to human existence, yet is deteriorating due to the discharge of industrial waste, urban sewage and garbage, and the use of pesticides and fertilizers containing heavy metals (Ren et al. 2021). Heavy metals are the main pollutants affecting the quality of agricultural land (Barsova et al. 2019). One investigation showed that there were about 330 severe heavy metal pollution areas in China with soil heavy metal contents seriously exceeding normal levels (Huang et al. 2019). There are many causes of soil heavy metal pollution, among which mining is one of the main ones (Davis et al. 1991; Zhang et al. 2016). Soil heavy metal pollution is characterized by concealment, persistence, and irreversibility. Heavy metal pollution not only reduces crop yields but also accumulates in the food chain and affects human health (Mclaughlin et al. 1999). Therefore, remediation of heavy metal-contaminated soil is challenging (Lacalle et al. 2018). Commonly used remediation technologies for contaminated soil can be divided into three categories: physical remediation, chemical remediation, and bioremediation (Ojuederie and Babalola 2017; Wang et al. 2020a, b, c; Xu et al. 2019a, b). Among them, in situ passivation technology for the remediation of heavy metal-contaminated soil has been widely studied because of its low cost, simple operation, and ready popularization. The selection of passivating agents is also important in soil remediation. Passivators can be divided into inorganic and organic passivators. At present, organic waste and biochar are the most widely used organic passivators (Mohan et al. 2014).
Biochar is a solid substance made from crop straw, various types of wood chips, and animal excrement subjected to high-temperature pyrolysis and carbonization under complete or incomplete hypoxia (Downie et al. 2012). Biochar not only improves soil physical and chemical properties but also contains an abundant pore structure, which provides habitats for microorganisms. Since biochar has been applied to agricultural production, many studies have shown that it not only improves the living environment of microorganisms but also enhances microbial activity (Zhu et al. 2017) and increases the abundance and diversity of microbial communities (Siedt et al. 2021). However, some studies have found that polycyclic aromatic hydrocarbons (Quilliam et al. 2013), volatile organic compounds (Dutta et al. 2017), and persistent free radicals can be produced during high-temperature pyrolysis, which will have toxic effects on soil microorganisms when biochar is applied to the soil (Tao et al. 2020). Biochar also has a strong adsorption capability; it can adsorb not only nutrients but also heavy metals, so it can be used for the remediation of heavy metal-contaminated soil (Li et al. 2017). The remediation function of biochar in heavy metal-contaminated soil has received much research attention. For example, Liu et al. (2020) found that both biochar and modified biochar can remove cadmium, with the removal rate of modified biochar being about 45% higher. Han et al. (2020) found that the specific surface area of biochar made from animal feces increased 3–6 times after treatment with NaOH, and the adsorption capacity of uranium(VI) was significantly increased. Biochar has many advantages as a potential soil conditioner, such as low price, availability of raw materials, multiple functions, and low pollution. It can remediate and improve heavy metal-contaminated soil by improving the soil’s physical and chemical properties and enhancing soil fertility (Shaaban et al. 2018; Wang et al. 2019).
Large accumulations of ore in mining areas are easily weathered and broken, releasing a large number of heavy metals that penetrate into the soil and enter rivers with rain runoff, causing soil and water pollution and seriously affecting environmental and food safety. Accordingly, the remediation of polluted mining areas is an urgent issue. This study investigates the use of biochar as a conditioner of contaminated soil. Through adsorption, complexation, ion precipitation, and other physical and chemical reactions, it may be able to change the availability of heavy metals, thus influencing soil microbial activity. Eventually, it may improve soil environmental quality and help to remediate heavy metal-contaminated soils. At present, many studies on the biochar remediation of heavy metal-contaminated soil have focused on the short-term removal of a single metal (Sun et al. 2021; Kameyama et al. 2021). However, many soils are contaminated with multiple heavy metals, especially in mining areas. It has also been found that the timing of biochar application influences its long-term effectiveness. Previous research has focused on the concentrations and morphological changes of heavy metal pollutants during the remediation process (Wang et al. 2020a, b, c; Xiao et al. 2020). There has been little exploration of remediation effects with consideration of soil microbial activity. An indoor pot experiment was conducted using contaminated soil from Chengmenshan Copper Mine at the Nanchang Institute of Technology in March 2020. In a 180-day indoor experiment, different doses of biochar were added to the contaminated soil to explore its effects on the forms and contents of heavy metals and the levels of microbial activity. The results provide a theoretical basis and practical guidance for using biochar to remediate heavy metal-contaminated soils.
Materials and methods
Materials
The tested soil was taken from the Chengmenshan Copper Mine. Its basic physical and chemical properties were as follows: pH = 3.85, organic carbon = 10.79 g·kg−1, total N = 0.94 g·kg−1, and total P = 1.17 g·kg−1. The contents of Cd, Pb, Cu, and Zn were 4.41 mg·kg−1, 122.53 mg·kg−1, 372.44 mg·kg−1, and 328.57 mg·kg−1, respectively. These are all higher than the soil pollution risk-control values for agricultural land stipulated in standard GB15618-2018 (2018).
Biochar was purchased from Sanli New Energy Company in Shangqiu. It was made with wheat straw under anaerobic conditions at 350–500 ℃. The physicochemical properties of the biochar were: pH = 10.35, organic carbon = 467.20 g·kg−1, total N = 5.90 g·kg−1, total P = 14.43 g·kg−1, and total K = 11.56 g·kg−1.
