Abstract
Modelling bioprocesses is an essential aspect in process design of reactor systems in the context of wastewater treatment. Designing a biological reactor for simultaneous nitrification and denitrification requires consideration of both substrate and microbial kinetics along with the effect of other experimental parameters. Nitrogen removal from wastewaters can be economically and efficiently achieved using this single-staged process that has proved to be advantageous over nitrification and denitrification, occurring separately. For the last few decades several models have been developed to estimate and predict outcome of such processes based on both experimental results and modifications of classical mathematical models including activated sludge models. Models have been established for a number of different suspended and attached growth reactors considering several influencing and inhibitory parameters. This paper exhaustively reviews the existing models analysing different considerations and assumptions thereby identifying the research gaps that can be further addressed to develop a more versatile model of simultaneous nitrification and denitrification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Simultaneous nitrification and denitrification (SND) is one of the most favourable methods for removing nitrogen from wastewater due to its several advantages over other multi-staged nitrogen removal systems. The process involves nitrification and denitrification occurring synchronously in a single reactor vessel, thus reducing reactor footprint, treatment time, fabrication and energy cost required for distinct nitrifying and denitrifying units and eradicates the necessity of separate monitoring systems for the two different units (Bhattacharya and Mazumder 2021). Nitrogen removal via SND has been reported to be quite high (within 80–96%) (Zeng et al. 2004; Jimenez et al. 2010), it reduces both treatment time and necessity of external carbon source and alkali requirement (Pochana et al. 1999). Almost 22–40% less carbon is needed along with 30% less sludge production (Seifi and Fazaelipoor 2012) as compared to separate nitrification and denitrification process. Plants with SND face significantly less design and operational challenges once the process is stabilized and proper evaluation is made (Pochana et al. 1999). Despite these advantages, SND has certain drawbacks including aeration cost required for aerobic nitrification and addressing the challenges of creating different environmental conditions to sustain two processes in a single reactor. Development of layers of different microbial niche inside biofilm and large flocs into aerobic and anoxic zones supporting these two reactions depends on the availability of oxygen penetrated or diffused, which needs to be controlled around an optimum range. Other combined pathways including SND over nitrite or shortcut nitrification and denitrification, partial nitrification-and-Anammox, simultaneous partial nitrification-Anammox,-and-denitrification (SNAD) have been investigated in quest for establishing a more economic and efficient nitrogen removal system. SND over nitrite offers several benefits in terms of energy (60% reduction with respect to conventional nitrification), carbon (40% reduced necessity) and other chemical requirements as compared to conventional SND by removing the step involving nitrate formation and utilization. Anammox does not require the presence of oxygen, rather is inhibited by it; thus, aeration cost is completely eliminated along with no possibility of producing nitrous oxide (N2O), a greenhouse gas. These, however, comes with their own disadvantages, important among which is complete control over DO, pH and organic carbon concentration that otherwise would completely mess up these sensitive processes. A crucial disadvantage of Anammox is the presence of excessive nitrate in the effluent, which requires further treatment before safe disposal (Rahimi et al. 2020).
The occurrence of SND has been studied in various biological reactors including sequencing batch reactors (SBR) (Pochana et al. 1999; Zeng et al. 2004), sequencing batch biofilm reactor (SBBR) (do Canto et al. 2008), moving bed sequencing batch reactor (MBSBR) (Cao et al. 2017), oxidation ditch (Sager 2016), rotating biological reactor (RBC) (Helmer and Kunst 1998), fluidized bed biofilm reactor (FBBR) (Seifi and Fazaelipoor 2012), packed bed reactor (Morita et al. 2008), hybrid bioreactor(HBR) (Jianlong et al. 2008), membrane bioreactor (MBR) (Sarioglu et al. 2009), membrane immobilized biofilm reactor (Ho et al. 2002), moving bed bioreactor (MBBR) (Chu and Wang 2011), membrane aerated biofilm reactor (MABR) (Hibiya et al. 2003), modified suspended carrier biofilm reactor (Xia et al. 2008) and upflow fixed bed reactor (Halling-Sørensen and Nielsen 1996). For the slow growing and sensitive nitrifiers, biofilm reactors are proved to be more efficient than suspended growth as they allow high loading rates (Nicolella et al. 2000).
The incidence of the two different biological processes within a stratified biofilm is based on the growth and activity of multiple microorganism species that facilitates these reactions simultaneously. With regards to the economic design of robust bio-reactors, modelling is considered as an efficient tool for better understanding of the interconnection and optimization of the concerned processes. Several models have been developed to describe the phenomenon occurring in multispecies biofilm (Wanner and Gujer 1986; Wanner and Reichart 1996) and spherical bacterial flocs (Daigger et al. 2007). The literature has been recorded for validation of the models developed in order to describe the process of SND along with carbon and/or phosphorus removal in biological flocs involved in suspended growth systems consequently with the effect of DO penetration (Pochana et al. 1999; Daigger et al. 2007; Layer et al. 2020). As for biofilm reactors, several models have been developed to simulate the growth of biomass in support media and its effect on the mechanism of SND (Halling-Sorensen and Nielsen 1996; Sarioglu et al. 2008, 2009; He et al. 2009; Seifi and Fazaelipoor 2012).
Inclusion of factors like nonlinear and time-dependent characteristics of microorganisms, flow rates and inlet concentrations makes the predictability of the model more accurate (Ostace et al. 2011). As the background of developing these mathematical or statistical models in various biological reactors, a number of empirical models have been proposed describing the diffusion and transfer of substrates and DO in the microbial flocs and biofilms (Wanner and Gujer 1986; Pérez et al. 2005). All those models are developed emphasizing on oxygen transfer into large flocs and deep biofilms and consequent development of oxic and anoxic zones. Development of models describing the mechanism of SND based on relative concentrations of various nitrogen species and optimisation of the process with respect to substrate transfer rates along with spatial distribution of various bacterial species and their effect on nitrogen removal process is quite limited. These existing models are either empirical models or mechanistic models along with modified activated sludge models (ASM) for SND (Wett and Rauch 2003; Hiatt and Grady 2008). The process of conventional nitrification and denitrification is based on the concept of consecutive reactions, where the by-product of nitrification is used as the substrate for denitrification along with the formation of intermediate products in the sub-steps of the reactions. The rate equation of all these steps along with the respective concentrations of all substrates and products influence the rate of SND reaction as a whole. Thus effective model requires quantification of every parameter involved in all these steps.
Though the concept for SND in different microbial reactors has long been developed and modelled, an exhaustive review of the existing models is not available, which can bring about the areas of further research. In spite of this broad research, modelling of SND for wastewaters characterised by low carbon content with respect to high ammonium concentration is still an area remotely investigated as most of the models are based on combined carbon oxidation and nitrogen removal. The present paper aims to provide a comprehensive idea about mathematical modelling associated with SND analysing considerations of the existing models. It has never been attempted to summarize and critically evaluate the gradual progress of modelling over the years in the context of SND in various biological reactor systems. The review in this paper looks into the areas in which different biological models predict output with respect to various nitrogen species, discussing the advantages and limitations of the developed models on SND in biofilm reactor systems and highlighting the capacities as well as prospects for further investigation.
Mechanism of conventional SND
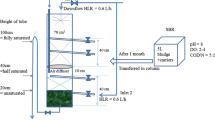
Traditional nitrogen removal pathway involves nitrification using oxygen as electron acceptor, with subsequent denitrification in anoxic conditions using organic matter as carbon source. Nitrification involves conversion of ammonium nitrogen to nitrite and nitrate in aerobic conditions by nitrifiers. The nitrate thus formed is then converted by denitrifiers in absence of oxygen into molecular nitrogen gas. Figure 1 demonstrates the schematic representation of conventional SND in large flocs and thick biofilms. The optimum pH and temperature range for the first phase of nitrification, known as the nitritation, is 6.5–8.2 and 30–40 °C, respectively, whereas the next phase or nitratation is carried out at an optimal pH range of which is 7.2–8.0 (Aslan and Dahab 2008). The bacterial species involved in these two phases are broadly termed as ammonia oxidising bacteria (AOB) and nitrite oxidising bacteria (NOB) that converts NH4–N to nitrite and nitrite to nitrate, respectively. Heterotrophic denitrification includes the conversion of nitrate to nitrogen gas via nitrite. The nitrification rate is generally the limiting factor for nitrogen conversion via SND as the by-products of nitrification, mainly nitrate is used as the substrate of denitrification (dos Santos et al. 1996).
