Abstract
This paper discusses the result obtained from the investigation on the performance, emission, and combustion characteristics of multi-cylinder SI engines using ethanol-n butanol-unleaded gasoline blends (EB5, EB10, EB15, and EB20) as fuel. The tests were conducted at 10 Nm and 15 Nm load conditions. The speed varied between 1200 and 2400 rpm. Increased blend percentage increases brake thermal efficiency, and the brake thermal efficiency of 16.7%, 17.8%, 18.3%, and 19.2% are achieved for EB5, EB10, EB15, and EB20, respectively, at 15 Nm and 2400 rpm. The tremendous reduction in hydrocarbon and carbon monoxide emissions is observed with an increase in load and speed conditions with blends. The lowest hydrocarbon and carbon monoxide emissions of 164 ppm and 0.01569%, respectively, are obtained at 15 Nm and 2400 rpm. However, oxides of nitrogen emissions increased in all blends and load conditions. The EB20 blend produced the highest in-cylinder pressure of 27.2 bar at 15 Nm and 2400 rpm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing population and per-person energy use have dramatically grown, which has led to an increase in both air and water pollution. Automotive vehicles account for a large portion of poor air quality on a mass scale. Based on this, many scientists are concentrating on environmentally friendly energies like renewable energy, bioenergy and working on cutting back on the use of fossil fuels in automobiles. (Akansu et al. 2017). Currently, biofuels account for nearly 3% of road transportation fuel consumption, and by 2030, the share will rise to 4 to 6% (Abdellatief et al. 2022). Alcohol is becoming more prominent biofuel for automobiles due to the industrial revolution in the twentieth century. Several oxygenates are examined as fuel additives to improve the efficiency and performance of the fuel. However, oxygenates have been used primarily due to their ability to increase the octane number of fuel and to reduce exhaust emissions (Dhamodaran and Esakkimuthu 2019; Lim et al. 2019). Besides its high-octane rating, ethanol's laminar flame speed improves combustion efficiency and reduces knocking. As a result of its oxygen content, hydrocarbon emissions are reduced due to its increased efficiency in primary combustion and post-oxidation (Dhamodaran et al. 2022a, 2022b; Wang et al. 2017). Most notably, because of its advantageous characteristics for use in internal combustion engines and also because it is produced from renewable sources, ethanol has the potential to achieve remarkable thermal efficiency and very low exhaust emissions (Hua et al. 2022). Ethanol has a higher latent heat of vaporization, which promotes higher volumetric efficiency by allowing low-temperature dense air into the intake manifold. Also, the higher latent heat of vaporization, reduces the oxides of nitrogen (NOx) emission in SI engines by absorbing more heat from the cylinder wall and combustion products.
Researchers are now focusing more attention on higher alcohols because of their high energy density, their greater blend stability, and the fact that they are more hygroscopic than lower alcohols. Alcohol molecules develop an ignition quality as their carbon chains become longer (Yaman et al. 2022). Alcohols with greater carbon contents (such as n-butanol) have also been the subject of research and recommendations (Dhamodaran et al. 2022a, b; Moxey et al. 2016). The performance investigation of ethanol-gasoline blends in SI engines was looked into by Thakur et al. (2017) under various engine load, speed, and compression ratio conditions. When the ethanol blends E20, E25, E30, E75, and E100 were employed, the brake specific fuel conception (BSFC) improved by 5.17%, 10%, 20%, 37%, and 56%, respectively. But, for E10, E20, and E40, the increased brake thermal efficiency (BTE) was observed 2.5%, 3.5%, and 6%.
The performance and emission characteristics of the SI engine were studied with the fuel containing oxygen from 3.5 to 20 wt.% using ethers, alcohols, and carbonate blends. The oxygen content and specific gravity positively affect carbon monoxide (CO) and carbon dioxide (CO2) emissions and negatively affects NOx emissions. Moreover, by operating the engine with a higher compression ratio, the blend's oxygen content can cut CO and HC emissions, improve the knock index, and promote higher engine efficiency (Schifter et al. 2017).