Experimental design
Five treatments were set up: 1) K (contaminated soil, as a control), 2) KC1 (contaminated soil + 1% biochar), 3) KC2 (contaminated soil + 2% biochar), 4) KC3 (contaminated soil + 4% biochar), and 5) KC4 (contaminated soil + 10% biochar). According to the experimental design, the biochar was applied to the contaminated soils at the same time and then mixed evenly. Some 1 kg of the mixture was placed in each pot. Each treatment had five replicates, making a total of 25 pots. The temperature in the incubation chamber was 26 ± 0.2 °C. The soil water content was adjusted daily with ultrapure water to 60% of the soil saturated water content by weight. After incubation for 180 days, the soil was collected to analyze the soil microbial activity and the forms and contents of heavy metals.
Methods
Forms and contents of heavy metals
The forms and contents of heavy metals in the soil were determined by a modified European Community Bureau of Reference (BCR) sequential extraction method (Quevauv et al. 1993). A 1.00 g soil sample was weighed and added into a 50 mL centrifuge tube, then the acid-soluble heavy metals were extracted with 0.11 mol·L−1 acetic acid, the reducible heavy metals were extracted by 0.5 mol·L−1 NH2OH·HCl (pH = 2.0), the oxidizable heavy metals were extracted with 30% hydrogen peroxide and 1 mol·L−1 ammonium acetate, and the residual heavy metals were extracted with 6 mol·L−1 hydrochloric acid and 14 mol·L−1 nitric acid. Finally, inductively coupled plasma emission spectrometry (ICP-MS) was used to identify the metals.
Soil microbial activity
Soil basal respiration was measured by the alkali absorption method (Bolat 2019). Soil microbial carbon was determined by chloroform fumigation-K2SO4 extraction (Brookes et al. 1985). The soil respiratory quotient (qCO2) was calculated based on the soil basal respiration rate and soil microbial biomass carbon content using the formula of Anderson and Domsch (1993). The qCO2 indicates the microbial biomass’s efficiency in using effective carbon for biosynthesis and is considered an important indicator of biological activity and substrate quality (Wardle and Ghani 1995). Soil fluorescein diacetate hydrolase activity was determined by the optimized fluorescein diacetate lipid hydrolysis method (Adam and Duncan 2001). Soil dehydrogenase activity was measured by the triphenyte trazoliumchloride reduction method (Wittling et al. 1996). Soil catalase activity was measured by potassium permanganate titration (Johnson and Temple 1964). Soil urease activity was determined by a colorimetric method using brilliant phenol blue (Apha 1998).
Data analysis
The data were collated and plotted using Microsoft Excel 2013. SPSS 19.0 was used for a one-way ANOVA analysis of differences between treatments. Least significant differences were used with the significance level set at p < 0.05. All data are presented as means ± standard deviation (n = 5).
Results and discussion
Effects of different doses of biochar on the species and contents of heavy metals in contaminated soil
The content of acid-soluble Cd was highest in control soil (K) and was significantly lower by 71.20%, 65.60%, 65.47%, and 64.80% in groups KC1, KC2, KC3, and KC4, respectively. Hence, biochar application decreased the content of acid-soluble Cd in soil (Table 1). The content of reducible Cd was highest in controls and was significantly lower by 74.15%, 71.46%, 72.32%, and 68.66% in groups KC1, KC2, KC3, and KC4, respectively. Hence, biochar application also decreased the amount of reducible Cd in soil. The content of oxidizable Cd was highest in KC4, and was significantly lower than in controls in groups KC1 and KC2, by 36.88% and 21.88%, respectively. Biochar addition increased the amount of residual Cd in soil. The contents in KC1, KC2, KC3, and KC4 were significantly higher than in controls, by 44.16%, 42.38%, 43.17%, and 39.92%, respectively.
The content of acid-soluble Pb was highest in controls and was significantly lower, by 71.45%, 61.82%, 49.89%, and 74.40%, in KC1, KC2, KC3, and KC4, respectively. Hence, biochar application reduced the content of acid-soluble Pb (Table 1). The reducible Pb content was highest in controls and was significantly reduced, by 53.13%, 44.44%, 63.57%, and 76.00%, in KC1, KC2, KC3, and KC4, respectively. Hence, biochar application reduced the reducible Pb content. The content of oxidizable Pb was highest in KC4. Compared with controls, its contents in KC1, KC2, KC3, and KC4 were 17.83%, 16.93%, 20.53%, and 45.85% higher, respectively. The contents of residual Pb were significantly higher than in controls, by 10.97%, 23.54%, 18.51%, and 22.12%, in KC1, KC2, KC3, and KC4, respectively. Hence, biochar addition increased the content of residual Pb.
The content of acid-soluble Cu was highest in controls, and significantly lower, by 50.58%, 38.12%, 34.64%, and 60.23%, in KC1, KC2, KC3, and KC4, respectively (Table 1). The content of reducible Cu was highest in controls and significantly lower, by 47.63%, 38.48%, 57.83%, and 65.06%, in KC1, KC2, KC3, and KC4, respectively. The content of oxidizable Cu was highest in KC1 and was 25.99% and 23.04% higher than in controls in KC1 and KC4, respectively. Biochar addition increased the content of residual Cu in soil; its contents in KC1, KC2, KC3, and KC4 were significantly higher, by 14.95%, 33.55%, 37.85%, and 32.70%, compared to controls, respectively.