Now, SND in single-staged reactor occurs basically due to the difference in DO gradient within flocs or biofilms or in aerated/ non-aerated zones in the same reactor, as observed in Fig. 1. Oxygen being a limiting substrate does not generally penetrate more than a few hundred µm because of its limited diffusion in biofilms and flocs (Oliveira et al. 2017). The regions exposed to high concentration of dissolved oxygen aids the growth of aerobic nitrifiers whereas zones under limiting oxygen concentration, forms the environment for the denitrifying microorganisms to thrive actively. Maintaining an optimum DO concentration in the reactor is crucial as both extreme DO concentrations would impair the process. High DO concentration would create less anoxic zones in the biofilm thus impairing denitrification. Apart from an optimum DO level, other prerequisite factors determining SND are sufficient SRT for slow growing nitrifiers and adequate concentration of electron donors for denitrification (Dey 2010). Nitrifiers are slow growing organisms, the growth rate and activity of which directly affect denitrification leading to lower removal rates and accumulation of by-products to an inhibitory level (Holman and Wareham 2005). Insufficient SRT also leads to washout of nitrifiers from the system thus weakening the process efficiency (Poduska and Andrews 1975). HRT plays a very prominent role in SND efficiency and it has been observed to decrease proportionately with decrease in HRT up to an optimum value, beyond which there is no significant increase in SND efficiency (Gupta et al. 1994). Optimum HRT varies with reactor systems and initial substrate concentrations and is generally recorded to vary between 6 and 48 h (Gupta et al. 1994; Wang et al. 2017). It has been observed that increase in HRT increases heterotrophic biomass concentration in the deeper layers of biofilm even when there is no considerable organic carbon concentration in the reactor (Nogueira et al. 2005).
One of the important features of SND includes maintaining a neutral pH within the system that eliminates the requirement of periodical pH adjustment, making it less cumbersome and economical. This property of SND favours the activity of both the groups of microorganisms, which act at a pH range of (7–8.6). Organic carbon serves as the electron donors for denitrification, which necessitates the presence of readily available organic carbon during SND. This dosing of carbon is an essential factor for successful SND as inadequate carbon may cause nitrite accumulation, whereas overdosing might lead to the presence of residual carbon in the treated effluent requiring further treatment. Thus, process optimization and modelling is immensely important for effective nitrogen removal via SND in biological reactors.
Occurrence of SND in single biological reactor
Conventional SND, in general, involves aerobic nitrification and anoxic denitrification under identical operating conditions, the major objective being the establishment of nitrification rates similar to those in aerobic systems, along with the aim of achieving significant nitrogen removal via denitrification at the same time (Pochana et al. 1999; Chu and Wang 2011) and thus the design of efficient SND requires deep understanding of inter-influencing parameters responsible for the processes (Jimenez et al. 2010). Considering only nitrification, both ammonium nitrogen concentration as well as DO directly influences microbial structure as well as reaction kinetics (Vannecke and Volcke 2015). In case of larger flocs and granules, it is observed that gradient of diffused DO decreases regularly with depth and reaches near-zero values, which leads to the formation of anoxic cores facilitating nitrification and denitrification in aerobic reactors (Bakti and Dick 1992; Daigger et al. 2007). Thus, the coexistence of aerobic nitrifiers, anaerobic denitrifiers as well as facultative microorganism aid in simultaneous nitrification and denitrification (Pochana et al. 1999), which is observed to occur mainly in case of activated sludge reactors with large granular sludge (Layer et al. 2020). The floc, size and DO penetration depth are the key factor in the formation and thickness of the anoxic zone in such cases (Li et al. 2008). Other than DO concentration in reactor, characteristics of the solution including diffusional coefficient of substrate in liquid is also found to directly influence reactions in flocs and granules (Pochana et al. 1999).
Conventional SND in biofilm reactor systems is suggested to occur in different strata developed inside the biofilm similar to the large flocs. Difference in growth conditions of various microbial species along with diffusional gradient of different electron acceptor concentrations leads to the formation of multispecies biofilm, which facilitates the process of SND in a single biological reactor unit. The autotrophic nitrifiers grow in proximity to the bulk reactor concentration with high ammonium and nitrite (if present) nitrogen and dissolved oxygen (DO) thus forming the outer layer of the biofilm. The deeper layer is anoxic for oxygen being diffusion limited and favours the growth of heterotrophic denitrifiers even in aerobic reactors facilitating denitrification (Tal et al. 2003; Holman and Wareham 2005). Various aspects of multispecies modelling in general have been developed (Wanner and Gujer 1986), studied and modified (Spengel and Dzombak 1992; Furumai and Rittmann 1994; Wanner and Reichart 1996). Apart from that, in case of biofilms, shear also plays an important role in net growth rate of microorganisms and biofilm thickness thereby affecting SND (Rao Bhamidimari and See 1992).
The depth of oxygen penetration in flocs and biofilms vary between 50 and 800 μm depending on bulk DO concentration, hydrodynamics and density of the biofilms, diameter of bioflocs (Hibiya et al. 2003). Liu et al. (2007) by using photo-lithography, concluded that up to a depth of 150 μm from the surface of sludge granules, nitrifying biomass was active. For understanding DO diffusion in aerobic granules and its corresponding concentration profile, a one-dimensional model has been developed, which confirmed the occurrence of SND within such granular solid having an anoxic core (Li et al. 2008). Similar results were obtained in case of biofilms, where in nitrifying reactors, heterotrophic bacterial culture was observed to develop towards the biocarrier surface with low DO concentration (Nogueira et al. 2005). The formation of aerobic and anoxic zones in flocs and biofilms due to diffusional gradient of DO is represented schematically in Fig. 2. Studies show that nitrifiers are found to exist on the outer surface of biofilms and microbial flocs in proximity of DO (Pochana et al. 1999; Layer et al. 2020). On contrary to this, Furumai and Rittmann (1994) and Okabe et al. (1995) hypothesized multi-species biofilm, where heterotrophic organisms reside in the outer layers of the biofilm and guard the inner nitrifying population against shearing action when sufficient carbon concentration is present in the reactor. It is due to the fact that heterotrophic organisms have a higher growth rate than autotrophic nitrifiers and thrive in regions of greater availability of substrates. They are prone to more shearing action but the biofilm volume is retained due to higher growth rates. But, this arrangement is observed more in case of wastewater with higher organic carbon content, where aerobic heterotrophs outcompete slow growing nitrifiers for space and oxygen (Fdz-Polanco et al. 2000). In such cases, both autotrophic and heterotrophic nitrification as well as anoxic and aerobic denitrification contributes for nitrogen removal (Wang et al. 2017).
Classic mathematical models establishing SND
Microbial distribution within biofilm layers are governed by microbial conversion of substrates, expansion of biomass in volumes and molecular diffusion of substrates that enables penetration and availability at deeper depths of biofilm. A number of models developed for SND are based on multispecies biofilm concept that quantifies the impact of oxygen gradient on development of various species responsible for nitrification and denitrification. Considering diffusive transport of substrates inside the biofilm and simultaneous attachment and detachment of cells, Wanner and Reichart (1996) have modified the pre-existing multispecies biofilm model (Wanner and Gujer 1986). Several models have been developed through years that predict the presence of different microbial species that brings about SND. Cohabitation of different species often is responsible to maintain the stability of systems undergoing SND (Volcke et al. 2008). However, most of the developed models considered nitrifiers or at most AOB and NOB as a single group of microorganisms and thus have a unified kinetic characteristic. However, considering only nitrification, multispecies model for aerobic biofilm has been developed that comprises of 60 different AOB and NOB species each, which predicts their spatial distribution (Vannecke and Volcke 2015). Empirical models are found to be inadequate for evaluating two-step nitrification and denitrification processes explicitly.
Reactions or experimentally developed models have limited versatility as they specify a particular set of operating conditions (Spengel and Dzombak 1992). In order to develop a mathematical model, a more general concept, the basic step is to establish mass balance equations based on the principle of mass conservation involving the different substrates, intermediates and by-products for the concerned reaction. Semi-empirical models are based on these substrate mass balance equations. This approach ultimately gives a clear picture of the various substances involved and their appropriate proportions of formation/degradation during the process. Considering time, the rate of change (degradation/formation) of one or more substances can be linked with the other parameters affecting the reaction. The resulting differential equations can be integrated to demonstrate the gradual change in concentration for each substrate and by-product (Wett and Rauch 2003).
