Abstract
Coal fly ash (CFA) management has become a global environmental concern due to their impact on the environment and the quantities of the waste generated. However, the utilization of CFA as feedstock in industrial processes is considered a promising option for managing the waste sustainably. The physical, chemical and mineralogical properties of CFA such as morphology, surface area, porosity, and chemical composition (silica, alumina, iron oxide, titania, etc.) makes it a suitable feedstock for several industrial processes. Few reports have attempted to summarize the utilization of the waste solely in individual sectors. However, little is known about the application of the waste in other sectors such as metallurgy, rare earth metals recovery and oil and gas. A multi-industry review of CFA utilization is needed to fully appreciate the myriad uses of the waste and identify new knowledge gaps. This review exposes the interconnections between different utilization options and identifies research gaps and new possible uses. In addition to the utilization of CFA across multiple industries, the current work also highlights the regulations and health implications associated with management of the waste. The findings give insight about how existing utilization options can be improved, identifies new sectors where the waste can be applied and exposes knowledge gaps to enhance research in the area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
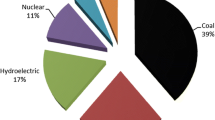
Coal, a source of fossil fuel is a vital resource needed to meet the global energy demand. Consequently, coal combustion residues (CCRs) generation is in an annual exponential growth (Asokan et al. 2005). The USA (Mushtaq et al. 2019; Harkness et al. 2015), China (Asokan et al. 2005) and India (Bhatt et al. 2019a) are known to combust the largest fraction of global coal mined as a source of energy in power plants for electric power generation, subsequently generating million tons of CCR as shown in Fig. 1. Among the million tons of CCRs globally generated per year, coal fly ash (CFA) forms about 65–95% (Matzenbacher et al. 2017). Therefore, CFA management as waste is an alarming issue that is causing environmental hazard in terms of soil, water and the air pollution that must be addressed (Mushtaq et al. 2019).

( Adopted from (Hower et al. 2017))
Scanning electron micrographs image of CFA.
Hower et al. (2017) and Blissett and Rowson (2012) reported that about 750 million tons of CFAs are globally generated per year with an average utilization of about 25%, leaving 75% of the waste unutilized. Thus, the rate of CFA production far outweighs utilization (Wang et al. 2021) and major efforts are needed to tackle this alarming situation. Nevertheless, industrial applications must be investigated in order to promote the recycling of the CFA output. New avenues and advances are being searched to sustainably make use of the waste to curb the adverse environmental and ecological effect associated with CFA generation and their disposal (Blanco et al. 2005, 2006). Major coal combustion and coal waste production rate at the global scale are shown in Table 1.
One way of dealing with the CFA disposal challenge is to reduce the huge percentage deficit of unutilized waste by adding value through scientific research for industrial reuse and recycling (Cao et al. 2008). Thus, a way of providing solution to this increasingly urgent problem is utilization for industrial purposes. Inadequate recycling and or conversion technologies are the major limiting factors of the waste utilization (Little et al. 2008). Efforts are being made to pay attention to high-value novel conventional research technologies for the conversion of the wastes into useful products for sustainable utilization (Mushtaq et al. 2019). Thus, there is the need to develop innovative utilization of the coal waste as raw materials for industrial application (Sokolar and Nguyen 2018).
Poor CFA disposal is linked to serious health hazards such as deoxyribonucleic acid repair mechanism complications from the polycyclic aromatic hydrocarbons when inhaled or consumed into living tissues (Matzenbacher et al. 2017). These poor disposal practices are equally causing significant economic and environmental challenges (Dash et al. 2018).
Currently, there is inadequate reviews in discussing the technological advancements in CFA utilization across multiple industrial sectors. There is a strong need to examine the applications of CFA holistically to advance research in the area, inform stakeholders and to identify the utilization gaps that are unexploited. This review investigates literature to discuss the characterization and composition of different types of CFAs and assesses the existing uses of CFA as feedstock for industrial processes. The study highlights the utilization of CFAs in various industries and concludes by reviewing the regulation and policies in CFA utilization and relevant recommendations to advance research in the various sectors investigated. The importance of this review article is to clarify the state of knowledge, explain apparent contradictions and identify needed research on CFA utilization and application in various industries. This study achieved this importance by compiling, summarizing, critiquing and synthesizing the available information on CFA utilization and application in various industries. This was done with the purpose of making this review article serve as a basis for knowledge development, create guidelines for policy and practice, provide evidence, highlight research gap and engender new ideas for future research directions. This review article also provides readers with interpretations and highlights complexities that exist in available literature of CFA utilization and application. Again, this review article sought to identify new patterns in CFA utilization and application in various industries that have not been previously recognized. This will provide readers a quick way of gaining some familiarity with the issues and major contributors in CFA utilization as well as providing useful syntheses and original insights. These insightful analyses in this review article addresses the difficulties in keeping up to date on the recent developments with the issues of CFA utilization and application due to the growing number of scholarly publications around the world.
Materials and methods
The study used literature reviews of multi-industrial studies on the coal fly ash to discuss the valorization of the waste material.
Results and discussion
Origin of coal fly ash (CFA) utilization and application
Coal fly ash (CFA) as a coal combustion residue of thermal power plants has been regarded as a problematic solid waste all over the world (Yu et al. 2022). CFA utilization and application originated as a result of the problems associated with the waste’s management processes such as large area of land required for disposal and the toxicity associated with heavy metal leached to groundwater (Ghazali et al. 2019). Since the twelfth century till recent past, large quantities of coal fly ashes have been stored in the form of waste heaps or deposits, whose contamination poses a serious threat to the environment as a major source of inorganic pollution (Akhai and Sharma 2019). In recent decades, due to the growth of urbanization and industrialization, CFA have not only increased in quantity but also undergone changes in properties and composition (Bhatt et al. 2019b). With the growing energy, demand and consequent increase in the use of coal, the problem of CFA disposal is expected to worsen (Jain and Dwivedi 2014). Therefore, the problem of CFA disposal has become a major challenge to environmentalists and scientists. At present, various treatment methods, including landfills, resource recycling, and incineration, have been applied but not widely investigated (Ghazali et al. 2019). In future, due to the increase in the energy demand, CFA production is expected to grow, making research into the use of this material a necessity and a large-scale application of this waste product may be possible for recovery of heavy metals, reclamation of wasteland and floriculture (Franus et al. 2015). However, it has been recognized worldwide that improper utilizations of the waste such as an enormous amount of fossil fuels have created various adverse effects on the environment, including acid rain and global warming (Ghazali et al. 2019). In recent times, there is a number of possible fly ash applications, on average, only 25% is utilized, the rest is considered a pollutant and disposed of as waste (Franus et al. 2015). Hence, the recent research onto CFA has been focused on its novel applications. In addition, most of the investigations on CFA utilization and application originated from exploratory coal samples that have been laboratory ashed for analytical investigations, not from coal fly ash stockpiles (Bhatt et al. 2019b).
Characterization of coal fly ash (CFA)
CFA are mostly characterized by their physical and chemical/mineralogical properties (Jayaranjan et al. 2014) and these properties have been investigated as shown in Table 1. The production and utilization of CFA cannot be durably isolated from their physical and chemical properties (Eze et al. 2013). CFA from different sources have different characteristics (Sokolar and Nguyen 2018). CFA may have different properties in terms of quantity and quality that is mostly influenced by method used in the coal mining and coal chemical content; properties of CFAs are not uniform in nature (Asokan et al. 2005). In CFA utilization, the physical and chemical properties are essential in order to determine their pozzolanic activity and reactivity (Blanco et al. 2005). These properties of CFA give wide freedom in planning their utilization and the properties can be further improved by incorporating value addition approaches. For example, the success of CFA value addition approach in sintering (Biernacki et al. 2008) was found to be strongly depended on the properties of the fly ash.