The content of acid-soluble Zn was highest in controls and was significantly lower, by 51.84%, 45.31%, 52.14%, and 61.65%, in KC1, KC2, KC3, and KC4, respectively (Table 1). The content of reducible Zn was highest in controls and was significantly lower, by 39.07%, 35.31%, 29.07%, and 27.88%, in KC1, KC2, KC3, and KC4, respectively. The content of oxidizable Zn was highest in KC4, and its contents in KC1, KC2, KC3, and KC4 were significantly higher than in controls, by 18.75%, 34.75%, 55.94%, and 86.98%, respectively. Hence, biochar application increased the content of oxidizable Zn. The contents of residual Zn in KC1, KC2, KC3, and KC4 were significantly higher than in controls, by 20.00%, 31.57%, 41.94%, and 35.47%, respectively. Biochar addition increased the content of residual Zn in soil.
Effects of different doses of biochar on microbial activity in contaminated soil
Effects on soil basal respiration
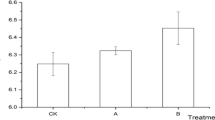
Soil basal respiration increased firstly and then decreased with increases in biochar dose. The soil basal respiration of each treatment was ranked KC1 > KC4 > KC2 > K > KC3 (Fig. 1). Soil basal respiration in KC1 was significantly higher, by 20.07%, compared with controls, but there were no significant differences between KC2, KC3, and KC4. Soil basal respiration in KC2 and KC3 was significantly lower, by 15.29% and 20.67%, respectively, compared with that of KC1. Hence, the application of low-dose biochar (1%) significantly increased soil basal respiration.
Effects of biochar on soil basal respiration in contaminated soil. K: contaminated soil; KC1: contaminated soil + 1% biochar; KC2: contaminated soil + 2% biochar; KC3: contaminated soil + 4% biochar; KC4: contaminated soil + 10% biochar. Data are displayed as means ± SD (n = 5). Different letters indicate a significant difference between treatments at p < 0.05
Effects on soil microbial carbon
Soil microbial carbon increased with biochar dose. The amount of soil microbial carbon in each treatment was ranked KC4 > KC3 > KC1 > KC2 > K (Fig. 2). Soil microbial carbon in KC1, KC2, KC3, and KC4 were significantly higher, by 22.75%, 11.93%, 29.70%, and 50.59%, respectively, compared with controls. Soil microbial carbon in KC4 was significantly higher, by 34.55%, compared with that of KC2. Hence, biochar application can increase soil microbial carbon, with a high dose (10%) increasing it the most.
Effects of biochar on MBC in contaminated soil. K: contaminated soil; KC1: contaminated soil + 1% biochar; KC2: contaminated soil + 2% biochar; KC3: contaminated soil + 4% biochar; KC4: contaminated soil + 10% biochar. Data are displayed as means ± SD (n = 5). Different letters indicate a significant difference between treatments at p < 0.05
Effects on microbial respiration entropy (qCO2)
Microbial respiration entropy (qCO2) increased first and then decreased with increases in biochar dose. The order of qCO2 in each treatment was KC1 > K > KC2 > KC3 > KC4 (Fig. 3). The qCO2 in KC3 and KC4 were significantly higher, by 23.41% and 24.75%, respectively, compared with controls, and there was no significant difference between KC1 and KC2. The qCO2 of KC3 and KC4 was significantly lower, by 29.43% and 30.66%, compared with KC1, respectively. The application of high-dose biochar (10%) had the greatest effect on qCO2.
Effect of biochar on microbial respiration entropy in contaminated soil. K: contaminated soil; KC1: contaminated soil + 1% biochar; KC2: contaminated soil + 2% biochar; KC3: contaminated soil + 4% biochar; KC4: contaminated soil + 10% biochar. Data are displayed as means ± SD (n = 5). Different letters indicate a significant difference between treatments at p < 0.05
Effects on soil enzyme activity
Soil FDA hydrolase activity decreased with increases in biochar dose, with the treatments ranked K > KC1 > KC3 > KC4 > KC2 (Fig. 4A). FDA hydrolase activity in KC1, KC2, KC3, and KC4 was significantly lower, by 18.83%, 34.78%, 25.84%, and 28.95%, respectively, compared with controls, while that of KC2 was significantly lower, by 19.66%, compared with KC1. Biochar application reduced FDA hydrolase activity, with the medium dose (2%) having the greatest effect.
Effects of biochar on the activities of FDA hydrolysis (A), dehydrogenases (B), catalase (C), and urease (D) in contaminated soil. K: contaminated soil; KC1: contaminated soil + 1% biochar; KC2: contaminated soil + 2% biochar; KC3: contaminated soil + 4% biochar; KC4: contaminated soil + 10% biochar. Data are displayed as means ± SD (n = 5). Different letters indicate a significant difference between treatments at p < 0.05
Soil dehydrogenase activity increased with biochar dose, and the treatments were ranked KC4 > KC3 > KC2 > K > KC1 (Fig. 4B). Dehydrogenase activity in KC2, KC3, and KC4 were significantly higher, by 24.00%, 27.68%, and 73.96%, respectively, compared with controls. Low-dose biochar (1%) did not significantly enhance dehydrogenase activity, while medium and high doses (2%, 4%, 10%) did, with the high dose having the best effect.