In a four-stroke, single-cylinder SI engine, Wen et al. (2010), examined the impact of adding dimethyl carbonate (DMC) and ethanol on exhaust emission for various engine speeds. The results indicate that DMC and ethanol-gasoline blends boost fuel consumption while lowering hydrocarbon (HC) and CO emissions. Because of enhanced combustion, the CO2 emission rose. However, it was negligible because NOx emission is dependent on engine operating conditions rather than the blending ratio. Due to the higher latent heat of vaporization of oxygenates, engine efficiency increased marginally. Under lean conditions, ethanol and DMC accelerate combustion by 5–10% compared to neat gasoline (Schifter et al. 2016). The effects of alcohol-gasoline fuel blends on the operation and combustion characteristics of a SI engine were investigated by Eyidogan et al. (2010) at various vehicle speeds and wheel power conditions. The findings indicate that brake-specific fuel consumption has increased when a vehicle is fueled with ethanol-gasoline (E5, E10) and methanol-gasoline (M5, M10) mixes as compared to pure gasoline fuel. This occurs because alcohols have a lower heating value (37–54 percent lowers than pure gasoline).
Mohammed et al. (2021) experimented on a single-cylinder SI engine using different proportions of ethanol and gasoline. The results show a significant reduction in exhaust emissions with an increase in ethanol percentage with gasoline. The highest reduction of CO was found in E30 (ethanol 30% + gasoline 70%), and of CO2, HC, and NOx emissions was found in E40 (ethanol 40% + gasoline 60%) by 26.33%, 25%, 31.50%, and 20.91%, respectively. The ethanol produced from pomegranate blended with gasoline in different proportions at different speeds and constant load conditions was tested on a multi-cylinder spark ignition engine. The maximum thermal efficiency of 28.33% was obtained in the PE15 blend at 1500 rpm. Also, the lowest HC and CO emissions are recorded in PE15 and PE20 blends at the speed range between 1400 to 1600 rpm (Dhande et al. 2021). According to the results of the study carryover by Lihak et al., it was found that the experiments with ethanol and acetylene showed a notable reduction in HC emission. The reduction was observed from 4 to 2.3 g/kWh for ethanol and 0.23 g/kWh for acetylene (İlhak et al. 2020).
Thangavel et al. (2016) investigated spark ignition engine by simultaneous injection of ethanol-gasoline and n-butanol-gasoline in the intake manifold. Two separate injectors are mounted in the intake manifold to inject gasoline and oxygenates separately. The engine operated with ethanol-gasoline produced higher torque and engine efficiency. E30S blend produced 5.2% higher torque than gasoline, and efficiency also increased from 28 to 29%. Using 60% n-butanol by mass with gasoline increases HC emission and reduces efficiency and torque because of the poor vaporization of n-butanol. Overall, the torque and efficiency of the ethanol-gasoline blend are comparatively higher than n-butanol-gasoline blend operation. In contrast, Tian et al. (2020) found that adding n-butanol to gasoline reduces in-cylinder temperatures and increases brake thermal efficiency. However, when the n-butanol blending ratio rises can achieve greater anti-knocking tendency and permit earlier ignition timing, improving peak cylinder pressure and heat release rate (Feng et al. 2018; Liu et al. 2018). Also, Dhamodaran et al. (2017), investigated n-butanol and gasoline blends in MPFI SI engine and observed increased in-cylinder pressure and higher heat release rate for all the blending ratios of N10, N20, and N30. The NOx and CO emissions decreased when n-butanol was added to neat gasoline. Also, the HC emissions initially reduced and then increased (Yu et al. 2018). Under different alcohol ratios, equivalency ratios, and engine loads, Li et al. (2017), studies compared the combustion, performance, and emissions properties of methanol, ethanol, and butanol in a spark ignition engine. The study discovered that ethanol-gasoline blends produce lower HC emissions due to higher oxygen content in alcohols, which improves combustion quality. Butanol-gasoline blends produce higher latent heat of vaporization (LHV), reducing brake-specific fuel consumption (BSFC). Saraswat and Chauhan (2020), carried out the comparative investigation of gasoline-butanol and gasoline-algae oil as fuel in SI engines. The lower calorific value of butanol and algae blends increases BSFC higher than gasoline for all the operating conditions. Besides brake power, torque, HC, and CO are improved while adding butanol. Acetone, butanol, and ethanol are the fuels blended with gasoline in terms of 88% gasoline, 5% ethanol, 2% butanol, and 5% acetone (ABE5) and 83% gasoline, 5% ethanol, 2% butanol, and 5% acetone (ABE10). The highest BTE of 29.96% was observed for ABE5 blends, but it is slightly lower than pure gasoline (Dinesha et al. 2022). Tang et al. (2021), experimented to study four-stroke SI engine fueled with n-butanol/gasoline blends on different proportions, speed conditions, and throttle positions. The results found that brake power, brake thermal efficiency, and BSFC increased with the n-butanol/gasoline blending ratio. Most importantly, NOx emission decreased notably, but a slight increase in CO emission.