Physical characteristics of coal fly ash
CFAs exist as solid powder at room temperature and are composed of microscopic spheres (as shown in Fig. 1) (Dai et al. 2010). The physical properties that include morphology, surface area and porosity make them effective material for wastewater treatment (Mushtaq et al. 2019). Other physical properties of CFA such as sphericity (as shown in Fig. 2), porosity, nontoxicity, lightweight, and high strength is makes the waste effective material for different applications (Joo et al. 2014). The ranges of the physical properties of CFA include 2.10–2.81 for specific gravity, 0.001–0.075 mm for particle size distribution (Prezzi and Kim 2008), 7.75 wt% for moisture content, 1.12–1.28 g cm−3 for bulk density and 1.0–9.44 m2 g−1 for specific surface area (Theis and Gardner 2009).

(Adopted from (Asokan et al. 2005))
Microstructure of CFAs showing spherical, hollow shaped and cenospheres in nature.
Matsunaga et al. (2002) classified CFA into two types based on their physical properties; precipitator and cenosphere. The precipitators are solid and spherical in shape with a density range of 2.0–2.5 g cm−3. The cenospheres are hollow in shape (as shown in Fig. 3) which have densities less than 1.0 g cm−3. The precipitator fly ash has been identified to improve various quality parameters of selected matrix materials, including stiffness, strength, wear resistance and density while the cenosphere fly ash can be used for the synthesis of ultra-light composite materials due to its significantly low density (Matsunaga et al. 2002).

(Adopted from (Chen et al. 2010))
FESEM image of cenosphere CFA.
Chemical and mineralogical properties of coal fly ash
The chemical and mineralogical compositions of CFA differ enormously from one source to another as shown in Table 2. CFA contain largely silica, iron, aluminum, calcium and other inorganic elements. Mineralogically, CFAs are typically composed of amorphous materials and crystalline mineral components (Dai et al. 2010). The chemical and mineralogical compositions of CFA particles have effect on the mechanical properties of recycled products (Aldahri et al. 2017; Manz 1997). The presence of certain elements such as quartz, mullite, calcite (Dai et al. 2010), corundum (Asl et al. 2018), hematite, graphite, Sillimanite (Flores et al. 2008), magnetite, ferrite, rutile (Vassilev and Vassileva 2007) are important properties that have effect in CFA utilization. Thus CFA is a silico-aluminate material consisting of SiO2, Al2O3, Fe2O3 as the major constituents and varying amount of CaO, MgO, SO3− (Izquierdo et al. 2008; Khatri and Rani 2008; Mardon et al. 2008). According to the (U.S., Department of Energy 2011), CFAs are potential sources of rare earth elements. However, a literature survey by Belardi et al. (2014) revealed that not much work has been done on characterization of rare earth elements in CFAs. Rare earth elements are useful resources that are recovered and widely utilized for their benefits as presented in Table 3. Another important chemical composition of CFA is the Carbon component.
Aldahri et al. (2017) reported that CFAs containing less than 5 wt% carbon are valuable raw material to produce construction products. The Carbon content of CFA as a chemical property makes it necessary to characterize CFAs into unburnt coal fly ash (UCFA), which is obtained from an inefficient combustion of the coal (Hower et al. 2017). UCFA has been studied as a non-uniform, monotone entity possessing some adsorbent properties (Bartoňová et al. 2011). However, UCFA has been identified with a number of limitations in its utilization compared to pure CFA in terms of serving as a precursor for cement production (Hower et al. 2017).
Class C coal fly ash
CFAs that are classified as class C fly ash contains 15–30% of calcium hydroxide or lime and they are produced from the combustion of lignite and sub-bituminous coals (Li et al. 2013). Class C CFAs possess pozzolanic and cementitious properties with lower content of silica and alumina, (Gupta et al. 2020; Yu et al. 2022). These properties of class C fly ash have been identified to lead in the formation of calcium silicate hydrate compounds in addition to the geopolymer gel products in augmenting the mechanical strength of the hardened matrix (Diaz et al. 2010).
Class F coal fly ash
Class F CFAs contain more silica, alumina and iron oxide and they are produced as a result of combustion of anthracite, bituminous or sub-bituminous coal in a low lime (0–7%) (López-Antón et al. 2006). The amount of calcium is one main feature that distinguish between class F fly ash and class C fly. Class F contains higher amount of calcium than class C (Li et al. 2013). F-type fly ash has been found to contain more than 70% of SiO2 and Al2O3, thus they are more enriched in silica, alumina, ferric oxide, calcium oxide and other metal oxides. Class F are mostly being used in research works as solid support for loading MgO (Jain and Rani 2011). The chemical and mineralogical properties of different CFA are shown in Table 2.
Coal fly ash utilization and application
The utilization and application of CFA has been identified as the most promising method to manage the waste and reduce environmental pollution (Gitari and Akinyemi 2018; Chindaprasirt et al. 2009). Reusing and recycling these hazardous waste through development of value-addition is helpful in reducing environmental pollution (Mushtaq et al. 2019). However, global utilization and application of CFA has been pegged at 15% (Sokolar and Nguyen 2018). This section therefore investigates CFA utilization and application in various industrial sectors.
Construction
About 23% of the total CFA utilization in the construction industry is used in producing Portland cement, concrete, wallboard, roofing granules, segregate for paving materials and asphalt filler (Tenenbaum 2009). Using CFA to partially replace cement saves energy because, unlike Portland cement, it does not rely on heating for its cementitious properties (Sokolar and Nguyen 2018). The use of CFA in concrete to replace cement also reduces the emission of about 60% CO2 that is produced in the process of cement production as a by-product of calcination (Tenenbaum 2009).
In construction, CFA are used to optimize the strength, durability, and economics, reduce heat generation during setting, reduce permeability to keep chlorides away from steel reinforcement and prevent corrosion (Sokolar and Nguyen 2018). Acar and Atalay (Acar and Atalay 2016) discovered the potential use of CFAs for the production and recovery of cenospheres for fillers and reinforcements. However, depending on the different characteristics of the CFA, the yield and recovery potential of the recovered cenospheres may vary significantly in contents through density and size, hence there is the need to further conduct investigations into the recovery potentials to maximize the quality.
CFA has also been innovatively used in the manufacturing of alternative binders termed geopolymer that is resistant to fire and acid with high compressive strength, low shrinkage, and solidification of heavy metal wastes (Bakharev 2005). The mechanical properties and microstructural characteristics reveals that CFA-based geopolymers increases in compressive strength after curing at 70 °C for 24 h (Li et al., 2013). Presently, efforts are being made to investigate the consistency of the compression strength when different classes of CFA are used (Li et al., 2013).
CFAs that are high in carbon content and have large particle mean diameter have been identified as reject fly ash (rFA) (Yu et al. 2022). These rFA were previously unable to be used as producing cement materials due to their characteristics, but current studies are developing novel ways to upgrade the coarse part of rFA to reuse them as cement systems by blending the coarse fraction of the waste with ordinary cement to enhance the mechanical and hydration properties of the cementitious materials. Results have revealed that these novel ways of upgrading CFAs in the construction industry is relatively cheap and drastically improves the overall performances of the waste containing cements (Yu et al. 2022).