As for the various classical models describing various wastewater treatment processes, ASM 2, followed by ASM 2D and ASM 3 describe carbon oxidation, nitrification–denitrification and phosphorus removal but nitrogen and phosphorus are described as a fraction of soluble COD in the wastewater (Gujer et al. 1999; Iacopozzi et al. 2007; Kaelin et al. 2009; Ostace et al. 2011). ASM1 has been frequently utilised as a basic model for nitrogen removal, which has been further modified by researchers in establishing SND. ASM1describes carbon oxidation along with nitrification and denitrification, where nitrite is not considered as an intermediate product in nitrification. No detailed description of denitrification is quantified from the viewpoint of anoxic heterotrophs that have different kinetic coefficients than aerobic heterotrophs (Henze et al. 1987a, b; Barker and Dold, 1997; Ostace et al. 2011).The basic model expression is given according to Monod’s microbial growth kinetics considering the effect of DO as a switching function and can be stated as: process rate, \(\rho = \mu \left( {\frac{{S_{S} }}{{K_{S} + S_{S} }}} \right)\left( {\frac{{S_{O} }}{{K_{O} + S_{O} }}} \right)X\).where µ, SS, SO, KS, KO and X denote microbial growth rate, initial substrate and DO concentrations, half saturation coefficients of substrate and DO and biomass concentration, respectively. Since bacterial diversity was not considered, all microbial parameters were based on that of heterotrophic organisms. The subsequent ASM models are modifications of ASM 1 with incorporations in each model. ASM 2 does not incorporate denitrification kinetics which has been addressed in its extension, ASM 2D (Henze et al. 1999). On the foundation of these equations used in ASM 1, modifications with respect to various substrates, microbial species and their kinetics and other physical processes are undertaken to make the models more conclusive. As such, similar equations were formulated for ASM 2 and ASM 2D, with consideration of separate kinetic parameters exclusively for nitrification by nitrifying organisms and denitrification by denitrifying heterotrophs, respectively (Henze et al. 2000).
Though the original ASM models are based on deriving average kinetics based on specific functional groups of microorganisms, a modification of the model incorporates a multispecies bacterial culture including five different species of autotrophic nitrifiers, seven heterotrophs, three hydrolysers along with a number of kinetic and stoichiometric coefficients (Dey 2010). The parameters associated with the microbial activity tends to follow logarithmic probability density functions and the values are observed to remain more or less constant, thus can be directly utilised in models based on ASM1 (Cox 2004). Moreover, the concept of endogenous decay was incorporated in ASM 3 model for the first time, which can be used to describe the source of organic carbon in case of limited external carbon source for denitrification process, although nitrogen removal does not consider nitrite as an intermediate product (Gujer et al. 1999). Iacopozzi et al. (2007) and Kaelin et al. (2009) separately modified ASM3 assuming both nitrification and denitrification with nitrite as the intermediate by-product, considering the separate kinetics for AOBs and NOBs. In the light of Monod’s substrate utilization kinetics, Iacopozzi et al. (2007) introduced two steps, one for each during nitrification and denitrification, based on nitrite as substrate. The equations for process rates of nitrite oxidation (\({\rho }_{{n}_{{NO}_{2}}})\) and reduction (\({\rho }_{d{n}_{{NO}_{2}}})\) are, respectively, stated as follows, the latter considering stored product within cells for carbon requirement for denitrification:
and
where \(\mu_{nb} , \mu_{{\text{H }}}\) are respective growth rates of nitrite oxidising bacteria and heterotrophs, \(S_{{{\text{NO}}_{2} }} , S_{{{\text{NH}}_{4} }} , S_{{{\text{Alk}}}} , S_{{\text{O}}}\) denotes initial nitrite, ammonium, alkalinity and DO concentration, K denotes saturation coefficients in aerobic (denoted with subscript A) and anoxic conditions for various substrates and \(X_{nb} , X_{{\text{H}}} , X_{{{\text{STO}}}}\) denote concentration of nitrite oxidising bacteria, heterotrophs and cells that are utilized for stored products. Similar process rate equations were formulated based on Monod’s kinetics for ammonium oxidation and nitrate reduction. Kaelin et al. (2009) used analogous expressions, also considering aerobic and anoxic endogenous respiration for individual bacterial species. A detailed study of various ASM models with their respective assumptions, theoretical considerations and applicability for SND processes are outlined in Table 1.
Dold’s general model (Barker and Dold 1997) was developed considering nitrification as two-step process for biological nutrient removal in activated sludge systems with nitrite as an intermediate substrate. The model was integrated with an anaerobic digestion model. A similar model Mantis 2 has been developed, which includes carbon, nitrogen and phosphorus removal along with anaerobic digestion in multi-staged activated sludge reactors. Considering SND as conventional two-step nitrification and four-step denitrification process, Hiatt and Grady (2008) modified the ASM as ASMN incorporating a number of factors including substrates, intermediates and inhibitory components that affect the reactions. A series of reaction equations were suggested based on stoichiometry that covers a wide range of parameters including organic inhibitors, salt, temperature functions, free ammonia and free nitric acid as substrate and inhibitors. These early models gave an idea about the quantitative aspects of basic mechanisms of SND, which have led to further modifications to make the models exhaustive considering as many factors responsible for the process. Proper modification and inclusion of influencing parameters may lead to the extension of activated sludge models for SND in biofilm reactors.
Mathematical models for SND developed so far
In understanding the mechanism of SND and developing its model for biochemical reactors, one of the basic similar characteristics between flocs and biofilms is observed to be the mechanism of diffusion of substrates within depth of biofilms and large flocs (Pérez et al. 2005). Flocs in large aggregates are hypothesized to have similar spatial distribution of microbes as in biofilms (Rittmann and Langeland 1985). Development of gradients acts as the driving force behind penetration to substrates through diffusion and as such, leads to the development of diversified regions in biofilm or microbial flocs. Although the mechanism of diffusion and as such its model equations can be similar in case of both floccular aggregates and biofilms, the key difference lies in the phenomenon of biomass loss. In case of biofilms, biomass is lost from the layer exposed to substrate feed due to shearing actions expressed as specific detachment rate. For suspended biomass, the floc as a whole gets wasted altogether, which can be estimated as the reciprocal of solid retention time of the reactor (Furumai and Rittmann 1994).
The models for establishing SND in different biological reactors aim to predict particular outputs with respect to either substrate or biological parameters or both for a particular set to input parameters. The process of SND is largely dependent on the bioreactor configuration, bulk dissolved oxygen concentration, and microbial structure and characteristics either in the form of flocs or biofilms (Jimenez et al. 2010; Bhattacharya and Mazumder 2021). A number of models have been developed describing the transfer and diffusion of DO in the biofilms; in comparison with that, SND models based on substrate limitations are quite low. The models which mathematically describe variation of different nitrogen species focus on nitrogen removal with respect to a number of variables influencing the process. There is limitation of many models devised so far. All physical, chemical and biological parameters affecting SND could not be incorporated into a single model, which restricts its application in a wider range (Wett and Rauch 2003). This affects directly the input and output to be considered during formulation of the model. As for example, the Wanner-Reichert multispecies model predicts the biofilm thickness, which in case of SUMO biofilm model is an input parameter that is to be fixed initially (Layer et al. 2020). From the pre-existing models on SND, Table 2 shows at a glance, the various input and output parameters considered in developing a model.
Among the models describing SND, some, including ASM, consider both the processes of nitrification and denitrification as single-step processes, that is, conversion of ammonium to nitrate as nitrification and nitrate to nitrogen gas as denitrification, ignoring the intermediates of the reactions (Halling-Sorensen and Nielsen 1996; Henze et al. 2000; He et al. 2009). In reality, SND involves a formation of a number of intermediate by-products. Inclusion of these intermediates makes the model more exhaustive, critical and accurate with subsequent increases the complexity. As for nitrification, ammonium oxidation is considered as the rate limiting step and used as representative for the process (Henze et al. 1987b). However, this consideration limits this model from being applied directly to elevated nitrogen conditions (Henze et al. 1987a). In such cases, nitrite forms an important intermediate and accumulation of it directly effects the complete process (Hiatt and Grady 2008). Several modified models consider the kinetics of nitrite, which makes both nitrification and denitrification two-step processes (Pochana et al. 1999; Wett and Rauch, 2003; Iacopozzi et al. 2007; Daigger et al. 2007; Ostace et al. 2011). Hiatt and Grady (2008) in ASMN considered denitrification as four-step process with nitrite, nitrous oxide and nitric oxide as intermediates, whereas nitrification is a two-step process with only nitrite as by-product.