Fagundez et.al. (2019), investigated pure n-butanol and n-butanol/ethanol blend as fuel in spark ignition engines with exciting comparisons. The results of pure n-butanol were compared with hydrous ethanol, and the blends of n-butanol/ethanol were compared with the gasoline/ethanol blend. According to this study, pure n-butanol showed abysmal performance compared with hydrous ethanol. However, n-butanol/ethanol blends were comparable to gasoline in terms of similar engine performance, indicating that n-butanol is the potential fuel to replace gasoline when blended with ethanol.
The literature shows that many studies have carried out ethanol-gasoline and butanol-gasoline mixtures as fuel in spark ignition engines. However, only a few experimental works are done in ethanol-butanol/gasoline mixture in multi-cylinder spark ignition engine.
Ethanol and n-butanol, each with their benefits and drawbacks in terms of fuel quality and combustion characteristics, have much potential to replace conventional fuel in the future. The performance and emissions of the multiple-cylinder MPFI SI engine were tested in this paper using ethanol, n-butanol, and gasoline mixes under various speed and load conditions. The results are then contrasted with unleaded gasoline.
Materials and methods
Experimental setup
The experiments were conducted in four cylinder, four stroke water cooled multi-cylinder MPFI SI engine and the specifications of the engine are given in Table 1. The test engine has the 72 mm of bore and 61 mm of stroke and 9.4:1 of compression ratio.
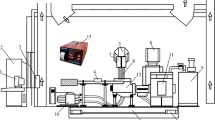
The test engine was loaded with an eddy current dynamometer with a maximum loading capacity of 45 Nm. Fuel consumption was measured by gravimetrically using an electro balance and air flow was measured by intake airflow sensor available in air tank. Emission was measured using Netel gas analyzer. Figure 1 shows the photographical view of experimental setup.
Fuel preparation
Commercial gasoline was obtained from a local gasoline station, ethanol of 99.5% and n-butanol of 99.9% per purity was purchased from a local chemical supplier (Lab Chemicals, Chennai, India). Ethanol, n-butanol and gasoline were mixed by volume basis and mixer ratios are 2.5:2.5:95 (E:B:G), 5:5:90 (E:B:G), 7.5:7.5:85 (E:B:G), 10:10:80 (E:B:G). These ratios were achieved by temperature controlled magnetic stirrer, and the individual properties of these blends are calculated using simple mixing rules. Table 2 shows the essential properties of ethanol and n-butanol fuels.
Experimental procedure
In this study, pure commercial unleaded gasoline was used as a base fuel and blended with ethanol and n-butanol to prepare four different blends on a volume basis. These are EB5 (Ethanol and n-butanol 5% + Gasoline 95%), EB10 (Ethanol and n-butanol 10% + Gasoline 90%), EB15 (Ethanol and n-butanol 15% + Gasoline 85%) and EB20 (Ethanol and n-butanol 20% + Gasoline 80%). Tests were conducted at four different speed conditions from 1200 to 2400 rpm and with load conditions of 10 Nm and 15 Nm, respectively. Engine performance and combustion parameters are measured for all individual experiments. Engine emissions of HC, CO, and NOx are measured with the help of five gas analyzer.
Error analysis
In order to assess the correctness of the experimental result, the uncertainty of the experiment must be evaluated. A number of errors may contribute to uncertainty regarding the experimental observation, including errors in reading, environment errors, instrument selection, calibration errors, and working conditions. A calculation of the uncertainty associated with each instrument was made according to the procedures laid down by Holman (2012). The uncertainty percentage of this experiment was calculated using the formula,
where Uxi is the uncertainty associated with each measured value using the corresponding instrument. Table 3 includes details about the parameters measured, the range and accuracy of the instrument, and the percentage of uncertainty associated with it. Performance measurement, properties measurement, and emission measurement instruments have calculated uncertainties of ± 0.886%, + 0.44%, and ± 0.4638%, respectively. The calculated uncertainty is 1.789% for the entire experiment.