Electronics
CFAs are potential sources of rare earths (Binnemans et al. 2013). Rare earth elements recovered from CFAs makes the coal waste essential raw material in electronics and many high-tech components (Xie et al. 2014). Hatch (2012) revealed that the global demand of rare earth elements is about 105 kt per year as shown in Table 4 and this demand is expected to grow. Rare earth elements are mostly needed in the production of magnets, metal alloys, catalysts, polishing powders, phosphors, glass additives, ceramics and energy-efficient fluorescent lighting (Chakhmouradian and Wall 2012). There were however, efforts to fund projects to develop technologies needed to separate and extract rare earths from CFA (Erickson 2018). It has been deduced that if researchers can develop technology to extract them economically, the generated revenue could offset some of the cleanup costs currently borne by the coal industry and some of the revenues could be used to fund other CFA utilization projects (Erickson 2018). Rare earth extraction process was dependent on a number of factor such as the acid strength, time and temperature (Wu et al. 2012). Xie et al. (2014) has reviewed that rare earth separation and recovery in CFA is possible with cation or anion exchangers and solvation extractants. The U.S. Department of Energy agency is currently funding many major projects in the area of rare earth extractions from coal wastes and their characterization in various coal-based materials. Additional research is required to demonstrate that these extraction processes are feasible, efficient and economical when scaled up for commercial purposes (Erickson 2018). Recovery efforts need to focus on optimizing the separation and extraction of rare earths from CFA with the highest concentrations of the rare earths.
Mining
CFA is widely recognized as a mineral resource because of its high alumina content that makes the waste a substitute for bauxite in mining areas (Ding et al. 2016). Alumina extraction from CFA has received a global attention but studies reveal that the recovery technologies have been slowly developed due to inadequate studies (Ding et al. 2016). Together with the environmental impact of CFA utilization that is now fully recognized, alumina recovery from fly ash is currently an important CFA utilization in the mining industry. However, innovative methods are required to for the alumina extraction but this is again limited by the little knowledge in the characteristics of the various coal fly ash produced in different parts of the world (Eze et al. 2013). It is therefore necessary for research institutions to fund and conduct more investigations to characterized coal fly ash from different production sites to enhance an optimal alumina recovery. Other mining utilization of CFA include dumping and refilling mining cavities with the waste but environmental specialists mostly criticize this application as an environmental hazard. For example, it has been observed that when CFAs are used to fill abandoned mines and quarries, the leachates that are generated which are mostly toxic acids often drain from these sites into nearby waters or into the groundwater resulting in significant contaminations (Theis and Gardner 2009).
Wastewater treatment
Pollution of water bodies with dye chemicals and heavy metals from industrial wastewater remains a critical environmental problem. Research and innovation is required to develop plausible techniques to remove the pollutants prior discharge (Visa et al. 2015). Economically, pollutants removal using adsorbents developed from cheap but efficient materials such as CFA is very attractive (Visa et al. 2010). Shannon et al. (2010) investigated water purification technologies and explained that there is the need for incredible studies to be carried out to develop robust and novel treatment techniques at low cost and minimal energy. The use of CFA in wastewater remediation is an essential technique to remove pollutants from wastewaters (Mushtaq et al. 2019). Most of the commercially available adsorbents used for water treatment require additional separation system that increases treatment cost. The incorporation of CFA in adsorbent fabrication through polymer matrix can produce stable and reusable adsorbent material for water treatment without any need for additional separation system (Joo et al. 2014). The properties of CFA in adsorbent fabrication can further be improved by incorporating them into polyurethane membrane fibers as increasing surface-area-to volume ratio could provide a sufficient area for interaction without agglomeration of nanoparticles during water treatment (Joo et al. 2014). CFA utilization in wastewater treatment is therefore a potential cost effective material that could help reduce environmental pollution (Mushtaq et al. 2019).
In the sanitation and waste management industry, dry materials of CFA are often added to stabilize sludge and as a cheaper source of adsorbents to remove heavy metals prior to transport into landfill storage (Khatri et al. 2010a). CFA utilization in wastewater treatment also provides an economic value and a cheaper chemical conditioning mechanism when used in activated sludge technology to dewater sludge by destroying the colloidal frame and flocculate the sludge flocs. Evidences of this mechanism by Yang et al. (2005) has proven that sewage sludge conditioned with CFA had the dewaterability greatly improved; whereas an advanced investigation by Chen et al. (2010) after an acid modification of the CFA prior sludge conditioning demonstrated superior dewatering properties. However, there is inadequate information on the characteristics of the residues after dewatering with the modified CFA acid and this exposes a research gap to determine the fate of the residues.
Another investigation in the use of CFA as microspheres in water treatment via a layer-by-layer (LBL) assembling presented an efficient photocatalytic degradation efficiency (Chen et al. 2018). This activity was attributed to a fast charge-carrier transfer, a reduction of charge recombination rate, an improved light harvesting and larger specific surface area characteristics of the CFA (Chen et al. 2018). The study provided insights into further investigations required for the synthesis of high-performance photocatalysts and structural design of composites from CFAs that could yield optimal separation properties of nanomaterials anchored as pollutants in wastewater. The increasing demand for these CFA based porous catalysts has led to more resounding investigations as alternative materials with comparative efficiencies.
Studies have also revealed that apart from providing cost effective benefits in the sanitation and waste management industry, CFA possess unique properties of high porous structure, large surface area, tunable pore size and alumino-silicate compounds that makes the waste a potential material to replace commercially used expensive supports such as pure SiO2, TiO2, ZrO2 and Al2O3 (Jala and Goyal 2006). In order to develop selective catalytic materials from CFA for various wastewater treatment applications, such as acylation reactions (Rani et al. 2013), benzylation (Khatri et al. 2010a), oil of wintergreen (Khatri and Rani 2008), some types of condensation reactions studied under liquid phase conditions (Jain and Rani 2011), catalytic combustion of methane (Arandiyan et al. 2013a, 2013b) syngas production (Khalesi et al. 2008) and photo-degradation of RB (Wang et al. 2013), addition of proper catalyst promoters and conducting suitable activation process needs to be extensively studied.
An evaluation demonstrated that, the amorphous geopolymer is effective and have much higher removal efficiency compared to the raw fly ash as a sorbent material for copper (Al-harahsheh et al. 2015) and lead (Al-zboon et al. 2011). However, the removal efficiency is affected by solid/liquid ratio, temperature, reaction time, and Cu2+ initial concentration (C0). Therefore, further research in optimizing these factors could yield a CFA product with a superior removal capacity for the sanitation industry. A feasibility study, using CFA to indigenously develop fly ash membrane in municipal wastewater treatment has also been reported (Namburath et al. 2015). Reported results revealed that the fly ash membranes could withstand pressures of 760 mm Hg. At a constant filtration rate, the membrane bioreactor was found to delay the fouling process as expected in an efficient membrane while the membrane fouling took longer time in the systems to operate at higher air flowrates due to better scouring action of the air bubbles. It is however crucial to further investigate the durability and the compression strength of the Fly Ash Membranes for industrial use especially as the loads in the wastewater industry tend to be huge and highly turbid.
The low carbonation rate and efficiency properties of CFA without pre-treatment makes the waste a potential source of highly reactive feedstock for CO2 mineral carbonation or sequestration by direct mineralization in wastewaters but this phenomenon is highly sensitive to temperature, solid to liquid ratio and gas flow rate (Ji et al. 2016). Hence, wide investigations are required to optimize the conditions to improve the CFAs ability to sequestrate CO2 by direct mineralization.