Soil catalase activity increased with biochar dose, and the treatments were ranked KC4 > KC3 > KC2 > K > KC1 (Fig. 4C). Catalase activity in KC3 and KC4 was significantly higher, by 90.25% and 251.21%, respectively, compared with controls. Catalase activity in KC1 was significantly lower, by 33.68%, compared with controls. Low-dose biochar (1%) significantly decreased catalase activity, while medium and high doses (2%, 4%, 10%) significantly increased it, with the high dose (10%) having the greatest effect.
Soil urease activity increased first and then decreased with increases in biochar dose. Urease activity in each treatment was ranked in the order of KC3 > KC1 > KC2 > K > KC4 (Fig. 4D). Urease activity in KC1 and KC3 was significantly higher, by 9.54% and 57.72%, respectively, compared with controls. Urease activity in KC4 was significantly lower, by 10.81%, compared with controls. Medium and low doses of biochar (1%, 2%, 4%) increased urease activity, while the high dose (10%) decreased it.
Correlations between biochar application, microbial activity, and heavy metal content
A correlation analysis was conducted on the biochar application, microbial activity, and heavy metal content data (Table 2).
Biochar application showed significant positive correlations with soil microbial biomass carbon, the activities of soil dehydrogenase and soil catalase, oxidizable-state Cd and Zn, and residual-state Cd, Cu and Zn (p < 0.01 or < 0.05 in all cases). It was negatively correlated with microbial respiration entropy, soil FDA hydrolase activity, acid-soluble-state Cd, Pb, Cu and Zn, and reducible-state Cd, Pb and Cu (p < 0.01 or < 0.05). Soil basal respiration showed positive correlations with microbial respiration entropy and oxidizable-state Cu (p < 0.01 or < 0.05), and negative correlations with acid-soluble-state Pb and Cu, and reducible-state Zn (p < 0.05). Soil microbial biomass carbon was positively correlated with soil dehydrogenase activity, soil catalase, oxidizable-state Cu and Zn, and residual-state Cd, Pb, Cu and Zn (p < 0.01 or < 0.05), and negatively correlated with microbial respiration entropy, soil FDA hydrolase activity, reducible-state Cd, Pb and Cu, and acid-soluble-state Cd, Pb, Cu and Zn (p < 0.01 or < 0.05). Microbial respiration entropy was positively correlated with soil FDA hydrolase activity and reducible-state Pb and Cu (p < 0.05), and negatively correlated with soil dehydrogenase activity, soil catalase, oxidizable state Cd, Pb and Zn, residual state Pb, Cu and Zn (p < 0.01 or < 0.05). Soil FDA hydrolase activity was positively correlated with acid-soluble-state Cd, Pb, Cu and Zn, and reducible-state Cd, Pb, Cu and Zn (p < 0.01), and negatively correlated with soil dehydrogenase activity, residual-state Cd, Pb, Cu and Zn, and oxidizable-state Pb and Zn (p < 0.01). Soil dehydrogenase activity was positively correlated with soil catalase, oxidizable-state Cd, Pb and Zn, and residual-state Pb, Cu and Zn (p < 0.01), and negatively correlated with reducible-state Pb and Cu, and acid-soluble-state Cu and Zn (p < 0.01 or < 0.05). Soil catalase activity was positively correlated with oxidizable-state Cd, Pb and Zn and residual-state Pb, Cu and Zn (p < 0.01 or < 0.05), and negatively correlated with reducible-state Pb and Cu, and acid-soluble-state Cu and Zn (p < 0.01 or < 0.05). Soil urease activity was positively correlated with residual-state Zn (p < 0.05) and negatively correlated with oxidizable-state Cu (p < 0.01).
Discussion
Heavy metals not only include elements with both strong biotoxicity (Hg, Cd, Pb, Cr) and moderate biotoxicity (Zn, Cu, Co, Sn) (Agostini et al. 2020). Studies have shown that the mobility, toxicity, and bioavailability of heavy metals in contaminated soil are not only related to the total amount of heavy metals, but also to their speciation (Carbonell et al. 2011). According to the modified BCR extraction method (Bolat 2019), heavy metals in soil can be in acid-soluble, reducible, oxidizable, and residue states. Acid-soluble states include exchangeable states and carbonate binding states, which are readily absorbed by plants and can cause great harm. Reducible states are ferric and manganese oxide-bound, which are readily absorbed by plants. Oxidizable states include organic matter and sulfide-bound, which are not readily used by plants. Residual states are strongly combined with mineral lattices; they are not readily used by plants and are less harmful. Acid-soluble and reducible states are regarded as available states; the higher their contents, the greater the environmental pollution. Wang et al. (2020b) found that the effect of biochar on the passivation rate of heavy metals varied with its addition amount. After adding 3%, 5%, and 7% biochar to pig manure, the highest passivation rates of Ni, As, and Pb were observed in the 5% biochar group, while 7% biochar caused the highest passivation rates of Cd, Cr, Mn, and Zn. The results of this study show that biochar can significantly reduce the contents of Cd, Pb, Cu, and Zn in acid-soluble and reducible states, and significantly increase the contents of Cd, Pb, Cu, and Zn in oxidizable and residual states. The amount of biochar applied was negatively correlated with the availability of heavy metals. This indicates that biochar can passivate heavy metals, which is consistent with the results of many other studies (Ahmad et al. 2016; Igalavithana et al. 2017). Xu et al. (2019b) used macadamia nutshell biochar to improve Cd- and Pb-spiked soils. Their study showed that biochar application can reduce the availability of heavy metals. The main reason can be attributed to biochar’s rich pore structure, oxygen-containing functional groups, and strong adsorption, which gives it a strong ability to adsorb and fix heavy metals in soil and, thus, a passivation effect (Igalavithana et al. 2017). Ahmad et al. (2016) proposed that biochar's effect on heavy metals is not only due to physical adsorption but also to various chemical reactions, such as complexation, precipitation, and ion exchange. These physical and chemical reactions interact and, eventually, change the forms of heavy metals. Meanwhile, biochar can also promote the precipitable formation of heavy metals, which will reduce the bioavailability of heavy metals and passivate them, inhibit their transfer from soil to plants, inhibit the diffusion and enrichment of heavy metals in the environment, and reduce their environmental harm.