Results and discussion
Brake thermal efficiency
The BTE shows an engine conversion efficiency of chemical energy of fuel into mechanical energy (Barboza et al. 2022; Li et al. 2016). Fig. 2 shows variation of BTE with different load and speed conditions. The results show lower BTE compared to EB10, EB15 and EB20, this is due EB5 having lower oxygen content than others, lower the oxygen content reduces the combustion efficiency and reduces the BTE. At higher engine speed condition, fuel absorb the heat from engine cylinder to get vaporize and reduces the compression work of the piston to get better mixing, so it will increase the BTE of the engine (Eyidogan et al. 2010). Higher the flame velocity leads to reduce the combustion duration and increases the thermal efficiency of all higher EB blends compared with gasoline. EB20 produced higher BTE compared to other blends because of higher oxygen content supplied into the engine leads to improved combustion. Also, the higher engine speeds enhance the evaporation of blends and improve the mixing of air and fuel at compression stroke and increases the BTE of the engine. The BTE of 16.7%, 17.8%, 18.3% and 19.2% is achieved for EB5, EB10, EB15 and EB20, respectively, at 15 Nm and 2400 rpm. All the blends produced higher BTE as compared to gasoline at all the speed and load conditions.
Hydrocarbon
The HC emission shows the engine's combustion quality, which means lower HC emissions indicate enhanced engine combustion. Some significant causes of HC emissions are the non-stoichiometric air–fuel ratio, incomplete combustion, and crevice volume of the combustion chamber (Akansu et al. 2017; Thomas et al. 2016). Figure 3 shows that HC emission was reduced in all blends while increasing the engine speed. The oxygen content in alcohols improves combustion efficiency and reduces HC emissions. The ethanol-n-butanol-gasoline blend gives lower HC emissions than unleaded gasoline due to ethanol and n-butanol having a higher laminar flame speed and thus producing the lowest concentration of HC emissions in the exhaust. The engine speed increases the turbulent effect and enhances the oxidation of HC to H2O and CO2 (Sathyanarayanan et al. 2022).
Further, the higher latent heat of vaporization of ethanol and n-butanol reduces combustion wall temperature, and this lower wall temperature suppresses flame velocity by rapid cooling. This cooling effect interrupts the chain reaction of the flame and forms a flame-quenching layer on the wall surface (Tian et al. 2020). However, adding alcohol blends increases OH and HO2 radicals, reduces the phenomenon of flame cooling and wall quenching, and thus reduces HC emissions (Li et al. 2023). The lower amount of HC emission flame is observed at 15 Nm and 2400 rpm, and a reduction is 1.43%, 7.62%, 16.19%, and 21.43%tare observed with EB5, EB10, EB5, and EB20, respectively, as compared to gasoline.
Carbon monoxide
Incomplete combustion produces CO as an intermediate product during the combustion of hydrocarbons (Li et al. 2022). A zero percent reduction of CO is not possible; a small quantity of incomplete fuel combustion occurs in the combustion chamber (Wang et al. 2022). The observation results of CO emission show that increment of ethanol-n-butanol blends lowers CO concentration compared to pure gasoline. Furthermore, for all load and speed conditions, the CO reduction was notably lower than pure gasoline. Due to ethanol and n-butanol fuels contain more oxygen, enhancing blended fuel properties, creating a leaning effect in the air–fuel mixture, improving the combustion process, and decreasing CO emission (Wen et al. 2010).
Further, ethanol increases oxidizing activating groups, such as OH in the fuel–air mixture, which will convert CO produced at high temperatures to CO2 when the temperature drops (Zhao et al. 2022). Figure 4 shows the CO emissions from all ethanol-n-butanol blends and pure gasoline at various speed and load conditions. Compared to pure gasoline, ethanol-n-butanol blends EB5, EB10, EB5, and EB20 produced significantly lower CO emissions. The lowest CO concentration of 0.01569% is observed from the EB20 blend ratio at 15 Nm and 2400 rpm.