Chemical and pharmaceutical
The search of new applications of CFA for catalyst development to replace homogeneous acids such as H2SO4, H3PO4 (Khatri and Rani 2008) and hetero-poly acids such as H3PW12O40 (Peng and Song 2000) in esterification reactions is highly needed in the chemical and pharmaceutical industry. The catalytic role of activated fly ash for different reactions in the chemical and pharmaceutical industry such as oxidation, chlorination and condensation of short chain olefins (Ahubelem et al. 2015; Kastner et al. 2003) is well documented in literature. In pharmaceutical studies, CFA has been used to develop several solid acid catalysts in the synthesis of aspirin (Khatri and Rani 2007, 2008), oil of wintergreen (Khatri and Rani 2008), 3,4-dimethoxyacetophenone (Khatri et al. 2010b) (anti neoplastic) and diphenylmethane (Khatri et al. 2010a). CFA has been used as solid support in the synthesis of 2-mercaptobenzothiazole derivatives under microwave irradiation (Narkhede et al. 2007). However, the utilization of fly ash as heterogeneous solid acid catalyst is not fully exploited.
Esterification products such as Acetylsalicylic acid made from CFA have wide applications in the chemical and pharmaceutical industry for the production of flavors, perfumery chemicals and drugs (Mirafzal and Summer 2000). Acetylsalicylic acid is generally synthesized by esterification of salicylic acid with acetic anhydride using acid catalyst such as H2SO4, H3PO4 (Borer 2000). Result of a chemical activation of CFA using sulfuric acid followed by a thermal treatment at 600 °C has being reported to be successful in synthesizing nano-crystalline activated fly ash catalyst (AFAC) with crystallite size of 12 nm (Khatri and Rani 2008). The AFAC has proven to demonstrate superior characteristics of amorphous nature due to high silica content (81%) and high BET surface area (120 m2/g), making the catalyst a highly active product for industrial use. Rani et al. (2013) have successfully converted CFA into an efficient solid Lewis acid catalyst by loading scandium triflate in a thermal and chemical activation to achieve a conversion up of 84%.
CFA has also been successfully used in the synthesis of a fly ash-supported cerium triflate catalyst (CFT) by loading cerium triflate (7 wt%) on an efficient solid Lewis acid activated with fly ash with high silica content of 81% (Khatri et al. 2010b). The catalytic activity of the CFA product was very high, corresponding to high conversion (88%) of veratrole to 3,4-dimethoxyacetophenone. However, the increased concentration of silica surface hydroxyl groups on activated fly ash have a major influence on the quality and yield of the CFT and therefore further investigations are needed to optimize production.
CFA coated with chitosan, has been found to be effective as an adsorbent on chemical ions in aqueous solutions (Adamczuk and Kołodyńska 2015). Further investigation of the adsorption isotherms, initial concentration, pH, contact time, temperature and adsorbent dosage revealed that the CFA adsorption process was endothermic, favorable and spontaneous but the adsorption capacity decreased when temperature was increased. Similar application of CFA reuse in the preparation of porous fly ash/NiFe2O4 composite by calcination method has been reported (Sonar et al. 2014). The composites were used as adsorbents for the separation of Congo red dye from aqueous solution. The results revealed that the adsorption capacity of the CFA composites reached a magnitude of 23.33 mg g−1, which is much higher than individual fly ash, pure NiFe2O4 or other composites. This could be a very useful phenomenon in the chemical industry because the sorption data gave a good fit with a pseudo-second-order kinetic model (Sonar et al. 2014). This outcome establishes the fact that, CFA composites are promising adsorbents and hence the need to conduct further investigations in their sustainable use for the chemical and pharmaceutical industry and other possible applications.
CFA for industrial use could be easily regenerated and reused, giving similar conversion up to three reaction cycles (Rani et al. 2013). Literature has also shown that CFA utilization in catalyst development has the potential of replacing conventional environmentally hazardous homogeneous liquid acids, making it an ecofriendly, solvent free and a solid acid based catalytic process in the chemical and the pharmaceutical industry (Khatri and Rani 2008). However, further research is required to maximize the conversion rates to establish a sustainable and efficient industrial use.
Agriculture
CFAs are used as acid neutralizing agents in agriculture and as feedstock to produce sulfate fertilizers (Wang et al. 2021). The advantages and disadvantages of CFA applications in agriculture especially to improve soil fertility have also been investigated (Dzantor et al. 2015). However, ecological studies and environmental impact assessments on heavy metals and toxic element accumulations in the crops and agricultural products needs to be conducted to enable safe application of CFAs and CFA based products in agriculture.
A greenhouse study conducted in ameliorating the low pH of acidic coal mine soils showed that lime and fly ash could significantly increase soil pH above ground plant biomass and root biomass (Awoyemi et al. 2019). CFA has been utilized as a base for growing corn and results generated greater yield that was attributed to the higher water holding capacity of the fly (Wang et al. 2020). However, there was boron present in corn leaves when fly ash mix was used and therefore further evaluation studies in metal assessment needs to be conducted when using the waste to grow crops. Again, a study by Sale et al. (1996) demonstrated that the application of unweathered CFA from a western Canada power plant successfully increased both plant height and grain yield of Barley (Hordeum vulgare) which was grown on the mixtures of the waste in a greenhouse.
Ceramics
The production of conventional ceramics is an important utilization for CFA since this application uses large quantities of the waste as raw materials in the ceramic production (Sokolar and Nguyen 2018). This then demands a wide research on CFA utilization in ceramics to optimize the industrial use and to show its promising results of their utilization. A few of these studies have therefore been reviewed in this section.
Alumina could be harnessed from CFA to produce porous polyvinylpyrrolidone alumina ceramics that has been investigated to exhibit higher bending strength and Young's modulus compared to porous polymers (Feng et al. 2013). However, it is a challenge to retain the mechanical strength while a large volume of pores is introduced into ceramic structures and further investigations are needed to determine the quantity of CFA alumina and polyvinylpyrrolidone needed to produce porous alumina ceramics with optimum microstructure and mechanical strength. A study conducted by Oueralt et al. (1997) into the utilization of binary mixtures of fly ash to produce ceramic products with up to 50 wt% of mullite and 16 wt% of feldspars, demonstrated that the resulting ceramic bodies exhibited features that suggested possibilities for use in paving stoneware manufacture, tiling and conventional brickmaking. However, it was discovered that CFA sometimes contains high amounts of iron oxides or metals, which can pose a serious problem affecting properties and also the ceramic process, hence improved methods to remove excess amount of free iron oxides are needed.
CFA is also a potential source of natural aluminosilicates which is a raw material used in the substrates preparation for the development of support, active layer and tubular composite ceramic membranes. Composite ceramic membranes fabricated from CFA has been found to show better structural characteristics, porosities and hydraulic permeabilities than several inorganic commercial membranes and composite membranes obtained from other researches (Almandoz et al. 2015). The produced Composite ceramic membranes have been found to be suitable for microfiltration processes with high insoluble residue rejections (100%) and high bacterial removal (87–99%).
Metallurgy
Studies on CFA utilization in the metallurgy industry such as the use of the waste in the fabrication of fillers and reinforcements in metal-matrix composites (MMCs) and polymer-matrix composites (PMCs) have gained much attention (Matsunaga et al. 2002). This form of waste utilization in the industry can significantly reduce the material costs of composites by incorporating CFA into the matrices of polymers and metallic alloys. However, very little information is available on the physical and mechanical properties of the fly ash particles to aid in the design of the composite materials. Little has also been proven that these forms of waste utilizations in the metallurgical industry are environmentally friendly. The few studies available on CFA utilization in the metallurgy industry are therefore focused on areas that lock the ash and its toxic contaminants away from human exposure.