Soil microbial activity not only indicates soil quality and fertility directly but also interacts with other soil organisms to affect soil quality, thus affecting the agricultural production and sustainability of soil ecosystems (Małgorzata et al. 2018). Soil basal respiration depends on soil microbial respiration and is an important indicator of soil microbial activity (Baath 1989). The results of this study show that low-dose biochar addition significantly promoted basal respiratory in contaminated soil, while medium-to-high-dose biochar addition did not, which is consistent with many other studies (Li et al. 2019; Romero-Freire et al. 2016). This can be attributed to the fact that biochar is a form of aromatic carbon, which is extremely difficult for microorganisms to use. As small amounts of biochar are added to the soil over time, some of it degrades and becomes a carbon source for microbes, thus improving microbial activity and enhancing soil basal respiration (Prayogo et al. 2014). On the other hand, the stress of heavy metals on soil microorganisms is alleviated due to increases in biochar application. Microorganisms do not need to carry out additional respiration to resist stress and, thus, soil respiration under high-dose biochar application is not accelerated (Romero-Freire et al. 2016).
Soil microbial biomass carbon is not only the most active part of soil organic carbon, but is also the most variable component of soil and is an important indicator of the degree of soil pollution (Giller et al. 1998). Soil microbial biomass carbon directly reflects the number of soil microorganisms. Martins Filho et al. (2021) found that biochar application increased soil microorganism growth and microbial biomass carbon content. This is consistent with the present study, which showed that biochar application promoted the microbial biomass carbon content of polluted soil in a dose-dependent manner. This is mainly due to the porous and alkaline characteristics of biochar, which can improve the physical and chemical properties of soil and benefit the survival and reproduction of microorganisms (Fowles 2007). In addition, biochar itself contains a small amount of unstable carbon, which can provide additional nutrients for the growth of microorganisms, provide favorable conditions for their survival, promote their reproduction, and increase soil microbial biomass carbon (Hamer et al. 2004). Soil microbial biomass carbon showed a negative correlation with heavy metal effectiveness. Hence, biochar application can reduce the availability of heavy metals and increase the microbial activity in the soil. At the same time, the microbial abundance increases, leading to an increase in microbial biomass carbon.
Soil microbial respiration entropy is the intensity of respiration per unit of microorganisms per unit time. It reflects the ability of microorganisms to use the soil matrix and is an indicator of microbial activity (Anderson and Domsch 1993). According to Odum ecosystem theory (Odum 1985), the lower the microbial respiratory entropy, the more mature and stable the environment is for microorganism survival. In agricultural soil, qCO2 values are inversely associated with soil microbial activity. The better the soil quality, the lower the qCO2 value and the higher the soil microbial activity. Higher qCO2 values indicate lower soil microbial activity due to the degradation of soil quality by overutilization, pollution, and other reasons. The results of this study show that microbial respiratory entropy decreased gradually with increases in biochar application amount, indicating that biochar application can create a more mature and stable environment and alleviate the stress of heavy metals on soil microorganisms.