Oxides of nitrogen
Figure 5 shows the variation of NOx emission at different speeds and load conditions. The concentration of NOx emission increases with engine speed and fuel blending ratios. The combustion chamber temperature and air–fuel ratio are the critical parameters affecting the formation of NOx emission (Behçet and Yakin 2022). The increases in NOx emission indicate higher in-cylinder temperature due to enhancement in the combustion process by higher oxygen content in the blend, the formation of an excellent combustible mixture at higher speed, and higher fuel vaporization. Ethanol and n-butanol have higher oxygen content and tend to create a lean combustion charge. During the combustion process, the lean charge of the mixture enhances the reaction between oxygen and nitrogen at higher temperatures and forms NOx. Adding ethanol to the premixed mixture does not affect the temperature or pressure of the cylinder. Despite this, it suppresses the mole fraction of H and O radicals by suppressing the contributing reactions, resulting in a passive effect on NO formation. In addition, the effect is even more substantial when high levels of ethanol are present (Xie et al. 2022). The increased percentage of NOx emission was 6.93%, 12.70%, 13.05%, and 21.66% of EB5, EB10, EB15, and EB20, respectively, at 15 Nm and 2400 rpm, as compared to gasoline.
In-cylinder pressure
Figure 6 shows the variation of in-cylinder gas pressure with crank angles at 10 Nm and 15 Nm at 2400 rpm. As seen in the figure, for the all-loading condition, the cylinder gas pressure with EB5, EB10, EB15, and EB20 began to increase earlier than unleaded gasoline. The earlier increase in gas pressure reduced the combustion duration because of short ignition delay period (Fu et al. 2017; Sayin and Balki 2015). Short combustion duration reduces heat losses to the cylinder walls and increases the combustion charge temperature and pressure. The maximum in-cylinder gas pressure observed at 15 Nm is 26.6 bar, 26.7 bar, 27 bar, and 27.3 bar for EB5, EB10, EB15, and EB20, respectively. Compared to gasoline, in-cylinder pressure improved for all blended fuels due to more oxygen molecules in the blends, thus accelerating the combustion process and leading to peak pressure earlier. The position of OH radicals in butanol promotes the combustion process by breaking bonds with less energy requirement and also improves more participation of radicals in the combustion. This phenomenon is also the reason for earlier peak pressure in the combustion. (Edwin Geo et al. 2019).
Conclusion
In this study, the performance, combustion, and emission characteristics of ethanol and n-butanol blends with gasoline in multi-cylinder SI engine with the blends of EB5, EB10, EB15, and EB20 by volume basis are studied at 10 Nm and 15 Nm loads, with speed 1200 rpm, 1600 rpm, 2000 rpm, and 2400 rpm. The following results were obtained through the investigation.
-
The addition of EB blends improved the BTE at all speeds and load conditions, and EB20 produced higher BTE than all the others. The higher load and speed conditions increase the turbulence, and higher EB blends enhance oxygen molecules and accelerate the flame velocity, thus improving BTE. At 15 Nm and 2400 rpm, the BTE of 16.7%, 17.8%, 18.3%, and 19.2% are achieved for EB5, EB10, EB15, and EB20, respectively.
-
The lowest HC and CO emissions were observed at 15 Nm and 2400 rpm. The observed values are 153 ppm and 0.01569%, respectively, due to the higher oxygen content and laminar speed of EB blends which promote complete combustion.
-
The maximum percentage increase in NOx emission was 6.93%, 12.70%, 13.05%, and 21.66% for EB5, EB10, EB15, and EB20, respectively, at 15 Nm and 2400 rpm compared to unleaded gasoline. The reason was that the leaner combustion charge enhances the reaction between oxygen and nitrogen at a higher temperature.
-
The highest in-cylinder gas pressure measured was 27.3 bar at 15 Nm and 2400 rpm. The gas pressure reached its maximum value at 1° earlier crank angle than unleaded gasoline. The short combustion duration, surplus amount of oxygen content in the mixture, and position of OH radicals are the critical factors of improved in-cylinder pressure.
-
Overall, the EB20 blend produced higher performance and lower emission characteristics except for NOx. This exhibits that ethanol and n-butanol can be used as fuel additives for gasoline without much compromise in their effect on performance, combustion, and emission.
This study investigated engine performance, combustion, and emission values of ethanol-n-butanol and gasoline in partial loads. In other studies, more suitable operating conditions can be obtained for SI engine when comparatively ethanol-gasoline, butanol-gasoline and ethanol-butanol-gasoline mixtures are tested in detail.