Studies have also demonstrated the opportunity of gainfully utilizing CFA as a waste byproduct for industrial use while simultaneously providing a solution to its safe disposal (Ewulonu et al. 2016). For example, CFA has been successfully used as an extender in the development of protective and decorative industrial coatings as solvent borne, cold curing and two pack epoxy systems. These are to provide solutions to the problem of deterioration in metallic structures and components owing to corrosion and abrasion that is facing the metallurgy industry (Praharaj 2009). It was discovered that the properties of CFA that contributed to its usefulness as an extender shows abrasion resistance, chemical inertness, low oil absorption, and low specific gravity (Tiwari and Saxena 1999). Tiwari and Saxena (1999) established that CFA can be a cost-effective substitute for conventional extenders in high performance industrial coatings. However, the optimum results are reported to be obtained by using 35% fly ash in primers and 18% fly ash in enamels but further studies were needed to assess the optimum percentages to eliminate the potential disadvantages of water-based coatings that may limit their applications (Reitz 2008).
Oil and gas
Hydraulic fracturing technology increases the productivity of ultra-tight shale oil and shale gas reservoirs and the use of nanoparticles prior to the placement of larger proppants help prevent fluid loss into the formation, and increase the conductivity of the fissures and micro-sized fractures. Recovery of high value proppants from CFA has been proven feasible in providing a long-term strategic resource worth billions in revenue and increased oil and gas production (Robl and Oberlink 2018). A study by Bose et al. (2015) in an application of CFA as nanoproppants for fracture conductivity improvement to reduce fluid loss and packing of micro-fractures demonstrated that these nanoparticles generate significant conductivity and fracture permeability values ranging from 27 to 33 mD. Further studies are needed to investigate the size, nano-hardness, reduced elastic modulus, fluid loss prevention capabilities as well as their induced fracture conductivity when CFA are used as nano-proppants to optimize the conductivity and fracture permeability efficiencies.
The stimulated fracture network and natural fracture network provides the pathway for hydrocarbon flow from the matrix to the wellbore, which is critical to the success of oil/gas recovery from unconventional reservoirs (Robl and Oberlink 2018). The spherical shape and smaller sizes of CFA make the waste desirable to be used as propped fractures that have higher magnitude permeabilities than that of the unpropped fractures (Snellings et al. 2012). Propped fractures are essentially needed in the oil and gas industry for fracking because as reservoir pressure depletes with oil and gas production, the effective stress increases, causing the fractures to close. The fractures are classified as macro-fractures and microfractures. CFA as byproduct of the coal combustion process have been found to be more suitable for generating microfractures that are at the sub-micrometer scale, preventing conventional proppants from adequately penetrating those (Robl and Oberlink 2019).
This study proposes investigations into the use of fly ash as “micro-proppant” to prop open the microfracture, as well as its use as macro-fracture under effective stress conditions. The use of fly ash as a “micro-proppant” may enhance the stimulation of smaller fractures that are not effectively propped using available conventional techniques. The fly ash could be used with the fracturing pad during the initial stimulation process. The “fracturing pad” is the portion of the stimulation design that does not typically contain any proppant and creates a volume of fractures, which is stimulated but unpropped (Manchanda 2015). The innovative use of fly ash with the fracturing pad may therefore enhance the currently achieved fracture network by bypassing the macro-fractures and settling within the microfracture network ultimately improving the overall stimulated reservoir volume (Ghanbari and Dehghanpour 2016).
Regulations and health implications of CFA utilization and application
Regulations classify CFA as a prescribed waste and prohibit its disposal in regular landfill (Gupta et al. 2020). In order to lower air pollution caused by coal-fired power plants, CFA production is regularized as the waste is separated from flue gas in smokestacks by precipitators (Asl et al. 2018). CFA ponds and coal waste landfills have been found to leach toxins into streams and drinking water. An Environmental protection Agency study determined that a 10-acre landfill of CFA would leak 0.2–10 gallons leachate per day, or between 730 and 36,500 gallons over a ten-year period and this is an amount guaranteed to infiltrate the drinking water supply (Kuriakose and Nagaraju 2004).
Since long-term isolation from the weathering environment cannot be guaranteed, the weathering behavior of CFA disposal is crucial for its long- term environmental impact, hence current waste disposal regulations call for isolating the fly ash deposits in containment systems to prevent leaching of contaminants (Bhatt et al. 2019a). EPA regulations also supports the treatment of the fly ash through innovative ways such as sintering (Biernacki et al. 2008) and geopolymer matrix stabilization (Gupta et al. 2020) to reduce the leaching rate of metals in the waste during landfill disposal.
CFA reuse is mostly regulated under EPA’s Coal Combustion Residuals rule in most countries, but there have been some failures to provide adequate safeguards for the health of communities and the environment (SACE, Southern Alliance for Clean Energy 2020). Under USEPA’s coal ash rule, projects using more than 12,400 tons of CFA on the land, are required to make some health and safety demonstrations (SACE, Southern Alliance for Clean Energy 2020). Some environmental legislations seek to support identified innovative ways that can successfully reduce CFA production and promote its safe reuse or recycling as more environmentally friendly alternatives (Khatri and Rani 2008). For example, the Chinese government has imposed much stricter regulations on the rare earth recovery industries suspending the issuance of new licenses, capping production, and clamping down on illegal operations and environmental hazard recovery techniques (Hatch 2012). Clearly, as new technologies and processing techniques in CFA utilization and application come on stream, both manufacturers and consumers need to make informed and responsible choices regarding the waste reuse.
Summary of CFA utilization and application
Coal fly ash (CFA) management has become a global environmental concern due to their large global annual production, where less than 30% of the waste are being utilized due to inadequate information, challenges in the utilization processes and the slow rate at which viable new utilization options are created. Few studies have summarized CFA utilization and application in the attempt to bridge the information deficit but these reports are solely focused on specific individual sectors and are not enough to provide a complete assessment of the utilization technologies in varied sectors.
A more comprehensive step in curbing this problem is to provide a holistic review in varied sectors such as the construction, electronics and resource recovery, mining, wastewater treatment, chemical and pharmaceutical, agricultural, ceramics and the metallurgy. Besides the current and existing utilization and application of coal fly ash in the various sectors, highlighting other potential utilization options that are underdeveloped and needed to be explored is another stride of productively managing the coal waste.
It has been established that the characterization of CFA in terms of the physical, chemical and mineralogical properties considerably influence the technologies associated with the waste utilization in varied sectors. Studies have revealed that there is huge information deficit on the potential variations in the characteristics of CFA produced from diverse sources and this is affecting technology needed for the coal waste utilization. Further investigations in the physical and chemical characteristics of CFA from different sources are needed to inform stakeholders to facilitate the waste utilization.
Studies have demonstrated that innovative studies are needed in the construction industry to reduce carbon contents in UCFAs during the waste production to make them desirable for construction application. The electronics sector is experiencing inadequate technologies needed for the separation and extraction of rare earths from CFA. Rare earth elements recovery in the electronics industry from CFA are underutilized due to inadequate technologies and research to harness these minerals from the waste such as the use of biological metallurgy of microbial pathway for the rare earth metals recovery. Again, CFA has become a useful source of alumina mineral resource to substitute bauxite in the mining industry but the recovery processes have been inefficient due to inadequate technologies used in harnessing this mineral. The wastewater treatment sector has seen a wide CFA utilization and application of CFAs. The coal waste utilization in wastewater treatment promises potential cost effective ways of treating wastewater that could help reduce environmental pollution. Due to the unique characteristics such as high porous structure, large surface area, tunable pore size and alumino-silicate compounds, the coal waste is utilized commercially into adsorbents, catalysts, catalyst supports, sensors, filters, thermal insulators and electrodes for the pollutants removal in wastewater. The coal waste is also synthesized into amorphous geopolymer that have high adsorption efficiency and are applied for the removal of metals in wastewater. The application of CFA to soils to improve soil fertility in the agricultural sector needs to be further investigated to optimize the use of the waste for crop production. Similarly, more research is needed on the CFA utilization in the ceramic and the metallurgy industry to optimize the production of improved and enhanced products from the waste.