Mathematical models in suspended growth reactors
The phenomenon of substrate diffusion through suspended biological flocs has already been established (Bakti and Dick 1992; Matson and Characklis 1976). Identification of specific model parameters like growth rate of microorganisms and utilization rates, the IWA models have been modified to predict and establish simultaneous nitrification and denitrification occurring in various suspended systems including activated sludge reactors (Hiatt and Grady 2008). In an attempt to establish SND within flocs, Pochana et al. (1999) developed one of the earliest models in SBR where the individual kinetic constants are developed based on modified IAWQ Activated Sludge Model No. I using nitrite as intermediate substrate. The model initially considers mass balance for a single floc, thereafter calculating the overall reaction rate based on floc distribution. Rate of change of substrate in a single floc \(\left( {\frac{{{\text{d}}S_{i} }}{{{\text{d}}t}}} \right)\) is calculated as: \(\frac{{{\text{d}}S_{i} }}{{{\text{d}}t}} = D_{j} \left( {\frac{{{\text{d}}^{2} S_{i} }}{{{\text{d}}a^{2} }} + \frac{2}{a}\frac{{{\text{d}}S_{j} }}{{{\text{d}}a}}} \right) \pm \sum\nolimits_{k = 1}^{n} {r_{k} }\).where \(D_{j}\) = diffusivity in floc, a = radial distance from centre of spherical floc, \(\frac{{{\text{d}}S_{j} }}{{{\text{d}}a}}\) = concentration gradient at any point inside the floc and \(r_{k}\) = rate of kth reaction. To describe the process of SND occurring in microbial flocs, a mathematical model based on diffusion of dissolved oxygen, methanol, ammonia, nitrite, and nitrate through a spherical floc and utilization of DO by both autotrophic and heterotrophic microorganisms was developed that predicted the DO profile in flocs and a single model parameter, namely the concentration of heterotrophs was required to be adjusted (Daigger et al. 2007).The model was based on similar mathematical considerations as that developed by Pochana et al. (1999) with inclusion of a stoichiometric coefficient, mathematically expressed as:\(D_{f} \left( {\frac{{{\text{d}}^{2} S_{j} }}{{{\text{d}}a^{2} }} + \frac{2}{a}\frac{{{\text{d}}S_{j} }}{{{\text{d}}a}}} \right) = \pm \sum\nolimits_{k = 1}^{n} {C_{k} r_{k} }\).where Ck denotes the stoichiometric coefficient for reaction k and other symbols denote identical parameters as stated earlier. With the help of the model, DO decline within larger flocs could be estimated accurately. The statistical model was developed considering floc particles to be spherical, and boundary layer effects to be negligible. Following the same trend of understanding DO effect inside flocs, Dey (2010) attempted to develop a model for sustaining SND under a specific DO that would reflect the operational performance without considering the formation of flocs or existence of DO gradient across the floc. 0.4 mg/L DO and 15 day SRT were selected as optimum conditions for efficient nitrogen removal.
Perhaps, the most exhaustive mathematical model for SND in suspended reactors for wastewaters with low COD/N ratio (0.25–4) was developed by Wett and Rauch (2003) considering all the inhibitory parameters for nitrification and by calculating their influencing rate on overall SND process. Monod’s kinetics along with kinetics for substrate inhibition was utilized to modify IWA-activated sludge models to calculate the process rates of AOB and NOB growth and decay, nitrate and nitrite reduction along with CO2 stripping. Rate of CO2 stripping is evaluated from the expression denoting its dependency on CO2flux and biokinetic reaction rate of CO2 (\(R_{{CO_{2} }} )\) as
where \(\rho_{{CO_{2w} }} , \rho_{{CO_{2a} }}\) denotes partial pressure of CO2 in water and air, respectively, \(L_{{CO_{2} }}\) = solubility of CO2 in water, k = gas transfer velocity, a = water–gas interface area and \(V_{w}\) = working volume of the reactor. The model is developed for wastewater having a high ammonium nitrogen concentration as obtained from pig slurries. Hiatt and Grady (2008) modified the existing ASM as ASMN by incorporating individual reaction-specific parameters for two-step nitrification and four-step denitrification under elevated nitrogen concentration. The model was developed based on several substrates and intermediates of SND including nitrite, nitrate and nitrous oxide. The effect of inhibitory compounds like free ammonia and free nitrous acid was also taken into consideration. Magri and Floats (2008) developed a mathematical model considering SND as a two-step process of nitrification and denitrification. The model was based on surface limited kinetics and suggested that anoxic growth rate is directly dependent on electron acceptors, where the process rates are based on Monod’s kinetics, similar to other models. The considerations that makes this approach novel, is the inclusion of individual liquid –gas transfer coefficients of DO, CO2, NH3 and N2. The occurrence of SND in granular sludge was modelled by Layer et al. (2020) to further understand the contribution of electron donors and formation of anoxic zones within granules that aids denitrification. The complete model consisted of three sub models including biofilm, biokinetic and reactor model and one-dimensional multispecies model was considered by subdividing the spherical granule into several layers. The salient features of the models are discussed in Table 3.
Mathematical models in attached growth systems
The availability of biofilm models for SND processes in wastewater treatment simulation enables an increased application of biofilm modelling in engineering practice. One of the challenges while modelling biofilms is the uncertainties involving dynamics of biofilm and rate of biofilm detachment due to various factors, one or more of which are often neglected. Most of the models emphasize on the concept of uniform thickness of biofilm, which holds true only up to a thickness of 300 \(\mu\)m (Rao Bhamidimarri and See 1992) and is also far less than that needed for SND (Bhattacharya and Mazumder 2021). Oyebamiji et al. (2018) devised a model to understand and quantify the effect of hydrodynamic shear on structural deformation of biofilm using Bayesian Poisson regression and linear kinetic models. Apart from that, there are a number of models for estimating shear stress on biofilms (Duddu et al. 2009; Jones and Buie 2019). However, extension of this concept in the scenario of multispecies model required for SND is yet to be implemented.
To investigate the mathematical approach for occurrence of SND in attached systems, Halling-Sorensen and Nielsen (1996) developed a kinetic model for SND as well as organic matter removal in a submerged fixed bed reactor with clinoptilolite clay as matrix. In the model, six state variables were used to study the removal of ammonia, nitrate and carbon by three groups of bacteria responsible for the removal of corresponding substrates. This model is an extremely simplistic one developed on the model formulated by Jorgensen (1991) based on substrate flux inside the biofilm and Monod’s kinetics to evaluate biomass growth and process rate equations. A kinetic model for SND was constituted on the basis of batch test result in a membrane bioreactor (He et al. 2009) by combining Lawrence–McCarty model (Lawrence and McCarty 1970) and ASM1. Autotrophic and heterotrophic biomass concentration (\(X_{a}\) and \(X_{h} )\) is calculated using Lawrence–McCarty model, expressed as:
where X = biomass concentration, \(\theta_{c}\) = SRT, Y = sludge yield, \(S_{i} , S_{e}\) = influent and effluent substrate concentration, respectively, b = decay coefficient and \(\theta\) = HRT. Using the respective data for autotrophic and heterotrophic biomass, a ratio of the two (\(\left( {\frac{{X_{a} }}{{X_{h} }}} \right)\) can be calculated in terms of their respective initial substrate concentration. Combining this with ASM1, HRT of the system is calculated using the following equation:
where k and A are constants, \(S_{{{\text{NO}}_{3} }}\) denotes nitrate concentration, \(k_{{{\text{NO}}_{3} }}\) = nitrate saturation coefficient. It was found that the simulation nitrate saturation coefficient was much higher than that in a single-sludge wastewater treatment system due to the limitation of mass transfer. Under the same bulking nitrate concentration, compared to single-sludge system, denitrification takes place slower for SND.
Insel et al. (2011) developed a model for SND in MBR systems describing the effect of dissolved oxygen on different kinetic parameters responsible for the growth of individual autotrophs and heterotrophs in the reactor system by considering half saturation coefficients of oxygen with Monod’s kinetic equations. The half saturations coefficients for autotrophs (\(\left( {K_{{{\text{OA}}}} } \right)\) and heterotrophs (\(K_{{{\text{OH}}}}\)) are determined using the following empirical equations:
and
where MLSS denotes autotrophic and heterotrophic biomass concentration for respective cases. The model also predicted the concentration of nitrite accumulation and total nitrogen removal from the system. The effect of biomass concentration on the reaction and that of mass transfer limitation on different microorganisms were also analysed using the model as well as experimental data. Another successful implication of the study was the optimization of MLSS concentration on reduction of reactor footprint and effective nitrogen removal from wastewater. The extent of SND was modelled in an MBR by Sarioglu et al. (2009) which was then calibrated with the experimental data and used to define significant parameters of an optimized MBR operation for nitrogen removal. It accounted for the diffusion limitation and the resulting simultaneous nitrification/denitrification in terms of the high half saturation constants. The contribution of soluble and inert COD was also incorporated in the model. Sarioglu et al. (2008) also modified the existing ASM1 model by incorporating endogenous decay model developed by Orhon and Artan (1994) for evaluating growth and decay rates of autotrophs and heterotrophs involved in SND in MBR. Both these models were based on kinetic equations of Monod’s theory. Zinatizadeh and Ghaytooli (2015) developed mathematical model based on experimental observations in treating municipal wastewater in MBBR. In the study both carbon oxidation and nitrogen removal was studied following SND process for wastewaters with COD:N:P ratio of 100:20:3. In the model the effect of three independent parameters, namely DO, HRT and type of carriers, was quantified for the evaluation of different output parameters using central composite design and equations to evaluate various effluent concentrations and removal percentages were established from ANOVA results.