Abbreviations
- BMEP:
-
Brake mean effective pressure
- BP:
-
Brake power
- BSFC:
-
Brake specific fuel consumption
- bTDC:
-
Before top dead center
- BTE:
-
Brake thermal efficiency
- CA:
-
Crank angle
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- CR:
-
Compression ratio
- EB:
-
Ethanol-butanol
- EB0:
-
Pure gasoline
- EB5:
-
5% Ethanol-butanol—95% gasoline
- EB10:
-
10% Ethanol-butanol—90% gasoline
- EB15:
-
15% Ethanol-butanol—85% gasoline
- EB20:
-
20% Ethanol-butanol—80% gasoline
- HC:
-
Hydrocarbon
- NO:
-
Nitrogen oxide
- NOx:
-
Oxides of nitrogen
- O2 :
-
Oxygen
- ppm:
-
Parts per million
- RON:
-
Research octane number
- RPM:
-
Revolution per minute
References
Abdellatief TMM, Ershov MA, Kapustin VM, Chernysheva EA, Savelenko VD, Salameh T, Abdelkareem MA, Olabi AG (2022) Uniqueness technique for introducing high octane environmental gasoline using renewable oxygenates and its formulation on Fuzzy modeling. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.149863
Akansu SO, Tangöz S, Kahraman N, İlhak Mİ, Açıkgöz S (2017) Experimental study of gasoline-ethanol-hydrogen blends combustion in an SI engine. Int J Hydrog Energy 42(40):25781–25790. https://doi.org/10.1016/j.ijhydene.2017.07.014
Barboza ABV, Mohan S, Dinesha P (2022) Influence of hydrogen peroxide emulsification with gasoline on the emissions and performance in an MPFI engine. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2022.05.229
Behçet R, Yakin A (2022) Evaluation of hydrogen-containing NaBH4 and oxygen-containing alcohols (CH3OH, C2H5OH) as fuel additives in a gasoline engine. Int J Hydrogen Energy 47(53):22316–22327. https://doi.org/10.1016/j.ijhydene.2022.04.258
Dhamodaran G, Esakkimuthu GS (2019) Experimental measurement of physico-chemical properties of oxygenate (DIPE) blended gasoline. Meas J Int Meas Confed 134:280–285. https://doi.org/10.1016/j.measurement.2018.10.077
Dhamodaran G, Esakkimuthu GS, Palani T (2022a) Feasibility of adding n-Butanol and di isopropyl ether with gasoline on its physico-chemical properties. Pet Sci Technol 40(4):486–503. https://doi.org/10.1080/10916466.2021.2003383
Dhamodaran G, Esakkimuthu GS, Pochareddy YK, Sivasubramanian H (2017) Investigation of n-butanol as fuel in a four-cylinder MPFI SI engine. Energy 125:726–735. https://doi.org/10.1016/j.energy.2017.02.134
Dhamodaran, G., Sundaram Esakkimuthu, G., Palani, T., & Krishnan, R. (2022b). Feasibility of adding fusel oil as an oxygenate to gasoline on reducing MPFI engine emissions. In: Environmental Engineering and Management Journal (Vol. 21, Issue 7). http://www.eemj.icpm.tuiasi.ro/; http://www.eemj.eu
Dhande DY, Sinaga N, Dahe KB (2021) Study on combustion, performance and exhaust emissions of bioethanol-gasoline blended spark ignition engine. Heliyon 7(3):5. https://doi.org/10.1016/j.heliyon.2021.e06380
Dinesha P, Mohan S, Kumar S (2022) Experimental investigation of SI engine characteristics using Acetone-Butanol-Ethanol (ABE): Gasoline blends and optimization using particle swarm optimization. Int J Hydrog Energy 47(8):5692–5708. https://doi.org/10.1016/j.ijhydene.2021.11.119
Edwin Geo V, Jesu Godwin D, Thiyagarajan S, Saravanan CG, Aloui F (2019) Effect of higher and lower order alcohol blending with gasoline on performance, emission and combustion characteristics of SI engine. Fuel. https://doi.org/10.1016/j.fuel.2019.115806
Elfasakhany A (2015) Investigations on the effects of ethanol–methanol–gasoline blends in a spark-ignition engine: performance and emissions analysis. Eng Sci Technol Int J 18(4):713–719. https://doi.org/10.1016/j.jestch.2015.05.003
Eyidogan M, Ozsezen AN, Canakci M, Turkcan A (2010) Impact of alcohol-gasoline fuel blends on the performance and combustion characteristics of an SI engine. Fuel 89(10):2713–2720. https://doi.org/10.1016/j.fuel.2010.01.032