CFA is also being used by the oil and gas industry as micro proppant for fracking. Further investigations are needed to improve the use of fly ash as “micro-proppant” to prop open the microfracture and studies are also required to understand the mechanisms of its use as macro-fracture under effective stress conditions. These areas of utilization of CFA in the oil and gas industry could increase oil and gas recovery especially in unconventional formations.
The leaching of toxins during CFA disposal and their health and regulatory implications is crucial for the waste’s long- term environmental impact, hence current waste disposal regulations call for the isolation of the fly ash deposits in containment systems to curb the leaching of contaminants into the environment. Regulation institutions are then supporting innovative treatment methods such as sintering and geopolymer matrix stabilization that aim to reduce the leaching rate of metals during landfill disposal. The knowledge gaps identified in current study would help to develop technologies needed to improve the current global utilization percentage of coal waste into useful resources.
Conclusion
Coal ash poses potential environmental hazards due to high heavy metal contents that may be leached out into the environment. Coal fly ash utilization and application in varied sectors such as construction, electronics and resource recovery, mining, wastewater treatment, agricultural, ceramics and metallurgy have been reviewed in this paper. The following findings were made;
-
The characteristics of CFA in terms of the physical and chemical/mineralogical properties are important factors that play a major role in the utilization of the waste.
-
The utilization and application of CFA is the most promising method to manage the waste and reduce environmental pollution.
-
The utilization of CFAs in the construction industry as alternative to cement is relatively cheap and drastically improves the overall performances of building products.
-
Recovery efforts are needed to focus on optimizing the separation and extraction of rare earths from CFA.
-
The use of CFAs in filling abandoned mines and quarries is environmentally unsuitable especially when the leachates drains into nearby waters or into the groundwater. Thus, properly engineered landfills may be used if necessary.
-
The application of CFAs as nanoproppants for fracture conductivity improvement by reducing fluid loss and packing of micro-fractures in oil and gas production.
The findings presented in this study reveals that, CFA could be developed into a number of valuable resources for industrial and domestic application. This would go a long way to reduce the negative impact of the waste on the environment while at the same time providing economic benefits.
References
Acar I, Atalay MU (2016) Recovery potentials of cenospheres from bituminous coal fly ashes. Fuel 180:97–105
Adamczuk A, Kołodyńska D (2015) Equilibrium, thermodynamic and kinetic studies on removal of chromium, cop- per, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem Eng J 274:200–212
Ahubelem N, Shahabi K, Moghtaderi B, Altarawneh M, Dlugogorski BZ, Page AJ (2015) Formation of chlorobenzenes by oxidative thermal decomposition of 1,3- dichloropropene. Combust Flame 162:2414–2421
Akhai S, Sharma V (2019) Trends in utilization of coal fly ash in India: a review. J Eng Des Anal 2(1):12–16
Aldahri T, Behin J, Kazemian H, Rohani S (2017) Effect of microwave irradiation on crystal growth of zeolitized coal fly ash with different solid/liquid ratios. Adv Powder Technol 28:2865–2874
Al-harahsheh MS, Al-makhadmeh L, Hararah M, Mahasneh M (2015) Fly ash based geopolymer for heavy metal removal : a case study on copper removal. J Environ Chem Eng 3:1669–1677
Almandoz MC, Pagliero CL, Ochoa NA, Marchese J (2015) Composite ceramic membranes from natural aluminosilicates for microfiltration applications. Ceram Int 41:5621–5633
Al-zboon K, Al-harahsheh MS, Hani BF (2011) Fly ash-based geopolymer for Pb removal from aqueous solution. J Hazard Mater 188:414–421
Arandiyan H, Chang H, Liu C, Peng Y, Li J (2013a) Dextrose-aided hydrothermal preparation with large surface area on 1D single-crystalline perovskite La0.5Sr0.5CoO3 nanowires without template: highly catalytic activity for methane combustion. J Mol Catal A Chem 378:299–306
Arandiyan H, Dai H, Deng J, Wang Y, Xie S, Li J (2013b) Dual-templating synthesis of three-dimensionally ordered macroporous La0.6Sr0.4MnO3-supported Ag nanoparticles: controllable alignments and super performance for the catalytic combustion of methane. Chem Commun 49(91):10748–10750
Asl SMH, Ghadi A, Baei MS, Javadian H, Maghsudi M, Kazemian H (2018) Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: a review. Fuel 217:320–342
Asokan P, Saxena M, Asolekar SR (2005) Coal combustion residues—environmental implications and recycling potentials. Resour Conserv Recycl 43:239–262
Awoyemi O, Adeleke E, Dzantor EK (2019) Arbuscular mycorrhizal fungi and exogenous glutathione mitigate coal fly ash (CFA)-induced phytotoxicity in CFA-contaminated soil. J Environ Manag 237:449–456
Bakharev T (2005) Geopolymeric materials prepared using class F fly ash and elevated temperature curing. Cem Concr Res 35:1224–1232
Bartoňová L, Juchelková D, Klika Z, Čech B (2011) On unburned carbon in coal ash from various combustion units. World Acad Sci Eng Technol 76:352–355
Belardi G, Ippolito N, Piga L, Serracino M (2014) Investigation on the status of rare earth elements contained in the powder of spent fluorescent lamps, vol 591. Elsevier BV, Amsterdam, pp 22–30
Bhatt A, Priyadarshini S, Acharath Mohanakrishnan A, Abri A, Sattler M, Techapaphawit S (2019a) Physical, chemical, and geotechnical properties of coal fly ash: a global review. Case Stud Constr Mater 11:e00263
Bhatt A, Priyadarshini S, Acharath A, Abri A, Sattler M, Techapaphawit S (2019b) Case studies in construction materials physical, chemical, and geotechnical properties of coal fly ash : a global review. Case Stud Constr Mater 11:e00263. https://doi.org/10.1016/j.cscm.2019.e00263
Biernacki JJ, Vazrala AK, Leimer HW (2008) Sintering of a class F fly ash. Fuel 87:782–792
Binnemans K, Tom P, Blanpain B, Gerven TV, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22
Blanco F, Garcia MP, Ayala J (2005) Variation in fly ash properties with milling and acid leaching. Fuel 84:89–96
Blanco F, Garcia MP, Ayala J, Mayoral G, Garcia MA (2006) The effect of mechanically and chemically activated fly ashes on mortar properties. Fuel 85:2018–2026
Blissett RS, Rowson NA (2012) Review article a review of the multi-component utilisation of coal fly ash. Fuel 97:1–23
Borer LL (2000) Experiments with aspirin. J Chem Educ 77(3):354–355
Bose CC, Fairchild B, Jones T, Gul A, Ghahfarokhi RB (2015) Application of nanoproppants for fracture conductivity improvement by reducing fl uid loss and packing of micro-fractures. J Nat Gas Sci Eng 9:1–8