Seifi and Fazaelipoor (2012) developed a model for SND in fluidized bed biofilm reactor that predicted removal efficiencies of COD, ammonium and nitrate with varying height of reactor, oxygen concentration supplied at inlet, oxygen mass transfer coefficient, specific surface area of biofilm and HRT. The conversion rates of ammonium (\({C}_{{\mathrm{NH}}_{4}})\), nitrate (\({C}_{{\mathrm{NO}}_{3}})\) and oxygen (\({C}_{{O}_{2}})\) in the biofilm due to microbial activities is evaluated from the model using the following equations:
where \(C_{{{\text{NH}}_{4} }} , C_{{{\text{NO}}_{3} }}\) are the concentrations of ammonium and nitrate in liquid phase, \(C_{{O_{2} }}\) = concentration of oxygen in gas phase, \(D_{{{\text{NH}}_{4} }} , D_{{{\text{NO}}_{3} }} , D_{{{\text{O}}_{2} }}\) = respective diffusion coefficients of ammonium, nitrate and oxygen, \(Y_{{\text{A }}} , Y_{{\text{H}}}\) = biomass yield for autotrophs and heterotrophs, respectively, \(X_{{\text{A }}} , X_{{\text{H }}}\) = autotrophic and heterotrophic biomass concentration, \(\mu_{{\text{A }}} , \mu_{{\text{H }}}\) = maximum specific growth rate of autotrophs and heterotrophs, \(\eta_{g}\) = anoxic reduction factor, \(K_{{{\text{NH}}_{4} }} , K_{{{\text{OA}}}} , K_{{{\text{NO}}_{3} }} , K_{{{\text{O}}_{2} }}\) = half saturation coefficients of ammonium, oxygen (for autotrophs), nitrate and oxygen (for heterotrophs), respectively. Using this model, the optimum values of respective parameters can be obtained for economical process design. This is a simplified approach, where the biological rate reactions are expressed in terms of Monod’s kinetic coefficients. Baek and Kim (2013) modified the ASM1 model to incorporate SND for oxygen limited membrane bioreactor. In case of denitrification, mass balance equation is considered to evaluate heterotrophic biomass yield \(\left( {Y_{{\text{H}}} } \right)\) as:
where XVSS = Volatile suspended solids concentration, \(S_{S}\) = influent substrate concentration, \(b_{H}\) = decay coefficient of heterotrophs, \(f_{ev}\) = ratio of COD of MLSS to MLVSS (taken as 1.42), \(f_{p}\) = inert fraction of biomass (assumed to be 0.08), \(\theta_{C}\) = SRT and XI = inert particulates of influent wastewater. The features of the models developed for attached growth reactors are discussed in Table 4.
Mathematical models under hybrid growth system:
Till date, detailed models describing SND in hybrid reactor systems are yet to be developed. Here lies a vast area of further research and development. Models on hybrid bioreactor are generally developed for both carbon and nitrogen removal. A few models have been developed for the simulation of the hybrid systems and mainly for steady-state conditions with carbon removal being the primary objective (Pastorelli et al. 1996). Very recently, a model has been developed in hybrid anoxic–oxic intermittent MBBR to illustrate the nitrogen removal via SND focussing nitrification along with carbon oxidation (Montecchio et al. 2022). It predicts the ammonium and TN removal efficiencies with respect to biofilm and suspended biomass in the reactor separately and is primarily based on mass balance considerations, variable biofilm thickness and diffusion processes. Although case specific, this work gives a quantitative insight about the occurrence of SND in hybrid reactors highlighting bacterial competition and distribution in the system for wastewaters with COD:N > 5. Mannina and Viviani (2009) developed a model on hybrid MBBR for carbon oxidation and nitrification referring to the shortcomings existing till date. The model relies on ASM1 for biokinetics of the process and comprises of two submodels each for suspended and attached growth system. The suspended growth sub model is developed based on ASM1 in Monod’s approach, similar to that developed by Henze et al. (1987a). For modelling biofilm, the approach by Rauch et al. (1999) was adopted for removal of multiple substrates by different bacterial species. The separate assessment of substrate diffusion allows to relate the penetration depth of substrates to a fraction of biomass that is active in conversion.
Nitrous oxide production in conjugation with SND models
Nitrous and nitric oxide are intermediate by-products formed during SND and their modelling is essential as it often defines the complete reaction and enables to estimate the possible greenhouse gas emissions, as both nitrification and denitrification can contribute to N2O production and utilization (Kampschreur et al. 2009). It has been established that nitrification, specifically ammonia oxidising bacteria contributes significantly more towards the production of nitrous oxide (Wunderlin et al. 2012; Guo et al. 2013) than that produced by heterotrophic denitrification (Guo and Vanrolleghem 2013). Incomplete denitrification often results in N2O emissions (Kampschreur et al. 2009) whereas nitrification at a low DO results in higher nitrous oxide accumulation (Foley et al. 2010) along with other factors including low C/N ratio and high nitrite concentration during the process (Kampschreur et al. 2009). Analysis using nitrous oxide transformation might explain the mechanisms undergoing in the reactor entitled for SND.
A modification of ASM 1 was developed to exhibit the production and utilization of nitrous oxide that takes place during aerobic nitrification and heterotrophic denitrification considering four successive steps of nitrification and denitrification each. This model gives a thorough quantification about various by-products during the process (Ni et al. 2011). Hiatt and Grady (2008) while modifying ASM for SND included the kinetics with respect to nitrous oxide as an intermediate during denitrification. This ASMN model defines the relationship between electron donor and acceptors in the anoxic reactor, which is considered to be the controlling factor for N2O emissions. Along with the activity of denitrifying bacteria, a modification of this model describes nitrous oxide formation emphasizing on the activity of AOB (Spérandio et al. 2016), a similar model of which was developed considering nitrifier denitrification and incomplete oxidation of hydroxylamine to nitrite during nitrification (Ni et al. 2013b), not relevant in this context of SND. However, it was later modified to include the impact of both nitrifiers and denitrifiers on nitrous oxide formation and validated in case of full scale treatment plants (Ni et al. 2013a). Based on ASM 3, a pseudo-mechanistic model was developed to extend the original model including nitrous oxide emission from full scale wastewater treatment plants. N2O formation during both autotrophic nitrification and heterotrophic denitrification was considered in this model along with N2O stripping (Blomberg et al. 2018).
Future scope in SND modelling
With an insight to the discussions regarding the existing models for conventional SND processes in biological reactors, it is clear that a number of models have been developed for suspended type reactors, specifically SBRs and recent development focuses on MBRs. All those models are based on ASM model structures with different modifications as required for variation of parameters concerned. In case of attached biofilm models, nitrogen removal in various cases has been studied using membrane bioreactors concerning diffusional limitations of substrates and DO (Insel et al. 2011; Baek and Kim 2013). With the advancement of cost-effective and efficient nitrogen removing technologies, MBBR for simultaneous nitrification and denitrification is gaining attention because of its several advantages over suspended growth systems as well as other attached growth reactors including MBR and a wide range of industrial pollutants can be degraded along with municipal effluent (Bhattacharya and Mazumder 2021). These reactors are generally preferred to others for their compact orientation and cost-effective nitrogen removal from wastewaters having low C/N ratio. It is observed that till date, no extensive model has been developed for conventional SND using this technology. The approach of Zinatizadeh and Ghaytooli (2015) used combined carbon oxidation and nitrogen removal, where the model is not strictly for SND. Thus, there lies a vast area of research in future. From the discussion, it is clear that models describing SND in hybrid reactor systems is yet to be developed, may be due to the complex characteristics of both suspended and attached biomass and the gaps in understanding their respective roles in SND.