Fagundez JLS, Golke D, Martins MES, Salau NPG (2019) An investigation on performance and combustion characteristics of pure n-butanol and a blend of n-butanol/ethanol as fuels in a spark ignition engine. Energy 176:521–530. https://doi.org/10.1016/j.energy.2019.04.010
Feng D, Wei H, Pan M, Zhou L, Hua J (2018) Combustion performance of dual-injection using n-butanol direct-injection and gasoline port fuel-injection in a SI engine. Energy 160:573–581. https://doi.org/10.1016/j.energy.2018.07.042
Fu J, Shu J, Zhou F, Liu J, Xu Z, Zeng D (2017) Experimental investigation on the effects of compression ratio on in-cylinder combustion process and performance improvement of liquefied methane engine. Appl Therm Eng 113:1208–1218. https://doi.org/10.1016/j.applthermaleng.2016.11.048
Hua Y, Xiang X, Qian Y, Meng S, Ye B (2022) Effect of diffusion oxygen enrichment on soot formation in coflow diffusion flames of gasoline-surrogate fuel doped with ethanol. Fuel 328:125306. https://doi.org/10.1016/j.fuel.2022.125306
İlhak Mİ, Doğan R, Akansu SO, Kahraman N (2020) Experimental study on an SI engine fueled by gasoline, ethanol and acetylene at partial loads. Fuel. https://doi.org/10.1016/j.fuel.2019.116148
Li D, Yu X, Guo Z, Zhang J, Wang T, Li Y (2023) Effects of isopropanol ratio at different excess air ratios on combustion and emissions characteristics of an isopropanol/gasoline dual-fuel combined injection SI engine. Fuel. https://doi.org/10.1016/j.fuel.2022.126507
Li X, Zhen X, Wang Y, Tian Z (2022) Numerical comparative study on performance and emissions characteristics fueled with methanol, ethanol and methane in high compression spark ignition engine. Energy. https://doi.org/10.1016/j.energy.2022.124374
Li Y, Gong J, Deng Y, Yuan W, Fu J, Zhang B (2017) Experimental comparative study on combustion, performance and emissions characteristics of methanol, ethanol and butanol in a spark ignition engine. Appl Therm Eng 115:53–63. https://doi.org/10.1016/j.applthermaleng.2016.12.037
Li Y, Meng L, Nithyanandan K, Lee TH, Lin Y, Cjia-fon FL, Liao S (2016) Combustion, performance and emissions characteristics of a spark-ignition engine fueled with isopropanol-n-butanol-ethanol and gasoline blends. Fuel 184:864–872. https://doi.org/10.1016/j.fuel.2016.07.063
Lim CS, Lim JH, Cha JS, Lim JY (2019) Comparative effects of oxygenates-gasoline blended fuels on the exhaust emissions in gasoline-powered vehicles. J Environ Manage 239:103–113. https://doi.org/10.1016/j.jenvman.2019.03.039
Liu K, Li Y, Yang J, Deng B, Feng R, Huang Y (2018) Comprehensive study of key operating parameters on combustion characteristics of butanol-gasoline blends in a high speed SI engine. Appl Energy 212:13–32. https://doi.org/10.1016/j.apenergy.2017.12.011
Mohammed MK, Balla HH, Al-Dulaimi ZMH, Kareem ZS, Al-Zuhairy MS (2021) Effect of ethanol-gasoline blends on SI engine performance and emissions. Case Stud Thermal Eng. https://doi.org/10.1016/j.csite.2021.100891
Moxey BG, Cairns A, Zhao H (2016) A comparison of butanol and ethanol flame development in an optical spark ignition engine. Fuel 170:27–38. https://doi.org/10.1016/j.fuel.2015.12.008
Saraswat M, Chauhan NR (2020) Comparative assessment of butanol and algae oil as alternate fuel for SI engines. Eng Sci Technol Int J 23(1):92–100. https://doi.org/10.1016/j.jestch.2019.04.002
Sathyanarayanan S, Suresh S, Saravanan CG, Vikneswaran M, Dhamodaran G, Sonthalia A, Josephin JSF, Varuvel EG (2022) Experimental investigation and performance prediction of gasoline engine operating parameters fueled with diisopropyl ether-gasoline blends: response surface methodology based optimization. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.133941
Sayin C, Balki MK (2015) Effect of compression ratio on the emission, performance and combustion characteristics of a gasoline engine fueled with iso-butanol/gasoline blends. Energy 82:550–555. https://doi.org/10.1016/j.energy.2015.01.064