Cao D, Selic E, Herbell J-D (2008) Utilization of fly ash from coal-fired power plants in China. J Zhejiang Univ Sci A 9(5):681–687
Chakhmouradian AR, Wall F (2012) Rare earth elements : minerals, mines, magnets. Elements 8:333–340
Chen C, Zhang P, Zeng G, Deng J, Zhou Y, Lu H (2010) Sewage sludge conditioning with coal fly ash modified by sulfuric acid. Chem Eng J J 158:616–622
Chen J, Yuan B, Shi J, Yang JE, Fu M (2018) Reduced graphene oxide and titania nanosheet cowrapped coal fly ash microspheres alternately as a novel photocatalyst for water treatment. Catal Today 315:247–254
Chindaprasirt P, Jaturapitakkul C, Chalee W, Rattanasak U (2009) Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag 29:539–543
Dai S, Zhao L, Peng S, Chou CL, Wang X, Zhang Y, Sun Y (2010) Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar power plant, Inner Mongolia, China. Int J Coal Geol 81(4):320–332
Dash S, Chaudhuri H, Gupta R, Nair UG (2018) Adsorption study of modified coal fly ash with sulfonic acid as a potential adsorbent for the removal of toxic reactive dyes from aqueous solution: kinetics and thermodynamics. Environ Chem Eng. https://doi.org/10.1016/j.jece.2018.05.017
Diaz EI, Allouche EN, Eklund S (2010) Factors affecting the suitability of fly ash as source material for geopolymers. Fuel 89:992–996
Ding J, Ma S, Shen S, Xie Z, Zheng S, Zhang Y (2016) Research and industrialization progress of recovering alumina from fly ash : a concise review. Waste Manag 60(June):375–387
Duan P, Yan C, Zhou W, Ren D (2016) Development of fly ash and iron ore tailing based porous geopolymer for removal of Cu(II) from wastewater. Ceram Int 42:13507–13518
Dzantor KE, Adeleke E, Kankarla V, Ogunmayowa O, Hui D (2015) Using coal fly ash in agriculture: combination of fly ash and poultry litter as soil amendments for bioenergy feedstock production. Coal Combust Gasif Prod 7:33
EGAT (2010) Personal communication with Mae Moh power plant, Thailand. Discussion notes with Mae Moh authrity during site visit made on 23–24 September 2011
Erickson BE (2018) From coal, a new source of rare earths. Am Chem Soc 96(28). Retrieved from https://cen.acs.org/materials/inorganic-chemistry/coal-new-source-rare-earths/96/i28
Ewulonu CM, Igwe IO, Onyeagoro GN (2016) Performance of local clay—titanuim dioxide core—shell extender pigments in alkyd paints. Adv Nanoparticles 5:90–102
Eze CP, Nyale SM, Akinyeye RO, Gitari WM, Akinyemi SA, Fatoba OO, Petrik LF (2013) Chemical, mineralogical and morphological changes in weathered coal fly ash: a case study of a brine impacted wet ash dump. J Environ Manag 129:479–492
Feng Y, Wang K, Yao J, Webley PA, Smart S, Wang H (2013) Effect of the addition of polyvinylpyrrolidone as a pore-former on microstructure and mechanical strength of porous alumina ceramics. Ceram Int 39(7):7551–7556
Flores Y, Flores R, Alvarez AG (2008) Heterogeneous catalysis in the fenton-type system reactive black 5/H2O2. J Mol Catal A Chem 281:184–191
Franus W, Wiatros-Motyka MM, Wdowin M (2015) Coal fly ash as a resource for rare earth elements. Environ Sci Pollut Res 22(12):9464–9474. https://doi.org/10.1007/s11356-015-4111-9
Ghanbari E, Dehghanpour H (2016) The fate of fracturing water: a field and simulation study. Fuel 163:282–294
Ghazali N, Muthusamy K, Wan Ahmad S (2019) Utilization of fly ash in construction. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/601/1/012023
Gitari MW, Akinyemi SA (2018) Introductory chapter: coal fly ash and its application for remediation of acid mine drainage, coal fly ash beneficiation-treatment of acid mine drainage with coal fly ash. In: Akinyemi S, Gitari M (eds) Beneficiation-treatment of acid mine drainage with coal fly ash. InTech, London
Gupta V, Pathak DK, Siddique S, Kumar R, Chaudhary S (2020) Study on the mineral phase characteristics of various Indian biomass and coal fly ash for its use in masonry construction products. Constr Build Mater 235:117413. https://doi.org/10.1016/j.conbuildmat.2019.117413
Harkness JS, Ruhl LS, Millot R, Kloppman W, Hower JC, Hsu-kim H, Vengosh A (2015) Lithium isotope fingerprints in coal and coal combustion residuals from the United States. Procedia Earth Planet Sci 13:134–137
Hatch GP (2012) Dynamics in the global market for rare earths. Elements 8:341–346
Hower JC, Groppo JG, Graham UM, Colin R, Kostova IJ, Maroto-valer MM, Dai S (2017) Coal-derived unburned carbons in fly ash: a review. Int J Coal Geol 179:11–27
Izquierdo M, Moreno N, Font O, Querol X, Alvarez E (2008) Influence of the co-firing on the leaching of trace pollutants from coal fly ash. Fuel 87:1958–1966
Jain M, Dwivedi A (2014) Fly ash–waste management and overview : a review. Recent Res Sci Technol 6(1):30–35
Jain D, Rani A (2011) MgO enriched coal fly ash as highly active heterogeneous base catalyst for claisen-schmidt condensation reaction. Am Chem Sci J 1(2):37–49
Jala S, Goyal D (2006) Fly ash as a soil ameliorant for improving crop production—a review. Biores Technol 97(9):1136–1147
Jayaranjan MLD, van Hullebusch ED, Annachhatre AP (2014) Reuse options for coal fired power plant bottom ash and fly ash. Rev Environ Sci Biotechnol 13:467–486
Ji L, Yu H, Wang X, Grigore M, French D, Gözükara M, Zeng M (2016) CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu. Fuel Process Technol 156:429–437
Joo H, Raj H, Hee J, Jung N, Sang C (2014) Fabrication of multifunctional TiO2–fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram Int 40(2):3023–3029
Kastner RJ, Das KC, Buquoi Q, Melear ND (2003) Low temperature catalytic oxidation of hydrogen sulfide and methanethiol using wood and coal fly ash. Environ Sci Technol 37:2568–2574
Khalesi A, Arandiyan HR, Parvari M (2008) Production of syngas by CO2 reforming on Mxla 1-xNi0.3Al0.7O3-d (M=Li, Na, K) catalysts. Ind Eng Chem Res 47(16):5892–5898
Khatri C, Rani A (2008) Synthesis of a nano-crystalline solid acid catalyst from fly ash and its catalytic performance. Fuel 87(13–14):2886–2892
Khatri C, Jain D, Rani A (2010a) Fly ash-supported cerium triflate as an active recyclable solid acid catalyst for Friedel–Crafts acylation reaction. Fuel 89(12):3853–3859
Khatri C, Mishra MK, Rani A (2010b) Synthesis and characterization of fly ash supported sulfated zirconia catalyst for benzylation reactions. Fuel Process Technol 91(10):1288–1295
Khatri C, Rani A (2007) Green catalytic process for aspirin synthesis using fly ash as heterogeneous solid acid catalyst. Indian Patent No. 1980/DEL/2007
Kizgut S, Cuhadaroglu D, Samanli S (2010) Stirred grinding of coal bottom ash to be evaluated as a cement additive. Energy Sources Part A 32:1529–1539
Kuriakose G, Nagaraju N (2004) Selective synthesis of phenyl salicylate (salol) by esterification reaction over solid acid catalysts. J Mol Catal A Chem 223:155–159
Levandowski J, Kalkreuth W (2009) Chemical and petrographical characterization of feed coal, fly ash and bottom ash from the Figueira power plant, Paraná, Brazil. Int J Coal Geol 77(3–4):269–281