Also, it is to be noticed that, the recent models for nitrogen removal are generally devised on the basis of Anammox-SHARON-CANON or SND over nitrite as they are more economical and also case specific (Azari et al. 2017). From that viewpoint, conventional SND is to be kept as an important option in cases where the experimental conditions are varied. Modelling nitrogen removal via these pathways is quite different from that in conventional aerobic nitrification and heterotrophic denitrification. The substrates modelled in the two cases are different along with a major difference in experimental parameters. A number of inhibiting conditions are to be reflected in models describing Anammox and SHARON. Biological perspective also varies in the two cases. SND over nitrite requires the accumulation of nitrite as an intermediate between the two processes and several models have been developed in this aspect (Volcke et al. 2008; Kaelin et al. 2009). Thus, research and model development in this regard is to be carried out for optimization of various parameters for SND process. Another gap in the approach of model development comes from the observation that those involving a large number of parameters seem to develop a more complicated model that requires highly advanced software. While the ones that are simplistic lacks a number of important parameters under concern. The need for development of a model both simplistic and exhaustive in nature must be a motivation for a number of future researches.
Aerobic nitrifiers are sensitive microorganisms that are easily affected by inhibitory compounds and factors including high temperatures, inappropriate pH, presence of free ammonia and free nitrous acid (Svenson et al. 2000). All these factors limit the applicability of existing generalized models for accommodating treatments of wastewater characterised by elevated nitrogen and insufficient carbon concentration such as effluents from anaerobic digester supernatant, piggery and concentrated animal feeding operations, industries manufacturing pharmaceuticals and fertilizers (Hiatt and Grady 2008). With a marked decrease in organic carbon content, the kinetics for denitrification will also need special attention as organic carbon might act as the limiting substrate in particular cases. There is hardly any model that accounts for SND dedicated to these types of effluents. Clearly the process design involving simultaneous carbon oxidation and nitrogen removal will be different from that of SND with very low organic carbon content.
Conclusion
Modelling in the aspect of biological reactors is of immense importance in optimizing an economical nutrient removal process. Selection of a biological model for a particular reactor is based on the simplicity of the model as also various aspects covered in the model. But the oversimplification causes a number of interacting parameters to be ruled out in case of biological systems, where co-interactive processes like nitrification and denitrification take place simultaneously causing heterogeneous system. To incorporate more and more parameters, advanced models are becoming complex and mathematical tools aid the investigation of parameters involved in larger flocs and thick biofilms (Nogueira et al. 2005). The major concern in identifying the true nature of the best fit model applicable for a defined case lies in the fact that there are several model structures each of which adequately describes the process (Reichert and Omlin 1997). The DO level in the reactor is a major factor that dictates the efficiency of SND and thus optimisation of DO and understanding diffusional distribution is essential for creating aerobic and anoxic environment simultaneously within a single reactor. Other influencing factors including pH, HRT, substrate concentrations are intertwined, making a change in one will affect the entire process. Effective models developed for any biological reactor address this concern. The majority of the developed models are based on the concept used in ASM 1 with inclusion and modification with respect to diffusion, biological distribution, inhibition kinetics and aerobic–anoxic layer depth. Models describing SND in hybrid reactor systems and several attached reactors including MBBR are yet to be developed. It is quite essential in the aspect that these reactors can efficiently treat low C/N wastewater. Nitrogen removal through any of the processes is impacted by development of inhibitory substances produced during the reaction, as well as different environmental factors. Thus, for successful implementation of the process, optimizing the parameters is essential for robust operation of the reactors.
References
Andrews JF (1969) Dynamic model of the anaerobic digestion process. J Sanit Eng Div 95:95–116. https://doi.org/10.1061/JSEDAI.0000943
Aslan S, Dahab M (2008) Nitritation and denitritation of ammonium-rich wastewater using fluidized-bed biofilm reactors. J Hazard Mater 156:56–63. https://doi.org/10.1016/j.jhazmat.2007.11.112
Azari M, Lübken M, Denecke M (2017) Simulation of simultaneous anammox and denitrification for kinetic and physiological characterization of microbial community in a granular biofilm system. Biochem Eng J 127:206–216. https://doi.org/10.1016/j.bej.2017.09.002
Baek SH, Kim HJ (2013) Mathematical model for simultaneous nitrification and denitrification (SND) in membrane bioreactor (MBR) under low dissolved oxygen (DO) concentrations. Biotechnol Bioprocess Eng 18:104–110. https://doi.org/10.1007/s12257-011-0419-6
Bakti NAK, Dick RI (1992) A model for a nitrifying suspended-growth reactor incorporating intraparticle diffusional limitation. Water Res 26:1681–1690. https://doi.org/10.1016/0043-1354(92)90168-4
Barker PS, Dold PL (1997) General model for biological nutrient removal activated-sludge systems: model presentation. Water Environ Res 69:969–984. https://doi.org/10.2175/106143097X125669
Batchelor B (1982) Kinetic analysis of alternative configurations for single-sludge nitrification/denitrification. J Water Pollut Control Feder 54:1493–1504. https://www.jstor.org/stable/25041741
Bhattacharya R, Mazumder D (2021) Simultaneous nitrification and denitrification in moving bed bioreactor and other biological systems. Bioprocess Biosyst Eng 44:635–652. https://doi.org/10.1007/s00449-020-02475-6
Blomberg K, Kosse P, Mikola A, Kuokkanen A, Fred T, Heinonen M, Mulas M, Lübken M, Wichern M, Vahala R (2018) Development of an extended ASM3 model for predicting the nitrous oxide emissions in a full-scale wastewater treatment plant. Environ Sci Technol 52:5803–5811. https://doi.org/10.1021/acs.est.8b00386
Cao Y, Zhang C, Rong H, Zheng G, Zhao L (2017) The effect of dissolved oxygen concentration (DO) on oxygen diffusion and bacterial community structure in moving bed sequencing batch reactor (MBSBR). Water Res 108:86–94. https://doi.org/10.1016/j.watres.2016.10.063
Chu L, Wang J (2011) Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in a moving bed biofilm reactor. Chem Eng J 170:220–225. https://doi.org/10.1016/j.cej.2011.03.058
Cox CD (2004) Statistical distribution of uncertainty and variability in activated sludge model parameters. Water Environ Res 76:2672–2685. https://doi.org/10.1002/j.1554-7531.2004.tb00229.x
Daigger GT, Adams CD, Steller HK (2007) Diffusion of oxygen through activated sludge flocs: Experimental measurement, modeling, and implications for simultaneous nitrification and denitrification. Water Environ Res 79:375–387. https://doi.org/10.2175/106143006X111835
Dey A (2010) Modeling simultaneous nitrification–denitrification process in an activated sludge continuous flow stirred-tank reactor: system optimization and sensitivity analysis. Environ Eng Sci 27:757–765. https://doi.org/10.1089/ees.2009.0413
do Canto CSA, Rodrigues JAD, Ratusznei SM, Zaiat M, Foresti E (2008) Feasibility of nitrification/denitrification in a sequencing batch biofilm reactor with liquid circulation applied to post-treatment. Bioresour Technol 99:644–654. https://doi.org/10.1016/j.biortech.2006.12.040