Soil enzyme activity determines the rate of soil metabolism and indicates the strength and weakness of various biochemical processes in soil (Gloria et al. 2021). FDA hydrolase exists widely in soil, mainly from the decomposition of microbial cells and animal or plant residues (Schumacher et al. 2015). Dehydrogenase plays a key role in catalyzing the decomposition of organic matter and is also an important indicator of soil organic matter content and microbial activity (Tan et al. 2017). Soil catalase is an indicator of soil aerobic microbial activity and reflects soil redox capacity (Qin et al. 2020). Soil urease is the only enzyme directly involved in the transformation of N-containing organic matter, and its activity reflects the amount of nitrogen transformation in soil (Jimenez et al. 2002). The results of this study show that heavy metal stress caused stress responses in microorganisms and accelerated their metabolism, thus enhancing FDA hydrolase activity. Meanwhile, biochar application significantly reduced FDA hydrolase activity, mainly because it reduced the bioavailability of heavy metals. Hence, the heavy metal stress response of soil microorganisms was alleviated and their metabolic rate was reduced, ultimately decreasing FDA hydrolase activity. Biochar application significantly increased dehydrogenase activity, mainly because biochar is stable and not readily mineralized (El-Naggar et al. 2019). Therefore, it can enhance the decomposition of organic matter in the soil, facilitate the decomposition of organic carbon, and significantly promote dehydrogenase activity. Biochar application can also promote soil catalase activity, mainly because it increases soil organic matter, porosity, and aeration, changes the soil aggregate structure, improves the habitat for microorganisms, and is conducive to the growth and reproduction of aerobic microorganisms, thus improving catalase activity (Czimczik and Masiello 2007). Some studies have also shown that biochar addition improves soil urease activity (Berglund et al. 2004; Tu et al. 2020). The reason may be that biochar provides a nitrogen source and a substrate for the enzymatic reaction of urease, thereby promoting urease activity (Berglund et al. 2004). On the other hand, biochar has a strong adsorption capacity for soil nutrients, which is conducive to nitrogen preservation and the formation of a "nitrogen pool", which indirectly enhances urease activity (Tu et al. 2020). According to these facts, the interactions between biochar and soil enzyme activity are complex. On the one hand, the large surface area of biochar provides binding sites for enzymatic reactions, thus improving soil enzyme activity. On the other hand, the sustained release of biochar forms protective films at these binding sites and plays a role in the adsorption and retention of enzyme molecules, thus reducing the speed of enzymatic reactions. The activities of soil dehydrogenase and soil catalase showed negative correlations with heavy metal availability, while soil FDA hydrolase activity showed a positive correlation with it. After it is incorporated into the soil, the strong adsorptivity of biochar allows it to bind with heavy metal ions from the soil by precipitation or complexation (Chen et al. 2020). Biochar can also activate or inhibit soil microbial activity. It also provides a microenvironment for enzymes, with different biochars providing different microenvironments and enzymatic effects (Wu et al. 2022). Some enzymes remain stable and highly active, while others may be inactivated via changes in their molecular structure or interactions with the microenvironment. These effects are highly dependent on the surface properties of the biochar and enzymes (Zhang et al. 2019). Therefore, biochar addition has different effects on different soil enzyme activities, and the different kinds of microbial activity further affect the passivation of heavy metals. Most studies have shown that heavy metals are not only transferred to the human body via the food chain, but also damage the local environment. Therefore, biochar remediation is used in mining areas with serious heavy metal pollution as it can reduce soil pollutant concentrations while restoring the natural environment.
Conclusion
Biochar addition can passivate heavy metals, significantly reduce the contents of Cd, Pb, Cu, and Zn in acid-soluble and reducible states, and significantly increase the contents of Cd, Pb, Cu, and Zn in oxidizable and residual states. Biochar addition can increase soil basal respiration and soil microbial carbon, decrease microbial respiration entropy and FDA hydrolase activity, and increase the activities of soil dehydrogenase, catalase, and urease. As a soil conditioner added to heavy metal-contaminated soil, biochar not only improves the soil nutrient content but also reduces the contents of available heavy metals and passivates them, thus improving soil microbial activity. Such effects are conducive to the improvement and remediation of heavy metal-contaminated soil. The purpose of the present study was to provide theoretical guidance for the management of contaminated soil in mining areas. However, the optimal timing of biochar remediation remains controversial. In the future, the long-term effects of biochar application to soil need to be better understood to determine the optimal application timeframe. This will optimize biochar’s special functions of absorbing and passivating heavy metals and provide a reference for the remediation of heavy metal-contaminated sites.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Ahmad M, Ok YS, Kim BY, Ahnc JH, Lee YH, Zhang M, Moon DH, Al-Wabel MI, Lee SS (2016) Impact of soybean stover and pine needle-derived biochar on Pb and As mobility, microbial community, and carbon stability in a contaminated agricultural soil. J Environ Manag 166:131–139