Schifter I, González U, Díaz L, Sánchez-Reyna G, Mejía-Centeno I, González-Macías C (2017) Comparison of performance and emissions for gasoline-oxygenated blends up to 20 percent oxygen and implications for combustion on a spark-ignited engine. Fuel 208:673–681. https://doi.org/10.1016/j.fuel.2017.07.065
Schifter I, González U, González-Macías C (2016) Effects of ethanol, ethyl-tert-butyl ether and dimethyl-carbonate blends with gasoline on SI engine. Fuel 183:253–261. https://doi.org/10.1016/j.fuel.2016.06.051
Tang Q, Jiang P, Peng C, Chang H, Zhao Z (2021) Comparison and analysis of the effects of spark timing and lambda on a high-speed spark ignition engine fuelled with n-butanol/gasoline blends. Fuel. https://doi.org/10.1016/j.fuel.2020.119505
Thakur AK, Kaviti AK, Mehra R, Mer KKS (2017) Progress in performance analysis of ethanol-gasoline blends on SI engine. In: Renewable and sustainable energy reviews (vol. 69, pp 324–340). Elsevier Ltd. https://doi.org/10.1016/j.rser.2016.11.056
Thangavel V, Momula SY, Gosala DB, Asvathanarayanan R (2016) Experimental studies on simultaneous injection of ethanol-gasoline and n-butanol-gasoline in the intake port of a four stroke SI engine. Renew Energy 91:347–360. https://doi.org/10.1016/j.renene.2016.01.074
Thomas R, Sreesankaran M, Jaidi J, Paul DM, Manjunath P (2016) Experimental evaluation of the effect of compression ratio on performance and emission of SI engine fuelled with gasoline and n-butanol blend at different loads. Perspect Sci 8:743–746. https://doi.org/10.1016/j.pisc.2016.06.076
Tian Z, Zhen X, Wang Y, Liu D, Li X (2020) Combustion and emission characteristics of n-butanol-gasoline blends in SI direct injection gasoline engine. Renew Energy 146:267–279. https://doi.org/10.1016/j.renene.2019.06.041
Wang C, Zeraati-Rezaei S, Xiang L, Xu H (2017) Ethanol blends in spark ignition engines: RON, octane-added value, cooling effect, compression ratio, and potential engine efficiency gain. In: Applied energy (vol. 191, pp. 603–619). Elsevier Ltd. https://doi.org/10.1016/j.apenergy.2017.01.081
Wang X, Gao J, Chen Z, Chen H, Zhao Y, Huang Y, Chen Z (2022) Evaluation of hydrous ethanol as a fuel for internal combustion engines: a review. In: Renewable energy (vol. 194, pp 504–525). Elsevier Ltd. https://doi.org/10.1016/j.renene.2022.05.132
Wen LB, Xin CY, Yang SC (2010) The effect of adding dimethyl carbonate (DMC) and ethanol to unleaded gasoline on exhaust emission. Appl Energy 87(1):115–121. https://doi.org/10.1016/j.apenergy.2009.06.005
Xie M, Li Q, Fu J, Yang H, Wang X, Liu J (2022) Chemical kinetic investigation on NOx emission of SI engine fueled with gasoline-ethanol fuel blends. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.154870
Yaman H, Yesilyurt MK, Uslu S (2022) Simultaneous optimization of multiple engine parameters of a 1-heptanol / gasoline fuel blends operated a port-fuel injection spark-ignition engine using response surface methodology approach. Energy. https://doi.org/10.1016/j.energy.2021.122019
Yu X, Guo Z, He L, Dong W, Sun P, Shi W, Du Y, He F (2018) Effect of gasoline/n-butanol blends on gaseous and particle emissions from an SI direct injection engine. Fuel 229:1–10. https://doi.org/10.1016/j.fuel.2018.05.003
Zhao Z, Yu X, Huang Y, Shi W, Guo Z, Li Z, Du Y, Jin Z, Li D, Wang T, Li Y (2022) Experimental study on combustion and emission of an SI engine with ethanol /gasoline combined injection and EGR. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.129903
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editorial responsibility: Samareh Mirkia.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palani, T., Esakkimuthu, G.S., Dhamodaran, G. et al. Experimental study on dual oxygenates (ethanol, n-butanol) with gasoline on MPFI engine performance and emission characteristics. Int. J. Environ. Sci. Technol. 21, 245–254 (2024). https://doi.org/10.1007/s13762-023-04852-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04852-6