dos Santos VM, Bruijnse M, Tramper J, Wijffels RH (1996) The magic-bead concept: an integrated approach to nitrogen removal with co-immobilized micro-organisms. Appl Microbiol Biotechnol 45:447–453. https://doi.org/10.1007/BF00578454
Duddu R, Chopp DL, Moran B (2009) A two-dimensional continuum model of biofilm growth incorporating fluid flow and shear stress based detachment. Biotechnol Bioeng 103:92–104. https://doi.org/10.1002/bit.22233
Fdz-Polanco F, Mendez E, Uruena MA, Villaverde S, Garcıa PA (2000) Spatial distribution of heterotrophs and nitrifiers in a submerged biofilter for nitrification. Water Res 34:4081–4089. https://doi.org/10.1016/S0043-1354(00)00159-7
Foley J, De Haas D, Yuan Z, Lant P (2010) Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res 44:831–844. https://doi.org/10.1016/j.watres.2009.10.033
Furumai H, Rittmann BE (1994) Evaluation of multiple-species biofilm and floc processes using a simplified aggregate model. Water Sci Technol 29:439–446
Gujer W, Henze M, Mino T, Van Loosdrecht M (1999) Activated sludge model no. 3. Water Sci Technol 39:183–193. https://doi.org/10.1016/S0273-1223(98)00785-9
Gujer W, Henze M, Mino T, Matsuo T, Wentzel MC, Marais GVR (1995) The activated sludge model No. 2: biological phosphorus removal. Water Sci Technol 31:1–11. https://doi.org/10.1016/0273-1223(95)00175-M
Gupta SK, Raja SM, Gupta AB (1994) Simultaneous nitrification-denitrification in a rotating biological contactor. Environ Technol 15:145–153. https://doi.org/10.1080/09593339409385414
Guo L, Vanrolleghem PA (2013) Calibration and validation of an activated sludge model for greenhouse gases no. 1 (ASMG1): prediction of temperature-dependent N2O emission dynamics. Bioprocess Biosyst Eng 37:151–163. https://doi.org/10.1007/s00449-013-0978-3
Guo L, Lamaire-Chad C, Bellandi G, Daelman M, Amerlinck Y, Maere T, Nous J, Flameling T, Weijers S, van Loosdrecht M, Volcke EI (2013) High-frequency field measurement of nitrous oxide (N2O) Gas emissions and influencing factors at WWTPs under dry and wet weather conditions. Proc Water Environ Fed 2013:621–629
Halling-Sørensen B, Nielsen SN (1996) A model of nitrogen removal from waste water in a fixed bed reactor using simultaneous nitrification and denitrification (SND). Ecol Model 87:131–141. https://doi.org/10.1016/0304-3800(95)00025-9
He SB, Xue G, Wang BZ (2009) Factors affecting simultaneous nitrification and de-nitrification (SND) and its kinetics model in membrane bioreactor. J Hazard Mater 168:704–710. https://doi.org/10.1016/j.jhazmat.2009.02.099
Helmer C, Kunst S (1998) Simultaneous nitrification/denitrification in an aerobic biofilm system. Water Sci Technol 37:183–187. https://doi.org/10.1016/S0273-1223(98)00102-4
Henze M, Grady CPL Jr, Gujer W, GvR M, Matsuo T (1987a) A general model for a single-sludge wastewater treatment system. Water Res 21:505–515. https://doi.org/10.1016/0043-1354(87)90058-3
Henze M, Grady C, Gujer W, Marais GvR, Matsuo T (1987b) Activated Sludge Model No.1, Scientific and Technical, Reports No.1, IA WPRC, London
Henze M, Gujer W, Mino T, Matsuo T, Wentzel MC, GvR M, Van Loosdrecht MC (1999) Activated sludge model no. 2d, ASM2d. Water Sci Technol 39:165–182. https://doi.org/10.2166/wst.1999.0036
Henze M, Gujer W, Mino T, van Loosdrecht MC (2000) Activated sludge models ASM1, ASM2, ASM2d and ASM3. IWA publishing, London (reprint edn)
Hiatt WC, Grady CL (2008) An updated process model for carbon oxidation, nitrification, and denitrification. Water Environ Res 80:2145–2156. https://doi.org/10.2175/106143008X304776
Hibiya K, Terada A, Tsuneda S, Hirata A (2003) Simultaneous nitrification and denitrification by controlling vertical and horizontal microenvironment in a membrane-aerated biofilm reactor. J Biotechnol 100:23–32. https://doi.org/10.1016/S0168-1656(02)00227-4
Ho CM, Tseng SK, Chang YJ (2002) Simultaneous nitrification and denitrification using an autotrophic membrane-immobilized biofilm reactor. Lett Appl Microbiol 35:481–485. https://doi.org/10.1046/j.1472-765X.2002.01225.x
Holman JB, Wareham DG (2005) COD, ammonia and dissolved oxygen time profiles in the simultaneous nitrification/denitrification process. Biochem Eng J 22:125–133. https://doi.org/10.1016/j.bej.2004.09.001
Iacopozzi I, Innocenti V, Marsili-Libelli S, Giusti E (2007) A modified activated sludge Model No. 3 (ASM3) with two-step nitrification–denitrification. Environ Model Softw 22:847–861. https://doi.org/10.1016/j.envsoft.2006.05.009
Insel G, Hocaoğlu SM, Cokgor EU, Orhon D (2011) Modelling the effect of biomass induced oxygen transfer limitations on the nitrogen removal performance of membrane bioreactor. J Membr Sci 368:54–63. https://doi.org/10.1016/j.memsci.2010.11.003
Jianlong W, Yongzhen P, Shuying W, Yongqing GAO (2008) Nitrogen removal by simultaneous nitrification and denitrification via nitrite in a sequence hybrid biological reactor. Chin J Chem Eng 16:778–784. https://doi.org/10.1016/S1004-9541(08)60155-X
Jimenez J, Dursun D, Dold P, Bratby J, Keller J, Parker D (2010) Simultaneous nitrification-denitrification to meet low effluent nitrogen limits: modeling, performance and reliability. Proc Water Environ Fed 2010:2404–2421
Jones AAD, Buie CR (2019) Continuous shear stress alters metabolism, mass-transport, and growth in electroactive biofilms independent of surface substrate transport. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-39267-2
Jørgensen SE (1991) Model of denitrification in a bark-ion exchange column. Water Treat 6:1–12
Kaelin D, Manser R, Rieger L, Eugster J, Rottermann K, Siegrist H (2009) Extension of ASM3 for two-step nitrification and denitrification and its calibration and validation with batch tests and pilot scale data. Water Res 43:1680–1692. https://doi.org/10.1016/j.watres.2008.12.039
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MS, van Loosdrecht MC (2009) Nitrous oxide emission during wastewater treatment. Water Res 43:4093–4103. https://doi.org/10.1016/j.watres.2009.03.001
Lawrence AW, McCarty PL (1970) Unified basis for biological treatment design and operation. J Sanit Eng Div 96:757–778. https://doi.org/10.1061/JSEDAI.0001126
Layer M, Villodres MG, Hernandez A, Reynaert E, Morgenroth E, Derlon N (2020) Limited simultaneous nitrification-denitrification (SND) in aerobic granular sludge systems treating municipal wastewater: mechanisms and practical implications. Water Res 7:100048. https://doi.org/10.1016/j.wroa.2020.100048
Li Y, Liu Y, Shen L, Chen F (2008) DO diffusion profile in aerobic granule and its microbiological implications. Enzyme Microb Technol 43:349–354. https://doi.org/10.1016/j.enzmictec.2008.04.005
Liu SY, Liu G, Tian YC, Chen YP, Yu HQ, Fang F (2007) An innovative microelectrode fabricated using photolithography for measuring dissolved oxygen distributions in aerobic granules. Environ Sci Technol 41:5447–5452. https://doi.org/10.1021/es070532g
Mannina G, Viviani G (2009) Hybrid moving bed biofilm reactors: an effective solution for upgrading a large wastewater treatment plant. Water Sci Technol 60:1103–1116. https://doi.org/10.2166/wst.2009.416
Magri A, Flotats X (2008) Modelling of biological nitrogen removal from the liquid fraction of pig slurry in a sequencing batch reactor. Biosys Eng 101:239–259. https://doi.org/10.1016/j.biosystemseng.2008.08.003
Magrí A, Sole-Mauri F, Colprim J, Flotats X (2007) Evaluation of the SHARON process (partial nitritation in a chemostat) using simulation. AFINIDAD-BARCELONA 529:378
Matson JV, Characklis WG (1976) Diffusion into microbial aggregates. Water Res 10:877–885. https://doi.org/10.1016/0043-1354(76)90022-1
Montecchio D, Mattei MR, Andreottola G, Esposito G, Ferrentino R (2022) Mathematical modelling of an intermittent Anoxic/Aerobic MBBR: estimation of nitrification rates and energy savings. Aerobic MBBR: estimation of nitrification rates and energy savings. Social Science Network Research. https://doi.org/10.2139/ssrn.4011567
Morita M, Uemoto H, Watanabe A (2008) Nitrogen-removal bioreactor capable of simultaneous nitrification and denitrification for application to industrial wastewater treatment. Biochem Eng J 41:59–66. https://doi.org/10.1016/j.bej.2008.03.008