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environment conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25(3):393–395
Apha A (1998) Standard methods for the examination of water and wastewater. APHA, Washington
Baath E (1989) Effects of heavy metals in soil on microbial processes and populations: a review. Water Air Soil Poll 47:335–379
Barsova N, Yakimenko O, Tolpeshta I, Motuzova G (2019) Current state and dynamics of heavy metal soil pollution in Russian Federation—a review. Environ Pollut 249:200–207
Berglund LM, Deluca TH, Zackrisson O (2004) Activated carbon amendments to soil alters nitrification rates in Scots pine forests. Soil Biol Biochem 36(12):2067–2073
Bolat L (2019) Microbial biomass, basal respiration, and microbial indices of soil in diverse croplands in a region of northwestern Turkey (Bartn). Environ Monit Assess 191(11):695
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Carbonell G, Imperial RMD, Torrijos M, Delgado M, Rodriguez JA (2011) Effects of municipal solid waste compost and mineral fertilizer amendments on soil properties and heavy metals distribution in maize plants (Zea mays L.). Chemosphere 85(10):1614–1623
Chen X, He HZ, Chen GK, Li HS (2020) Effects of biochar and crop straws on the bioavailability of cadmium in contaminated soil. Sci Rep 10:9528
Czimczik CI, Masiello CA (2007) Controls on black carbon storage in soils. Global Biogeochem Cy 21(3):1–8
Davis A, Olsen RL, Walker DR (1991) Distribution of metals between water and entrained sediment in streams impacted by acid mine discharge, Clear Creek, Colorado, USA. Appl Geochem 6(3):333–348
De Agostini A, Caltagirone C, Caredda A, Cicatelli A, Cogoni A, Farci D, Guarino F, Garau A, Labra M, Lussu M (2020) Heavy metal tolerance of orchid populations growing on abandoned mine tailings: a case study in Sardinia Island (Italy). Ecotox Environ Safe 189:110018
Downie A, Munroe P, Cowie A, Zwieten LV, Lau DMS (2012) Biochar as a geoengineering climate solution: hazard identification and risk management. Crit Rev Env Sci Tec 42(3):225–250
Dutta T, Kwon E, Bhattacharya SS, Jeon BH, Deep A, Uchimiya M, Kim KH (2017) Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: a review. GCB Bioenergy 9(6):990–1004
El-Naggar A, El-Naggar AH, Shaheen SM, Sarkar B, Chang SX, Tsang DCW, Rinklebe J, Ok YS (2019) Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: a review. J Environ Manag 241:458–467
Fowles M (2007) Black carbon sequestration as an alternative to bioenergy. Biomass Bioenergy 31(6):426–432
GB 15618–2018 (2018) Soil environmental quality-Risk control standard for soil contamination of agricultural land
Giller KE, Ernst W, Mcgrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30(10):1389–1414
Gloria MN, Bayo FL, Oyehan OO (2021) The effects of physicochemical parameters on analysed soil enzyme activity from Alice landfill site. Int J Environ Res Public Heath 18(1):221
Hamer U, Marschner B, Brodowski S, Amelung W (2004) Interactive priming of black carbon and glucose mineralization. Org Geochem 35(7):823–830
Han L, Zhang E, Yang Y, Sun K, Fang L (2020) Highly efficient U(VI) removal by chemically modified hydrochar and pyrochar derived from animal manure. J Clean Prod 264:121542
Huang Y, Wang LY, Wang WJ, Li T, He Z, Yang X (2019) Current status of agricultural soil pollution by heavy metals in China: a meta-analysis. Sci Total Environ 651(2):3034–3042
Igalavithana AD, Lee SE, Lee YH, Tsang DCW, Rinklebe J, Kwon EE, Ok YS (2017) Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 174:593–603
Jimenez MDLP, Horra ADL, Pruzzo L, Palma MR (2002) Soil quality: a new index based on microbiological and biochemical parameters. Biol Fertil Soils 35(4):302–306
Johnson JI, Temple KL (1964) Some variables affecting the measurement of catalase activity in soil. Soil Sci Soc Am J 28:207–216
Kameyama K, Miyamoto T, Iwata Y (2021) Comparison of plant Cd accumulation from a Cd-contaminated soil amended with biochar produced from various feedstocks. Environ Sci Pollut Res 28(10):12699–12706
Lacalle RG, Gómez-Sagasti MT, Artetxe U, Garbisu C, Becerril JM (2018) Effectiveness and ecotoxicity of zero-valent iron nanoparticles during rhizoremediation of soil contaminated with Zn, Cu, Cd and diesel. Data Brief 17:47–56
Li HB, Dong XL, Silva EBD, Oliveira LMD, Chen YS, Ma LQ (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478
Li WB, Yang YW (2021) Can environmental centralization help reduce pollution? Evidence from an administrative reform in China. J Clean Prod 314(2):127972
Li YS, Ding SS, Yin QY, Li JY, Zhou D, Liu GS (2019) Effect of long-term biochar application on soil respiration in flue-cured tobacco planting fields in Henan province. Environ Sci 40(2):915–923
Liu K, Li FB, Cui JH, Yang S, Fang L (2020) Simultaneous removal of Cd (II) and As (III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: synergistic effects and mechanisms. J Hazard Mater 395:122623
Małgorzata B, Jadwiga W, Jan K (2018) The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 27(9):1188–1202
Martins Filho AP, Medeiros EV, Lima JRS, Costa DPD, Duda GP, Silva JSAD, Oliveira JB, Antonino ACD, Menezes RSC (2021) Hammecker C. Impact of coffee biochar on carbon, microbial biomass and enzyme activities of a sandy soil cultivated with bean. An Acad Bras Cienc 93(4): e20200096
Mclaughlin MJ, Parker DR, Clarke JM (1999) Metals and micronutrients—food safety issues. Field Crop Res 60(1):143–163