Li X, Ma X, Zhang S, Zheng E (2013) Mechanical properties and microstructure of class C fly ash-based geopolymer paste and mortar. Materials 6(4):1485–1495
Little MR, Adell V, Boccaccini AR, Cheeseman CR (2008) Production of novel ceramic materials from coal fly ash and metal finishing wastes. Resour Conserv Recycl J 52:1329–1335
López-Antón MA, Díaz-Somoano M, Spears DA, Martínez-Tarazona MR (2006) Arsenic and selenium capture by fly ashes at low temperature. Environ Sci Technol 40(12):3947–3951
Manchanda R (2015) A general poro-elastic model for pad-scale fracturing of horizontal wells (Doctoral dissertation)
Manz OE (1997) World wide production of coal ash and utilization in concrete and other products. Fuel 76:691–696
Mardon SM, Hower JC, Keefe JMKO, Marks MN, Hedges DH (2008) Coal combustion by-product quality at two stoker boilers: Coal source versus fly ash collection system design. Int J Coal Geol J 75:248–254
Matsunaga T, Kim JK, Hardcastle S, Rohatgi PK (2002) Crystallinity and selected properties of fly ash particles. Mater Sci Eng 325:333–343
Matzenbacher CA, Garcia ALH, dos Santos MS, Nicolau CC, Premoli S, Corrêa DS, de Souza CT, Delgado TV (2017) DNA damage induced by coal dust, fly and bottom ash from coal combustion evaluated using the micronucleus test and comet assay in vitro. J Hazard Mater 324:781–788
Mirafzal GA, Summer JM (2000) Microwave irradiation reactions: synthesis of analgesic drugs. J Chem Educ 77(3):356–357
Mushtaq F, Zahid M, Bhatti IA, Nasir S, Hussain T (2019) Possible applications of coal fly ash in wastewater treatment. J Environ Manag 240:27–46
Namburath M, Joshi G, Cholemari M, Shet C, Sreekrishnan TR, Veeravalli S (2015) Feasibility Study of indigenously developed fly ash membrane in municipal wastewater treatment. Aquat Procedia 4:1492–1499
Narkhede HP, More UB, Dalal DS, Pawar NS, More DH, Mahulikar PP (2007) Fly-ash-supported synthesis of 2-mercaptobenzothiazole derivatives under microwave irradiation. Synth Commun 37:573–577
Oueralt I, Ouerol X, Lpez-soler A, Plana F (1997) Use of coal fly ash for ceramics : a case study for a large Spanish power station. Fuel 76(8):787–791
Peng Y, Song G (2000) Heteropolyacid-catalyzed synthesis of aspirin. Chem Educ 5:144
Praharaj S (2009) Processing and characterization of fly ash–quartz coatings.
Prezzi M, Kim B (2008) Compaction characteristics and corrosivity mixtures of Indiana class-F fly and bottom ash. Constr Build Mater 22(4):694–702
Rani A, Khatri C, Hada R (2013) Fly ash supported scandium triflate as an active recyclable solid acid catalyst for Friedel–Crafts acylation reaction. Fuel Process Technol 116:366–373
Reitz W (2008) A review of: “Organic Coatings—Science and Technology, 3rd edition, Z.W. Wicks, Jr., F.N. Jones, S.P. Pappas, and D.A. Wicks.” Mater Manuf Process 23(5):544–544
Robl TL, Oberlink AE (2018) Beneficiated fly ash as a micro proppant for oil and gas production from fracking. https://worldofcoalash.org/wpcontent/uploads/2018/11/7
Robl TL, Oberlink AE (2019) Proppant for use in hydraulic fracturing to stimulate a well. https://uknowledge.uky.edu/caer_patents/57%0A
SACE, Southern Alliance for Clean Energy (2020) Coal ash reuse. Retrieved October 7, 2020, from http://www.southeastcoalash.org/about-coal-ash/coal-ash-reuse
Sale LY, Naeth MA, Chanasyk DS (1996) Growth response of barley on unweathered fly ash-amended soil. J Environ Qual 25:684–691
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2010) Science and technology for water purification in the coming decades. Nanosci Technol Collect Rev Nat J 452(3):337–346
Snellings R, Mertens G, Elsen J (2012) Supplementary cementitious materials. Rev Mineral Geochem 7(74):211–278
Sokolar R, Nguyen M (2018) The fly ash of class C for ceramic technology. IOP Conf Ser Mater Sci Eng 385(1):012053
Sonar SK, Niphadkar PS, Mayadevi S, Joshi PN (2014) Preparation and characterization of porous fly ash/NiFe2O4 composite: promising adsorbent for the removal of Congo red dye from aqueous solution. Mater Chem Phys 148:371–379
Tenenbaum DJ (2009) TRASH OR TREASURE?: putting coal combustion waste to work. Environ Health Perspect. https://doi.org/10.1289/ehp.117-a490
Theis TL, Gardner KH (2009) Environmental assessment of ash disposal. Crit Rev Environ Control 20(1):37–41
Tiwari S, Saxena M (1999) Use of fly ash in high performance industrial coatings. Br Corros J 34(3):184–191
U.S., Department of Energy (2011) Critical Materials Strategy. DIANE publishing, Collingdale
Vassilev SV, Vassileva CG (2007) A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 86:1490–1512
Visa M, Bogatu C, Duta A (2010) Applied surface science simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash. Appl Surf Sci 256:5486–5491
Visa M, Andronic L, Duta A (2015) Fly ash-TiO2 nanocomposite material for multi-pollutants wastewater treatment. J Environ Manag 150:336–343
Wang Y, Dai H, Deng J, Liu Y, Arandiyan H, Li X, Xie S (2013) 3DOM InVO4-supported chromia with good performance for the visible-light-driven photodegradation of rhodamine B. Solid State Sci 24:62–70
Wang N, Sun X, Zhao Q, Yang Y, Wang P (2020) Leachability and adverse effects of coal fly ash: a review. J Hazard Mater 396:122725
Wang C, Xu G, Gu X, Gao Y, Zhao P (2021) High value-added applications of coal fly ash in the form of porous materials: a review. Ceram Int 47(16):22302–22315. https://doi.org/10.1016/j.ceramint.2021.05.070
Wu X, Wu S, Qin W, Ma X, Niu Y, Lai S, Ren L (2012) Reductive leaching of gallium from zinc residue. Hydrometallurgy 113–114:195–199
Xie F, Zang AT, Dreisinger D, Doyle F (2014) A critical review on solvent extraction of rare earths from aqueous solutions. Miner Eng 56:10–28
Yang CH, Zhao LM, Liu BG (2005) Use of powdered coal ash for conditioning of specific resistance of sludge water from water works. China Water Wastewater (in Chinese) 21:56–58
Yu X, Cui Y, Chen Y, Chang I-S, Wu J (2022) The drivers of collaborative innovation of the comprehensive utilization technologies of coal fly ash in China: a network analysis. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-19816-5
Zevenbergen C, Bradley JP, Reeuwijk VLP, Shyam AK, Hjelmar O, Comans RNJ (1999) Clay formation and metal fixation during weathering of coal fly ash. Environ Sci Technol 33(19):3405–3409
Acknowledgements
The authors would like to express their gratitude to the Provost of College of Engineering, Kwame Nkrumah University of Science and Technology, Kumasi for his support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Editorial responsibility: Sivakumar Durairaj.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nsiah-Gyambibi, R., Sokama-Neuyam, Y.A., Boakye, P. et al. Valorization of coal fly ash (CFA): a multi-industry review. Int. J. Environ. Sci. Technol. 20, 12807–12822 (2023). https://doi.org/10.1007/s13762-023-04895-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04895-9