Nicolella C, van Loosdrecht MC, Heijnen SJ (2000) Particle-based biofilm reactor technology. Trends Biotechnol 18:312–320. https://doi.org/10.1016/S0167-7799(00)01461-X
Ni BJ, Yuan Z, Chandran K, Vanrolleghem PA, Murthy S (2013a) Evaluating four mathematical models for nitrous oxide production by autotrophic ammonia-oxidizing bacteria. Biotechnol Bioeng 110:153–163. https://doi.org/10.1002/bit.24620
Ni BJ, Ruscalleda M, Pellicer-Nacher C, Smets BF (2011) Modeling nitrous oxide production during biological nitrogen removal via nitrification and denitrification: extensions to the general ASM models. Environ Sci Technol 45:7768–7776. https://doi.org/10.1021/es201489n
Ni BJ, Ye L, Law Y, Byers C, Yuan Z (2013b) Mathematical modeling of nitrous oxide (N2O) emissions from full-scale wastewater treatment plants. Environ Sci Technol 47:7795–7803. https://doi.org/10.1021/es4005398
Nogueira R, Elenter D, Brito A, Melo LF, Wagner M, Morgenroth E (2005) Evaluating heterotrophic growth in a nitrifying biofilm reactor using fluorescence in situ hybridization and mathematical modeling. Water Sci Technol 52:135–141. https://doi.org/10.2166/wst.2005.0192
Okabe S, Hirata K, Watanabe Y (1995) Dynamic changes in spatial microbial distribution in mixed-population biofilms: experimental results and model simulation. Water Sci Technol 32:67–74
Oliveira ACDG, Correa CZ, Prates KVMC, Lopes DD (2017) Nitrifying, denitrifying and heterotrophic biomass present in moving bed-reactor. Am J Environ Sci 13:47–57
Orhon D, Artan N (1994) Modeling of activated sludge systems. Technomic Publishing Company, Lancaster
Ostace GS, Cristea VM, Agachi PŞ (2011) Cost reduction of the wastewater treatment plant operation by MPC based on modified ASM1 with two-step nitrification/denitrification model. Comput Chem Eng 35:2469–2479. https://doi.org/10.1016/j.compchemeng.2011.03.031
Oyebamiji OK, Wilkinson DJ, Jayathilake PG, Rushton SP, Bridgens B, Li B, Zuliani P (2018) A Bayesian approach to modelling the impact of hydrodynamic shear stress on biofilm deformation. PLoS ONE 13:0195484. https://doi.org/10.1371/journal.pone.0195484
Pastorelli G, Andreottola G, Canziani R, de Fraja Frangipane E, De Pascalis F, Gurrieri G, Rozzi A (1996) Organic carbon and nitrogen removal in a moving-bed sequencing batch biofilm reactor. In: Proceedings of the 1st international specialized conference on sequencing batch reactor technology, pp 327–335
Pérez J, Picioreanu C, Van Loosdrecht M (2005) Modeling biofilm and floc diffusion processes based on analytical solution of reaction-diffusion equations. Water Res 39:1311–1323. https://doi.org/10.1016/j.watres.2004.12.020
Pochana K, Keller J, Lant P (1999) Model development for simultaneous nitrification and denitrification. Water Sci Technol 39:235–243. https://doi.org/10.1016/S0273-1223(98)00789-6
Poduska RA, Andrews JF (1975) Dynamics of nitrification in the activated sludge process. Journal (water Pollution Control Federation) 47:2599–2619
Rahimi S, Modin O, Mijakovic I (2020) Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol Adv 43:107570. https://doi.org/10.1016/j.biotechadv.2020.107570
Rao Bhamidimarri SM, See TT (1992) Shear loss characteristics of an aerobic biofilm. Water Sci Technol 26:595–600. https://doi.org/10.2166/wst.1992.0439
Rauch W, Vanhooren H, Vanrolleghem PA (1999) A simplified mixed-culture biofilm model. Water Res 33:2148–2162
Reichert P, Omlin M (1997) On the usefulness of over parameterized ecological models. Ecol Model 95:289–299. https://doi.org/10.1016/S0304-3800(96)00043-9
Rittmann BE, Langeland WE (1985) Simultaneous denitrification with nitrification in single-channel oxidation ditches. Journal (water Pollution Control Federation) 57:300–308
Sager AE (2016) Experimental studies of simultaneous nitrification denitrification and phosphorus removal at falkenburg advanced wastewater treatment plant. Graduate Theses and Dissertations
Sarioglu M, Insel G, Artan N, Orhon D (2008) Modelling of long-term simultaneous nitrification and denitrification (SNDN) performance of a pilot scale membrane bioreactor. Water Sci Technol 57:1825–1833. https://doi.org/10.2166/wst.2008.121
Sarioglu M, Insel G, Artan N, Orhon D (2009) Model evaluation of simultaneous nitrification and denitrification in a membrane bioreactor operated without an anoxic reactor. J Membr Sci 337:17–27. https://doi.org/10.1016/j.memsci.2009.03.015
Seifi M, Fazaelipoor MH (2012) Modeling simultaneous nitrification and denitrification (SND) in a fluidized bed biofilm reactor. Appl Math Model 36:5603–5613. https://doi.org/10.1016/j.apm.2012.01.004
Spengel DB, Dzombak DA (1992) Biokineticmodeling and scale-up considerations for rotating biological contactors. Water Environ Res 64:223–235. https://doi.org/10.2175/WER.64.3.6
Spérandio M, Pocquet M, Guo L, Ni BJ, Vanrolleghem PA, Yuan Z (2016) Evaluation of different nitrous oxide production models with four continuous long-term wastewater treatment process data series. Bioprocess Biosyst Eng 39:493–510. https://doi.org/10.1007/s00449-015-1532-2
Svenson A, Sandén B, Dalhammar G, Remberger M, Kaj L (2000) Toxicity identification and evaluation of nitrification inhibitors in wastewaters. Environ Toxicol Int J 15:527–532. https://doi.org/10.1002/1522-7278(2000)15:5%3c527::AID-TOX24%3e3.0.CO;2-F
Tal Y, Watts JE, Schreier SB, Sowers KR, Schreier HJ (2003) Characterization of the microbial community and nit126 rogen transformation processes associated with moving bed bioreactors in a closed recirculated mariculture system. Aquaculture 215:187–202. https://doi.org/10.1016/S0044-8486(02)00372-1
Vannecke TPW, Volcke EIP (2015) Modelling microbial competition in nitrifying biofilm reactors. Biotechnol Bioeng 112:2550–2561. https://doi.org/10.1002/bit.25680
Volcke EI, Sanchez O, Steyer JP, Dabert P, Bernet N (2008) Microbial population dynamics in nitrifying reactors: Experimental evidence explained by a simple model including interspecies competition. Process Biochem 43:1398–1406. https://doi.org/10.1016/j.procbio.2008.08.013
Wang J, Gong B, Wang Y, Wen Y, Zhou J, He Q (2017) The potential multiple mechanisms and microbial communities in simultaneous nitrification and denitrification process treating high carbon and nitrogen concentration saline wastewater. Biores Technol 243:708–715
Wanner O, Gujer W (1986) A multispecies biofilm model. Biotechnol Bioeng 28:314–328. https://doi.org/10.1002/bit.260280304
Wanner O, Reichert P (1996) Mathematical modeling of mixed-culture biofilms. Biotechnol Bioeng 49:172–184. https://doi.org/10.1002/(SICI)1097-0290(19960120)49:2%3c172::AID-BIT6%3e3.0.CO;2-N
Wett B, Rauch W (2003) The role of inorganic carbon limitation in biological nitrogen removal of extremely ammonia concentrated wastewater. Water Res 37:1100–1110
Wunderlin P, Mohn J, Joss A, Emmenegger L, Siegrist H (2012) Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res 46:1027–1037. https://doi.org/10.1016/j.watres.2011.11.080
Xia S, Li J, Wang R (2008) Nitrogen removal performance and microbial community structure dynamics response to carbon nitrogen ratio in a compact suspended carrier biofilm reactor. Ecol Eng 32:256–262. https://doi.org/10.1016/j.ecoleng.2007.11.013
Zeng RJ, Lemaire R, Yuan Z, Keller J (2004) A novel wastewater treatment process: simultaneous nitrification, denitrification and phosphorus removal. Water Sci Technol 50:163–170. https://doi.org/10.2166/wst.2004.0635
Zinatizadeh AAL, Ghaytooli E (2015) Simultaneous nitrogen and carbon removal from wastewater at different operating conditions in a moving bed biofilm reactor (MBBR): process modeling and optimization. J Taiwan Inst Chem Eng 53:98–111
Acknowledgements
The authors are thankful to Ministry of Human Resource Development, Government of India, for financial assistance in form of research fellowship during this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Nour Sh. El-Gendy.
Rights and permissions
About this article
Cite this article
Bhattacharya, R., Mazumder, D. Mathematical modelling of simultaneous nitrification and denitrification in biological reactor systems – a review. Int. J. Environ. Sci. Technol. 20, 8105–8126 (2023). https://doi.org/10.1007/s13762-022-04359-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04359-6