Mohan D, Sarswat A, Yong SO, Pittman CU (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–a critical review. Bioresource Technol 160(5):191–202
Odum EP (1985) Trends expected in stressed ecosystems. Bioscience 35(7):419–422
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14(12):1504
Prayogo C, Jones JE, Baeyens J, Baeyens J, Bending GD (2014) Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol Fertil Soils 50(4):695–702
Qin X, Liu YT, Huang QQ, Zhao LJ, Xu YM (2020) Effects of sepiolite and biochar on enzyme activity of soil contaminated by Cd and atrazine. B Environ Contam Toxicol 104(5):1–7
Quevauv IP, Rauret G, Griep IB (1993) Single and sequential extraction in sediments and soils. Int J Environ Anal Chem 51(1–4):231–235
Quilliam RS, Rangecroft S, Emmet BA, Deluca TH, Jones DL (2013) Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 5(2):96–103
Ren CC, Jin SQ, Wu YY, Zhang B, Kanter D, Wu B, Xi XC, Zhang X, Chen D, Xu JM, Gu BJ (2021) Fertilizer overuse in Chinese smallholders due to lack of fixed inputs. J Environ Manag 293:112913
Romero-Freire A, Aragón SM, Garzón FJM, Peinado FJM (2016) Is soil basal respiration a good indicator of soil pollution. Geoderma 263:132–139
Schumacher TE, Eynard A, Chintala R (2015) Rapid cost-effective analysis of microbial activity in soils using modified fluorescein diacetate method. Environ Sci Pollut Res 22(6):4759–4762
Shaaban M, Zwieten LV, Bashir S, Younas A, Núñez-Delgado A, Chhajro MA, Kubar KA, Ali U, Rana MS, Mehmood MA, Hu R (2018) A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J Environ Manag 228:429–440
Siedt M, Schäffer A, Smith KEC, Nabel M, Roß-Nickoll M, van Dongen JT (2021) Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci Total Environ 751:19–28
Sun T, Xu Y, Sun Y, Wang L, Liang X, Zheng S (2021) Cd immobilization and soil quality under Fe-modified biochar in weakly alkaline soil. Chemosphere 280:130606
Tan XP, Wang ZQ, Lu GN, He WX, Wei GH, Huang F, Xu X, Shen W (2017) Kinetics of soil dehydrogenase in response to exogenous Cd toxicity. J Hazard Mater 329:299–309
Tao WM, Duan WY, Liu CB, Zhu DD, Si XX, Zhu RZ, Oleszczuk P, Pan B (2020) Formation of persistent free radicals in biochar derived from rice straw based on a detailed analysis of pyrolysis kinetics. Sci Total Environ 715:136575
Tu C, Wei J, Guan F, Liu Y, Sun YH, Luo YM (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576
Wang GY, Pan XM, Zhang SR, Zhong QM, Zhou W, Zhang XH, Wu J, Vijver MG, Peijnenburg WJGM (2020a) Remediation of heavy metal contaminated soil by biodegradable chelator-induced washing: efficiencies and mechanisms. Environ Res 186:109554
Wang J, Hao X, Liu Z, Guo Z, Zhu L, Xiong B, Jiang D, Shen L, Li M, Kang B, Tang G, Bai L (2020b) Biochar improves heavy metal passivation during wet anaerobic digestion of pig manure. Environ Sci Pollut Res Int 28(1):635–644
Wang L, Wang YJ, Ma F, Tankpa V, Bai S, Guo X, Wang X (2019) Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci Total Environ 668:1298–1309
Wang YM, Wang SW, Wang CQ, Zhang ZY, Zhang JQ, Meng M, Li M, Uchimiya M, Yuan AX (2020c) Simultaneous Immobilization of Soil Cd(II) and As(V) by Fe-modified biochar. Int J Env Res Pub He 17(3):827
Wardle DA, Ghani A (1995) A critique of the microbial metabolic quotient (qCO2) asbioindicator of disturbance and ecosystem development. Soil Biol Biochem 27:1601–1610
Wittling SC, Houot S, Barriuso E (1996) Soil enzymatic response to addition of municipal solid-waste compost. Biol Fertil Soils 20:226–236
Wu R, Long M, Tai X, Wang J, Lu Y, Sun X, Tang D, Sun L (2022) Microbiological inoculation with and without biochar reduces the bioavailability of heavy metals by microbial correlation in pig manure composting. Ecotoxicol Environ Safe 248:114294
Xiao Y, Wang L, Zhao Z, Che Y (2020) Biochar shifts biomass and element allocation of legume-grass mixtures in Cd-contaminated soils. Environ Sci Pollut Res 27:10835–10845
Xu JW, Liu C, Hsu PC, Zhao J, Tong Wu, Tang J, Liu K, Cui Y (2019a) Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat Commun 10(1):1–8
Xu Y, Seshadri B, Sarkar B, Wang H, Rumpel C, Sparks D, Farrell M, Hall T, Yang X, Bolan N (2019b) Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci Total Environ 621:148–159
Zhang M, Riaz M, Zhang L, El-Desouki Z, Jiang C (2019) Biochar induces changes to basic soil properties and bacterial communities of different soils to varying degrees at 25 mm rainfall: more effective on acidic soils. Front Microbiol 10:1321
Zhang W, Long J, Wei Z, Alakangas L (2016) Vertical distribution and historical loss estimation of heavy metals in an abandoned tailings pond at HTM copper mine, northeastern China. Environ Earth Sci 75(22):1462
Zhu XM, Chen BL, Zhu LZ, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115
Acknowledgements
This work was funded by the National Natural Science Foundation of China (31460149; 41571225), the University Synergy Innovation Program of Anhui Province (GXXT-2020-075) and the Natural Science Foundation of Anhui Province (2108085MD128).
Funding
National Natural Science Foundation of China (41661065).
Author information
Authors and Affiliations
Contributions
YW, YMA and JHZ: Data curation, Writing- Original draft preparation. XPL, YMA, and CYZ: Visualization, Investigation. YW: Software, Validation. JHZ and SBZ: Conceptualization, Methodology, Supervision, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Ai, Y., Zhou, J. et al. Effects of biochar on heavy metal speciation and microbial activity in red soil at a mining area. Int. J. Environ. Sci. Technol. 20, 13491–13502 (2023). https://doi.org/10.1007/s13762-023-04904-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04904-x